Cosegregation of Bipolar Disorder and Autosomal-Dominant Medullary Cystic Kidney Disease in a Large Family
Abstract
OBJECTIVE: The authors report a large family in which bipolar disorder appears to cosegregate with autosomal-dominant medullary cystic kidney disease. METHOD: Information regarding diagnostic criteria for bipolar disorder and medullary cystic kidney disease were gathered from family members through formal research interviews, hospital admission records, imaging reports, and laboratory data. RESULTS: Of the seven members with medullary cystic kidney disease, five had bipolar I disorder, one had unipolar depression, and one had a hyperthymic phenotype. Information was not available on two members. CONCLUSIONS: The cosegregation in this family suggests a close proximity between genes for the two disorders. The two known loci of medullary cystic kidney disease are in regions of chromosomes 1 and 16 that have been previously linked to bipolar disorder and schizophrenia. This family may be a useful resource for positional cloning of bipolar candidate genes.
Bipolar disorder has been shown to have a strong genetic basis and is felt likely to be a complex genetic trait and an oligogenic disease. Regions on at least eight chromosomes have been linked to bipolar disorder (1, 2).
There are two known loci for medullary cystic kidney disease, a dominant condition leading to end-stage renal failure requiring transplantation in the third to fifth decade of life. Diagnosis is made from a combination of family history, polyuria with normal urinalysis, computerized tomography scan or ultrasound, and histological features, including tubular atrophy, interstitial fibrosis, and basement membrane thickening with material having a positive response to periodic acid–Schiff stain (3, 4).
We have found no previous reports of bipolar disorder cosegregating with medullary cystic kidney disease.
Method
This family was identified as part of a multisite consortium project to collect families with bipolar disorder for linkage studies. The ascertainment and diagnostic methods have been described in detail elsewhere (5). Written informed consent was obtained by using procedures approved by the University of California, San Diego, Human Research Protection Program. Following an interview with the Structured Clinical Interview for DSM-III-R (SCID) (6), best-estimate consensus diagnoses were made by a panel of experienced clinicians who reviewed the SCID, medical records, and information from family informants when available. The diagnoses of medullary cystic kidney disease were made by the treating nephrologist, based on direct examination of the patient, family history, autopsy report, histology done on organs after transplant, sonogram, clinical history, and the presence of an elevated uric acid level.
Results
The family’s pedigree is illustrated in Figure 1. No information was available on two siblings. All six half-siblings on whom information was available suffer from medullary cystic kidney disease. Members 1, 2, 6, and 7 had renal transplants between the ages of 24 and 37 years.
Members 2, 6, and 9 have bipolar I disorder according to the SCID interviews. Multiple family members, when questioned about members 4 and 8, were able to describe manic episodes meeting the DSM-IV criteria for duration, number of symptoms, and level of dysfunction. Member 1 received a diagnosis of major depressive disorder, recurrent. Member 7 reported a nonepisodic, lifelong history of high energy, successfully taking on multiple projects, and routinely needing only 4 hours of sleep per night. Member 7 met the criteria for hyperthymic temperament developed by Akiskal et al. (11), which were assessed by using the TEMPS-A instrument, a self-rated version of the TEMPS-I.
Discussion
The age-corrected lifetime risk of bipolar disorder is 0.3%–1.5% in the general population. First-degree relatives of an individual with bipolar disorder are seven times as likely to be affected as are people in the general population (12). Even with a bipolar parent, however, an individual with medullary cystic kidney disease in this family has a much higher risk of bipolar disorder than expected. Although four of the eight half-siblings may have a greater genetic loading because of a parent with depression, cosegregation is suggested in both sibships.
It is also possible that bipolar disorder is a result of medullary cystic kidney disease or its treatment. Although there are a few case reports of end-stage renal disease producing an organic mania syndrome (13–15) and transplant medications, such as glucocorticoids, have been associated with psychosis and depression, members of this pedigree met criteria for bipolar disorder before displaying evidence of medullary cystic kidney disease.
Cosegregation could be the result of the genes for medullary cystic kidney disease and bipolar disorder mapping close to each other on the same chromosome, with each gene carrying distinct point mutations. It is also possible that a mutation in one gene is responsible for both disorders. Finally, a chromosomal rearrangement, such as a deletion or inversion, could yield a breakpoint in genes for both disorders. Any of these possibilities could greatly facilitate the identification of a susceptibility gene for bipolar disorder.
Studies have narrowed the locus for type 1 medullary cystic kidney disease to a <650-kilobase (kb) interval centered on marker D1S303 on 1q21 (16–18). Brzustowicz et al. (9) reported a linkage 3 centimorgans (cM) away, between schizophrenia and marker D1S1653, with a maximum lod score of 6.50. Although members of the family reported here display bipolar disorder, and not schizophrenia, an overlap between the two disorders is seen in family studies and linkage analyses (19, 20).
The disease-causing gene for the locus for type 2 medullary cystic kidney disease on 16p has been confirmed as UMOD, which encodes the most common protein in urine, Tamm-Horsfall protein, or uromodulin (21). Linkage of bipolar disorder to marker D16S749, only 500 kb away from UMOD, was originally reported by Edenberg et al. (10) and has been replicated in an expansion of that study group with a peak lod score of 2.8 (7).
This family is striking not only for the cosegregation of these two illnesses but also for the high rate of transmission. This could be consistent with an unbalanced translocation or other rearrangement such that transmission of the alternative chromosome does not result in viable offspring. An initial low-resolution cytogenetic analysis failed to identify any gross rearrangement in one of the members. Higher-resolution cytogenetic and molecular studies are indicated.
This family may be a valuable asset in the effort to map genes for both traits. It also suggests the value of screening families with medullary cystic kidney disease for mood disorders in order to identify similar families for mapping studies.
Received July 10, 2003; revisions received Feb. 20, April 14, and Aug. 5, 2004; accepted Sept. 9, 2004. From the Department of Psychiatry, University of California, San Diego; the Department of Psychiatry, San Diego VA Healthcare System, La Jolla, Calif.; the Department of Molecular Medicine, Karolinska Institutet and Karolinska Sjukhuset, Stockholm; and the Department of Nephrology, St. Luke’s Hospital, Kansas City, Mo. Address correspondence and reprint requests to Dr. Kelsoe, Department of Psychiatry, 0603, University of California, San Diego, La Jolla, CA 92093-0603; [email protected] (e-mail). Supported by Novartis Pharma AG; by the VA Mental Illness Research, Education and Clinical Center of Veterans Integrated Service Network 22 (VISN22); by grants to Dr. Kelsoe from the VA, from NIMH (MH-47612, MH-59567), from the University of California, San Diego, Mental Health Clinical Research Center (MH-30914), and from the University of California, San Diego, General Clinical Research Center (M01 RR-00827); and by grants to Dr. Schalling from the Swedish Research Council, from the Karolinska Hospital and Institutet, from the Söderström Königska Foundation, and from the Söderberg Foundation. Dr. Kelsoe is a founder of and holds equity in Psynomics, Inc. The authors thank the family members who participated for making the study possible.
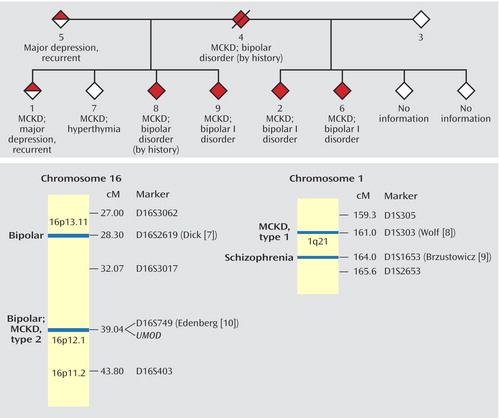
Figure 1. Relation of Medullary Cystic Kidney Disease (MCKD) and Bipolar Disorder Shown by Cosegregation in a Familya and by Correspondence in Regions of Chromosomes 16p and 1qb
aThe pedigree has been modified in order to protect subject confidentiality.
bThe chromosome map presents regions of chromosomes 16p and 1q for which nearby loci for bipolar disorder or schizophrenia have been reported. The first author of each linkage report is indicated next to the marker. The positions of genes for medullary cystic kidney disease and for Tamm-Horsfall protein (UMOD) are noted.
1. Johansson C, Jansson M, Linner L, Yuan QP, Pedersen NL, Blackwood D, Barden N, Kelsoe J, Schalling M: Genetics of affective disorders. Eur Neuropsychopharmacol 2001; 11:385–394Crossref, Medline, Google Scholar
2. Prathikanti S, McMahon FJ: Genome scans for susceptibility genes in bipolar affective disorder. Ann Med 2001; 33:257–262Crossref, Medline, Google Scholar
3. Hildebrandt F, Waldherr R, Kutt R, Brandis M: The nephronophthisis complex: clinical and genetic aspects. Clin Invest 1992; 70:802–808Crossref, Medline, Google Scholar
4. Scolari F, Viola BF, Prati E, Ghiggeri GM, Caridi G, Amoroso A, Casari G, Maiorca R: Medullary cystic kidney disease: past and present. Contrib Nephrol 2001; 136:68–78Crossref, Medline, Google Scholar
5. Kelsoe JR, Spence MA, Loetscher E, Foguet M, Sadovnick AD, Remick RA, Flodman P, Khristich J, Mroczkowski-Parker Z, Brown JL, Masser D, Ungerleider S, Rapaport MH, Wishart WL, Luebbert H: A genome survey indicates a possible susceptibility locus for bipolar disorder on chromosome 22. Proc Natl Acad Sci USA 2001; 98:585–590Crossref, Medline, Google Scholar
6. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
7. Dick DM, Foroud T, Edenberg HJ, Miller M, Bowman E, Rau NL, DePaulo JR, McInnis M, Gershon E, McMahon F, Rice JP, Bierut LJ, Reich T, Nurnberger J Jr: Apparent replication of suggestive linkage on chromosome 16 in the NIMH Genetics Initiative bipolar pedigrees. Am J Med Genet 2002; 114:407–412Crossref, Medline, Google Scholar
8. Wolf MTF, van Vlem B, Hennies HC, Zalewski I, Karle SM, Puetz M, Panther F, Otto E, Fuchshuber A, Lameire N, Loeys B, Hildebrandt F: Telomeric refinement of the MCKD1 locus on chromosome 1q21. Kidney Int 2004; 66:580–585Crossref, Medline, Google Scholar
9. Brzustowicz LM, Hodgkinson KA, Chow EW, Honer WG, Bassett AS: Location of a major susceptibility locus for familial schizophrenia on chromosome 1q21-q22. Science 2000; 288:678–682Crossref, Medline, Google Scholar
10. Edenberg HJ, Foroud T, Conneally PM, Sorbel JJ, Carr K, Crose C, Willig C, Zhao J, Miller M, Bowman E, Mayeda A, Rau NL, Smiley C, Rice JP, Goate A, Reich T, Stine OC, McMahon F, DePaulo JR, Meyers D, Detera-Wadleigh SD, Goldin LR, Gershon ES, Blehar MC, Nurnberger JI Jr: Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 3, 5, 15, 16, 17, and 22.Am J Med Genet 1997; 74:238–246Crossref, Medline, Google Scholar
11. Akiskal HS, Placidi GF, Maremmani I, Signoretta S, Liguori A, Gervasi R, Mallya G, Puzantian VR: TEMPS-I: delineating the most discriminant traits of the cyclothymic, depressive, hyperthymic and irritable temperaments in a nonpatient population. J Affect Disord 1998; 51:7–19Crossref, Medline, Google Scholar
12. NIMH: Report of the National Institute of Mental Health’s Genetic Workgroup. Biol Psychiatry 1999; 45:559–602Medline, Google Scholar
13. Baar JM: Organic mood disorder, manic type, associated with hyponatremia: a case report. Int J Psychiatry Med 1994; 24:223–228Crossref, Medline, Google Scholar
14. Jarecke CR, De Moya VF, Ware MR: A case of mania secondary to hemodialysis: successful treatment with clonazepam. J Clin Psychopharmacol 1990; 10:298–299Crossref, Medline, Google Scholar
15. Thomas CS, Neale TJ: Organic manic syndrome associated with advanced uraemia due to polycystic kidney disease. Br J Psychiatry 1991; 158:119–121Crossref, Medline, Google Scholar
16. Christodoulou K, Tsingis M, Stavrou C, Eleftheriou A, Papapavlou P, Patsalis PC, Ioannou P, Pierides A, Deltas CC: Chromosome 1 localization of a gene for autosomal dominant medullary cystic kidney disease. Hum Mol Genet 1998; 7:905–911Crossref, Medline, Google Scholar
17. Fuchshuber A, Deltas CC, Berthold S, Stavrou C, Vollmer M, Burton C, Feest T, Krieter D, Gal A, Brandis M, Pierides A, Hildebrandt F: Autosomal dominant medullary cystic kidney disease: evidence of gene locus heterogeneity. Nephrol Dial Transplant 1998; 13:1955–1957Crossref, Medline, Google Scholar
18. Stavrou C, Koptides M, Tombazos C, Psara E, Patsias C, Zouvani I, Kyriacou K, Hildebrandt F, Christofides T, Pierides A, Deltas CC: Autosomal-dominant medullary cystic kidney disease type 1: clinical and molecular findings in six large Cypriot families. Kidney Int 2002; 62:1385–1394Crossref, Medline, Google Scholar
19. Berrettini WH: Susceptibility loci for bipolar disorder: overlap with inherited vulnerability to schizophrenia. Biol Psychiatry 2000; 47:245–251Crossref, Medline, Google Scholar
20. Kelsoe JR: Recent progress in the search for genes for bipolar disorder. Curr Psychiatry Rep 1999; 1:135–140Crossref, Medline, Google Scholar
21. Wolf MT, Mucha BE, Attanasio M, Zalewski I, Karle SM, Neumann HP, Rahman N, Bader B, Baldamus CA, Otto E, Witzgall R, Fuchshuber A, Hildebrandt F: Mutations of the Uromodulin gene in MCKD type 2 patients cluster in exon 4, which encodes three EGF-like domains. Kidney Int 2003; 64:1580–1587Crossref, Medline, Google Scholar



