Risperidone and Haloperidol in First-Episode Psychosis: A Long-Term Randomized Trial
Abstract
OBJECTIVE: The first episode of psychotic illness is a key intervention point. The initial experience with medication can affect willingness to accept treatment. Further, relapse prevention is a treatment cornerstone during the first years of illness because active psychotic illness may affect lifetime outcomes. Thus, initial treatment of active symptoms and subsequent relapse prevention are central goals of pharmacotherapy. This study compared long-term effectiveness of risperidone versus haloperidol in first-episode psychosis patients. METHOD: First-episode psychosis patients (N=555, mean age=25.4 years) participated in a double-blind, randomized, controlled flexible-dose trial that compared risperidone (mean modal dose=3.3 mg) and haloperidol (mean modal dose=2.9 mg). The median treatment length was 206 days (maximum=1,514). RESULTS: Positive and Negative Syndrome Scale scores and Clinical Global Impression ratings improved significantly relative to baseline, with no significant differences between groups. Three-quarters of the patients achieved initial clinical improvement, defined as >20% reduction in total Positive and Negative Syndrome Scale score. However, among those who achieved clinical improvement, 42% of the risperidone group experienced a relapse compared with 55% of the haloperidol group. The median time to relapse was 466 days for risperidone-treated subjects and 205 days for those given haloperidol. These differences were statistically significant based on Kaplan-Meier survival analysis. Adverse effects distinguished the treatments: there were significantly more extrapyramidal signs and symptoms and adjunctive medication use in the haloperidol group and greater prolactin elevation in the risperidone group. There was less weight gain with haloperidol initially but no significant differences between groups at endpoint. CONCLUSIONS: Relatively low doses of antipsychotic drugs lead to significant symptom amelioration in the majority of first-episode psychosis patients. In the long term, risperidone prevents relapse in more patients and for a longer time and also induces less abnormal movements than haloperidol.
Following the first episode of psychosis, treatment with antipsychotic medication is associated with rapid improvement of symptoms in a majority of individuals (1–7). First-episode patients appear to respond to relatively low doses of antipsychotic medication (4, 8) and manifest high sensitivity to extrapyramidal signs and symptoms (9). Unfortunately, because of the chronic undulating course of psychosis in schizophrenia, the majority of first-episode patients experience a relapse within the first year after clinical improvement or remission, either because of medication discontinuation or despite continuous treatment (10). Although treating acute symptoms and preventing relapse are important at any time during the illness and at any age, it is particularly critical in adolescents and young adults and during the first few years of the illness. This is because the illness may be more active in the initial phases, with frequent and distinct cycles of remission and exacerbations (4). Also, late adolescents and early adulthood are critical years for social and vocational development. Hence, illness control might have an impact on life-long outcomes. Reducing the number of relapses and increasing the time spent with few or no symptoms is therefore a major goal of pharmacological treatment.
A number of meta-analyses have tried to determine to what extent the novel antipsychotics are superior to the older-generation antipsychotics (11, 12), specifically in terms of maintaining symptomatic improvement (13) and preventing relapse (14). A previous trial designed to compare the efficacy of risperidone versus haloperidol in preventing relapse in stable outpatients with chronic schizophrenia or schizoaffective disorder (15) reported significantly fewer occurrences of relapse with risperidone and after a significantly longer time of treatment. Patients treated with risperidone were about half as likely to experience relapse than those treated with haloperidol. The current study compared the efficacy of risperidone and haloperidol in preventing relapse in first-episode psychosis patients. We also compared long-term symptom efficacy and adverse effect profiles of the medications.
Method
The study, sponsored by Johnson & Johnson Pharmaceutical Research and Development, enrolled patients in 11 countries between November 1996 and January 2000 with planned treatment until the last enrolled participant completed 2 years of treatment. The study was conducted in accordance with good clinical practice after it was approved by the local institutional review boards.
Subjects
Consenting 16–45-year-old patients were enrolled into the trial if they 1) met Structured Clinical Interview for DSM-IV criteria for schizophrenia, schizophreniform disorder, or schizoaffective disorder for no more than 1 year during which period they had no more than two psychiatric hospitalizations for psychosis; and 2) had less than 12 weeks of cumulative exposure to antipsychotics and required antipsychotic treatment upon enrollment into the trial. Patients were excluded from the trial for any of the following reasons: 1) meeting DSM-IV criteria for another axis I diagnosis, including substance dependence or abuse; 2) needing another nonantipsychotic psychotropic medication at enrollment; or 3) having a serious or unstable medical illness.
Study Design and Procedures
Before entering the study, subjects provided informed consent after the procedure had been fully explained. Subjects admitted to this double-blind trial were randomly allocated to receive either risperidone or haloperidol according to a 1:1 randomization scheme balanced by site. Before administration of trial medication patients had a 3–7-day drug washout period that was waived for extremely ill patients. Subjects in both treatment groups started with a once daily dose of 1 mg that could be increased to 2 mg/day on day 4 and thereafter by 1 mg/day each week, up to a maximum daily dose of 4 mg. In exceptional cases (i.e., subjects showing insufficient response in whom not more than mild extrapyramidal signs and symptoms were observed at 4 mg/day), the dose could then be increased further by 1 mg a week up to a maximum daily dose of 8 mg. Concomitant psychotropic medications allowed were those addressing extrapyramidal signs and symptoms; chloral hydrate, zolpidem, or flurazepam for sleep; and lorazepam for agitation.
Assessments
Outcomes were measured in five domains: 1) relapse, 2) psychopathology, 3) safety, 4) quality of life, and 5) neurocognitive functioning (the latter two are reported in separate manuscripts). Psychopathology was assessed using the Positive and Negative Syndrome Scale (16) and Clinical Global Impression (CGI) severity and change scales (17).
Relapse was examined among patients who reached clinical improvement (decrease of more than 20% on total Positive and Negative Syndrome Scale score) and was defined according to Csernansky et al. criteria (15) as any one of the following occurring after clinical improvement: 1) 25% or more increase in score on the Positive and Negative Syndrome Scale (or a 10-point increase if initial score was 40 or less); 2) CGI change rating of “much worse” or “very much worse”; 3) deliberate self-injury (as a reported adverse event); 4) emergence of clinically significant suicidal or homicidal ideation (as a reported adverse event) or completed suicide; or 5) violent behavior resulting in significant injury to another person or significant property damage (as a reported adverse event).
Abnormal involuntary movements were assessed with the Extrapyramidal Symptom Rating Scale (18). Adverse effects were recorded with standard recording forms.
Assessments with the Positive and Negative Syndrome Scale, CGI, and Extrapyramidal Symptom Rating Scale were completed weekly during the first 4 weeks of the trial and then every 4 weeks for the next 5 months. During months 6–15, the instruments were completed every 2 months and every 3 months thereafter. Follow-up evaluations were conducted until the last patient enrolled had completed 2 years of treatment. The blind was broken when the study ended.
Statistical Analysis
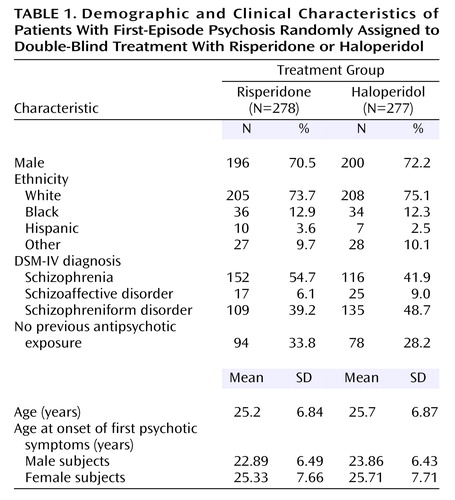
Five hundred fifty-nine patients from 11 countries were randomly assigned to receive either haloperidol or risperidone. Three patients assigned to risperidone and one patient assigned to haloperidol did not receive study medication and were thus excluded from the analysis. Therefore, 278 patients treated with risperidone and 277 with haloperidol were included in the analysis. The data from all randomized and treated patients were analyzed for safety. Before breaking the blind, 11 subjects receiving risperidone and 10 subjects receiving haloperidol, all from the same site, were excluded from the efficacy analyses because of violations of good clinical practice. Exclusion of these data did not change the findings. Baseline characteristics and duration of treatment were compared between the two groups by analysis of variance or chi-square test for categorical variables and summarized by descriptive statistics.
Differences between the groups in the degree of change from baseline in scores on the Positive and Negative Syndrome Scale and Extrapyramidal Symptom Rating Scale were evaluated with analysis of covariance after we controlled for baseline scores and tested for center-by-treatment interactions. For the Extrapyramidal Symptom Rating Scale, change for each patient was examined from baseline to maximum score at any time point. Differences on CGI change scale were also tested but with no baseline controls.
Further analysis of dyskinesia used the Extrapyramidal Symptom Rating Scale dyskinetic movement scale to operationalize dyskinesia on the basis of criteria of Schooler and Kane (19). Those criteria were originally developed for the Abnormal Involuntary Movement Scale (20), which contains almost identical items on a 5-point scale. Emergent dyskinesia was defined according to the Schooler and Kane criteria as an increase from baseline of 3 points or more on one item or 2 points or more on two items of the seven-item dyskinetic movement scale. Persistent dyskinesia was defined as emergent dyskinesia that met the criteria on two or more consecutive visits.
Relapse rates, which were part of the planned analysis, were analyzed according to the aforementioned Csernansky et al. criteria (15). As planned, the relapse analysis included only those patients who had reached clinical response as defined as a >20% decrease in score on the total Positive and Negative Syndrome Scale. A dichotomous variable was created that assigned a value of 1 once a patient experienced a relapse. Time to relapse was calculated as the number of days elapsing from clinical improvement to the first relapse using Kaplan-Meier survival analysis. Cox regression analysis was performed to test for possible interaction effects of treatment group and study center. The differences in time to relapse between treatment groups were analyzed by using Cox proportional hazards model and log rank test after we controlled for center. Follow-up evaluations for endpoint analyses ceased after the discontinuation of treatment. The analysis of time to relapse was therefore censored at the time of treatment discontinuation if it occurred before relapse. All statistical tests were two-tailed. All analyses tested for study center-by-treatment interactions; no such interactions were found.
Results
The characteristics of the 555 patients in the two treatment groups were similar (Table 1). About half had a diagnosis of schizophrenia, 70% were male, and their mean age was 25 years.
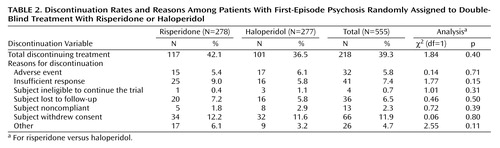
Subjects were treated with trial medication for a median of 192 days (range=2–1,502) in the risperidone group and for a median of 218 days (range=1–1,514) in the haloperidol group (Mann-Whitney z=0.116, p=0.90). The mean modal total daily dose was 3.3 mg for risperidone and 2.9 mg for haloperidol. The most commonly taken daily dose for each of the drugs (mode dose) was 3 mg. Two hundred eighteen subjects (117 in the risperidone group and 101 in the haloperidol group) discontinued double-blind treatment prematurely. As can be seen in Table 2, there were no significant differences between groups in overall discontinuation or specific reasons for discontinuation.
After 3 months, 73.6% (N=192) of patients randomly assigned to risperidone showed clinical improvement (>20% decrease in Positive and Negative Syndrome Scale score) as did 76.2% (N=199) of those receiving haloperidol (χ2=0.50, df=1, p=0.48). Kaplan-Meier survival analysis found that at study endpoint slightly more than three-quarters of the patients in each group (risperidone, N=197 [75.5%]; haloperidol, N=203 [77.8%]) met the predefined clinical improvement criterion, with a median time to clinical improvement of 26 days in the risperidone group and 22 days in the haloperidol group (log rank=1.49, p=0.22). As shown in Table 3, both groups showed clinical improvement according to Positive and Negative Syndrome Scale scores and CGI ratings, with no significant differences between the risperidone and haloperidol groups. According to CGI change ratings, 50.8% of the patients were “much” or “very much improved,” and 81.4% were at least minimally improved.
Among those patients who achieved clinical improvement (risperidone, N=197; haloperidol, N=203), there were significantly fewer relapses in the risperidone group (42.1%) than in the haloperidol group (54.7%). The time to relapse for the risperidone group was significantly longer than for the haloperidol group (risperidone median=466 days; haloperidol median=205 days). Figure 1 presents Kaplan-Meier plot of time from clinical response until relapse, the illustrated difference between the curves is highly significant (log rank=7.10, df=1, p=0.008). Significant differences between the groups emerged by 145 days, at which time there were 58 events of relapse in the risperidone group and 80 in the haloperidol group (mean days to relapse=114 for risperidone, 102 for haloperidol) (log-rank test comparing survival curves, p<0.04).
Safety
Treatment-emergent extrapyramidal signs and symptoms were significantly more frequent and more severe in the haloperidol-treated group as reflected by the scores on the Extrapyramidal Symptom Rating Scale (Table 4). There was significantly less emergent dyskinesia in the risperidone group than in the haloperidol group but no significant difference in persistent dyskinesia. On the specific Extrapyramidal Symptom Rating Scale subscales, the risperidone group had significantly lower maximum change in score from baseline on total, parkinsonism, and parkinsonism dystonia (due primarily to the difference in parkinsonism) symptoms. Significantly lower akathisia scores were seen in the risperidone-treated group as well as a tendency for lower dyskinesia scores.
Similarly, there were also differences in the rates of patients who received concomitant medications as treatments for extrapyramidal signs and symptoms. For the haloperidol- and risperidone-treated patients, 49.5% (N=137) and 41.7% (N=116), respectively, received anticholinergic agents (χ2=3.34, df=1, p<0.07); 61.7% (N=171) and 54.7% (N=152) received benzodiazepines to control agitation as well as extrapyramidal signs and symptoms (χ2=2.84, df=1, p<0.10); and 10.5% (N=29) and 5.0% (N=14) received beta blocking agents to control akathisia (χ2=5.73, df=1, p<0.02).
Forty-six patients expressed suicidal ideations during the trial. This was 9.4% (N=26) of the haloperidol group, in which there were three completed suicides, versus 7.2% (N=20) of the risperidone group, in which no suicides were completed.
Significantly more weight gain was observed in the risperidone group early in treatment. At the third month of treatment the risperidone group (N=180) had gained on average 4.6 kg (SD=4.96) and the haloperidol group (N=180) 3.5 kg (SD=4.42) (t=5.41, df=358, p=0.03). At endpoint the difference in weight gain between the treatment groups was no longer significant (risperidone [N=211]: mean=7.5 kg, SD=9.29; haloperidol [N=204]: mean=6.5 kg, SD=8.86) (t=1.13, df=413, p=0.26).
There were no notable differences between the treatment groups for vital signs, reported adverse events, or ECG parameters. On laboratory parameters, the only notable difference was that maximum prolactin levels (ng/ml) were higher in the risperidone group (women [N=73]: mean=73.69, SD=53.18; men [N=185]: mean=34.08, SD=21.90) than in the haloperidol group (women [N=71]: mean=48.16, SD=47.82; men [N=178]: mean=21.81, SD=14.54) (for women: t=3.03, df=142, p<0.003; for men: t=6.31, df=361, p<0.0001). There were abnormal prolactin values (males >18 ng/ml; females >25 ng/ml) in 73.8% (N=189 of 256) of the risperidone patients and in 49.8% (N=124 of 249) of the haloperidol-treated patients. Prolactin-related adverse effects were reported in 14 risperidone-treated patients and one haloperidol-treated patient. All patients with prolactin-related adverse effects had abnormal prolactin levels. Specifically, among the risperidone patients, there were three patients with gynecomastia, six with hyperprolactinemia, and six with galactorrhea. One patient had both hyperprolactinemia and galactorrhea. There was one case of hyperprolactinemia in the haloperidol group. Moderate hyperglycemia was reported as an adverse effect in one risperidone-treated subject.
Discussion
This study both confirms findings regarding treatment of the first episode of schizophrenia and extends our understanding of the role of medication during this critical period of the illness. In the present study we found initial symptom improvement in a carefully defined first-episode patient group treated with low doses of either a conventional antipsychotic (haloperidol) or an atypical medication (risperidone). This finding is in agreement with results of studies with older antipsychotic medications (1, 8, 21). It is also in accord with results of studies of second-generation antipsychotic medications (5, 7). However, even when dosage is appropriately managed for first-episode patients, haloperidol (at a mean dose of 2.9 mg/day) is associated with significantly greater acute extrapyramidal signs and symptoms and a greater need for concomitant medications to treat those side effects than risperidone. The favorable response rate to antipsychotic medication in this study was similar to rates reported by other trials treating recent-onset psychosis (7, 21).
The further and unique contributions of this study are a function of the long duration of treatment and the fact that it was a randomized, double-blind trial. To our knowledge this is the longest such trial to compare an older antipsychotic medication and an atypical agent. As a result, we were able to study the process of relapse after the initial response. The finding of an increased risk of relapse in haloperidol-treated subjects relative to those treated with risperidone is of great clinical significance. Because treatment for all participants continued until the last participant enrolled had an opportunity for 2 years of treatment, the earliest enrolled patients could be treated and followed for almost 6 years. Using this design we observed a significant increase in time to relapse for risperidone that emerged as early as 100 days after first clinical improvement and persisted until the end of the trial. The substantial delay in relapse with risperidone would not have been detected in a brief trial. Inspection of the survival curves also reveals that the magnitude of the difference does not decrease over time. Despite this substantial clinical advantage for risperidone, the extended observation period makes it clear that relapse does occur but that risperidone serves to delay and prevent that event.
The most frequent and disturbing adverse effects associated with treatment—specifically extrapyramidal signs and symptoms, including emergent dyskinesia and akathisia—were less prevalent in the risperidone-treated patients than in the haloperidol-treated patients. The lower prevalence of extrapyramidal signs and symptoms in the risperidone-treated patients has possible implications regarding adherence to medications, since extrapyramidal signs and symptoms are associated with poorer compliance (22). There are also possible implications for suicide prevention, since suicidality may also be related to extrapyramidal signs and symptoms (23). Reports of suicidal ideation as an adverse event did not differ significantly between the two treatment groups. Three completed suicides all occurred in the haloperidol group; this represents 1.08% over the course of the trial. The expected rate based on epidemiological studies of first-episode schizophrenia is estimated to be between 1% and 2% for the first year after initial hospitalization (24).
The novel atypical antipsychotics produce less extrapyramidal signs and symptoms than typical ones, but as seen in the present study, they are associated with a greater risk of weight gain (25). We found weight gain with both haloperidol and risperidone: significantly more gain with risperidone at the 3-month point (the first time point at which weight was measured following baseline), but no significant difference at endpoint. Furthermore, it appears that younger, recent-onset psychosis patients are more vulnerable to antipsychotic-induced weight gain than older patients with more chronic illness. Since most recent-onset patients are targeted with novel atypical agents, weight gain is a major consideration in selecting the specific atypical drug. A recent conference on antipsychotic drugs, obesity, and diabetes (26) concluded that clozapine and olanzapine are associated with the greatest weight gain and that risperidone and quetiapine have intermediate effects.
Risperidone treatment was associated with significantly greater elevation of prolactin in both men and women compared with haloperidol. Seventy-four percent of risperidone patients developed abnormal prolactin levels at some point during the trial, compared with 49% of the haloperidol-treated patients. These elevated prolactin levels were rarely associated with reports of prolactin-related adverse events.
A limitation of randomized controlled trials is that individuals who agree to participate in such trials may not be representative of the patients treated in routine clinical practice or of the general population of individuals suffering from schizophrenia, and therefore the results are not generalizable. To address this limitation, we compared the baseline characteristics of the subjects enrolled in this clinical trial to a large-scale epidemiological sample of first-episode patients previously collected in the United States (the Suffolk County Mental Health Project) (27). We found that 33% (N=59) of the epidemiological sample would not have met inclusion criteria for the present drug trial (because of antidepressant treatment, N=26; current substance abuse, N=18; recent suicide attempt, N=9; or for more than one reason, N=6). There were no significant differences between the two study groups on age at onset, age, gender, or premorbid functioning. Drug trial patients had more severe clinical symptoms, slightly lower CGI ratings, and less formal education than those in the epidemiological study. Although the epidemiological sample itself was from a limited geographic area, the general characteristics of these two study groups were similar on several key variables, supporting the generalizability of the findings. A second threat to generalizability concerns overall retention in the trial. As shown in Table 2 there were no treatment differences in withdrawal from the trial because of competing risks. Discontinuation due to relapse represented an outcome of the study. Overall, the percent discontinued was just under 40%.
The study raises many questions that call for further research. The first has to do with the determinants of relapse. In this trial, medication adherence was closely monitored. However, with oral medication in outpatients, lack of adherence to medication may have occurred. Robinson et al. (3), who followed first-episode patients regardless of treatment status, found that nonadherence was the strongest predictor of relapse in their study. It is clear that oral risperidone delays relapse substantially. Whether control for adherence through use of a long-term injectable form of medication, which is now available for risperidone, would delay relapse further needs to be investigated. A second important question is whether the delay of relapse seen in two long-term studies with risperidone is specific to risperidone or is a class effect that will be seen with other atypical antipsychotics such as olanzapine, quetiapine, ziprasidone, and aripiprazole. However, the combination of greater delay of relapse and a reduced emergent dyskinesia strongly support the use of risperidone early in a schizophrenic illness.
In summary, this report of the largest treatment study of first-episode psychosis patients, which also featured an unusually long duration, contributes to the emerging body of knowledge on the treatment response of first-episode psychosis patients and the comparative efficacy and safety of the atypical and conventional antipsychotic drugs. The present study results demonstrate a clear advantage for risperidone relative to haloperidol in terms of relapse prevention and in diminished incidence of extrapyramidal signs and symptoms.
Acknowledgments
The Early Psychosis Global Working Group comprised the following investigators: Australia—P. McGorry (Melbourne); T. Lambert (Bentley); J. Kulkarni (Dandenong); Austria—W. Fleischhacker (Innsbruck); Canada—D. Addington (Calgary); L. Kopala (Halifax, N.S.); R. Williams (Calgary); G. Chouinard (Montreal); A. Labelle (Ottawa); A. Malla (London, Ont.); S. Purdon (Edmonton); M. Saxena (Hamilton, Ont.); V. Nair (Montreal); R. Matte (Sherbrooke, Que.); S. Johnson (St. John’s, Nfld.); L. Beauclair (Montreal); Finland—K. Lehtinen (Tampere); France—E.R. Lombertie (Limoges); J-A. Meynard (La Rochelle); Germany—H. Freyberger (Stralsund); H-J. Möller (Munich); Israel—A. Caspi; M. Davidson (Ramat-Gan); A. Elitzur (Bat-Yam); M. Kotler (Beer Sheva); I. Treves (Hod-Hasharon); A. Weizman (Petach-Tikva); the Netherlands—P. Dries (Portugaal); New Zealand—D. Codyre (Auckland); South Africa—R. Emsley (Cape Town); C. Gagiano (Bloemfontein); United Kingdom—M. Reveley (Leicester); T. Sharma (London); United States—J. Csernansky (St. Louis); L. DeLisi (Stony Brook, N.Y.); J. Lauriello (Albuquerque); T. Manschreck (Fall River, Mass.); J. Pahl (Oklahoma City); N. Schooler, M. Keshavan (Pittsburgh); S. Schulz (Cleveland); S. Targum (Philadelphia); S. Risch (Charleston, S.C.).
 |
 |
 |
 |
Received Feb. 4, 2004; revision received April 22, 2004; accepted May 14, 2004. From the Early Psychosis Global Working Group. Address correspondence and reprint requests to Dr. Rabinowitz, Hurwich Professor, Bar Ilan University, Ramat Gan, Israel; [email protected] (e-mail). Supported by Johnson & Johnson Pharmaceutical Research and Development.
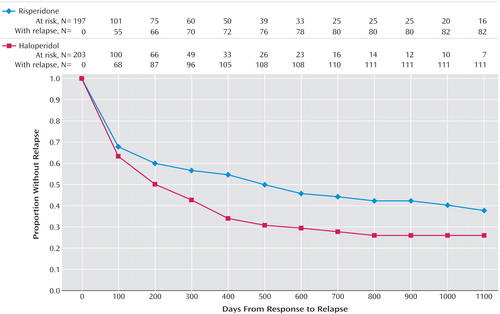
Figure 1. Time to Relapse Among Patients With First-Episode Psychosis Who Had Responded to Their Randomly Assigned Double-Blind Treatment With Risperidone or Haloperidol
1. May PR, Tuma AH, Dixon WJ, Yale C, Thiele DA, Kraude WH: Schizophrenia: a follow-up study of the results of five forms of treatment. Arch Gen Psychiatry 1981; 38:776–784Crossref, Medline, Google Scholar
2. Sheitman BB, Lee H, Strauss R, Lieberman JA: The evaluation and treatment of first-episode psychosis. Schizophr Bull 1997; 23:653–661Crossref, Medline, Google Scholar
3. Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241–277Crossref, Medline, Google Scholar
4. Lieberman JA, Sheitman B, Chakos M, Robinson D, Schooler N, Keith S: The development of treatment resistance in patients with schizophrenia: a clinical and pathophysiologic perspective. J Clin Psychopharmacol 1998; 18(2 suppl 1):20S-24SGoogle Scholar
5. Emsley RA (Risperidone Working Group): Risperidone in the treatment of first-episode psychotic patients: a double-blind multicenter study. Schizophr Bull 1999; 25:721–729Crossref, Medline, Google Scholar
6. Sanger TM, Lieberman JA, Tohen M, Grundy S, Beasley C Jr, Tollefson GD: Olanzapine versus haloperidol treatment in first-episode psychosis. Am J Psychiatry 1999; 156:79–87Link, Google Scholar
7. Lieberman JA, Tollefson G, Tohen M, Green AI, Gur RE, Kahn R, McEvoy J, Perkins D, Sharma T, Zipursky R, Wei H, Hamer MM (HGDH Study Group): Comparative efficacy and safety of atypical and conventional antipsychotic drugs in first-episode psychosis: a randomized, double-blind trial of olanzapine versus haloperidol. Am J Psychiatry 2003; 160:1396–1404; correction, 160:1901Link, Google Scholar
8. McEvoy JP, Hogarty GE, Steingard S: Optimal dose of neuroleptic in acute schizophrenia: a controlled study of the neuroleptic threshold and higher haloperidol dose. Arch Gen Psychiatry 1991; 48:739–745Crossref, Medline, Google Scholar
9. Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA: Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 1995; 152:1724–1729Link, Google Scholar
10. Nuechterlein GK, Subotnik KL, Ventura J, Mintz J, Fogelson DL, Bartzokis G, Aravagiri M: Clinical outcome following neuroleptic discontinuation in patients with remitted recent-onset schizophrenia. Am J Psychiatry 2001; 158:1835–1842Link, Google Scholar
11. Davis JM, Chen NY, Glick ID: A meta-analysis of the efficacy of second generation antipsychotics. Arch Gen Psychiatry 2003; 60:553–564Crossref, Medline, Google Scholar
12. Geddes J, Freemantle N, Harrison P, Bebbington P: Atypical antipsychotics in the treatment of schizophrenia: systematic overview and meta-regression analysis. BMJ 2000; 321:1371–1376Crossref, Medline, Google Scholar
13. Leucht S, Pitschel-Walz G, Abraham D, Kissling W: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophr Res 1999; 35:51–68Crossref, Medline, Google Scholar
14. Leucht S, Barnes TRE, Kissling W, Engel RR, Correll C, Kane JM: Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry 2003; 160:1209–1222Link, Google Scholar
15. Csernansky JG, Mahmoud R, Brenner R: A comparison of risperidone and haloperidol for the prevention of relapse in patients with schizophrenia. N Engl J Med 2002; 346:16–22Crossref, Medline, Google Scholar
16. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
17. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76–338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
18. Chouinard G, Ross-Chouinard A, Annable L, Jones B: Extrapyramidal Symptom Rating Scale (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
19. Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia (letter). Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
20. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76–338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
21. Robinson DG, Woerner MG, Alvir JMJ, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Bilder R, Goldman R, Lieberman JA: Predictors of treatment response from a first episode of schizophrenia or schizoaffective disorder. Am J Psychiatry 1999; 156:544–549Link, Google Scholar
22. Weiden P, Aquila R, Standard J: Atypical antipsychotic drugs and long-term outcome in schizophrenia. J Clin Psychiatry 1996; 57:53–60Medline, Google Scholar
23. Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer J-P, Potkin S (InterSePT Study Group): Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003; 60:82–91Crossref, Medline, Google Scholar
24. Mortensen PB: Suicide among schizophrenic patients: occurrence and risk factors. Clin Neuropharmacol 1995; 18:1–8Crossref, Medline, Google Scholar
25. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156:1686–1696Abstract, Google Scholar
26. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity: Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27:596–601Crossref, Medline, Google Scholar
27. Rabinowitz J, Bromet E, Davidson M: Are patients enrolled in first episode psychosis drug trials representative of those treated in routine clinical practice? Schizophr Res 2003; 61:149–155Crossref, Medline, Google Scholar



