Neuropsychological Performance in Schizotypal Personality Disorder: Importance of Working Memory
Abstract
Background: Cognitive deficits consistently have been reported in schizophrenia patients and in patients with schizotypal personality disorder. For this study, the authors wanted to identify which of the domains of cognitive impairment represent “core” deficits of schizophrenia, comparing subjects with schizotypal personality disorder to two comparison groups: healthy volunteers and patients with personality disorders unrelated to schizophrenia. METHOD: Three groups completed a neuropsychological battery: patients with DSM-III-R schizotypal personality disorder (N=82); patients with DSM-III-R personality disorders unrelated to schizophrenia (i.e., a personality disorder other than schizotypal, schizoid, or paranoid [N=44]); and healthy volunteers (N=63). The battery included the California Verbal Learning Test, Trailmaking Test parts A and B, the Dot test of working memory, the Stroop Color and Word Test, the Paced Auditory Serial Addition Test, the WMS visual reproduction test, and the WAIS-R vocabulary and block design. RESULTS: Normative standards for performance that controlled for age, gender, and education were created from the scores of the healthy volunteers. Overall, schizotypal personality disorder patients performed significantly worse than the healthy volunteers and those with personality disorders unrelated to schizophrenia. Specifically, patients with schizotypal personality disorder demonstrated impaired performance on the Paced Auditory Serial Addition Test, WMS visual reproduction test, Dot test, and California Verbal Learning Test. In addition, in a regression analysis, performance on the Paced Auditory Serial Addition Test demonstrated the largest effect size. Indeed, it accounted for unique variance above and beyond all other cognitive measures, since controlling for Paced Auditory Serial Addition Test performance abolished group differences across all other measures. CONCLUSIONS: Patients with schizotypal personality disorder demonstrated moderate cognitive impairment compared with healthy volunteers (significant for seven out of 11 measures). These differences reached statistical significance for tasks of working memory, episodic memory, and delayed recall. Working memory performance accounted for the group differences. This study supports the view that working memory represents a core deficit of schizophrenia spectrum disorders.
There is ample evidence that schizophrenia patients demonstrate cognitive impairment (1). Moreover, cognitive impairment is more predictive of long-term daily functioning than the severity of psychotic symptoms (2, 3). Extensive research has attempted to characterize the nature of cognitive impairment in schizophrenia to develop strategies to improve cognitive function in these patients. Indeed, cognitive impairment in schizophrenia has become not only a focus in the research arena but also a treatment target for the novel antipsychotic medications, which no longer focus on just ameliorating psychotic symptoms but also on improving cognitive function.
It appears that cognitive impairment represents a common factor that is shared across the schizophrenia spectrum disorders. Schizotypal personality disorder represents the best characterized of the schizophrenia-related personality disorders and shares with schizophrenia genetic, biological, and treatment response characteristics (4–9). Unlike schizophrenia patients, who exhibit severe cognitive impairment across most cognitive functions, patients with schizotypal personality disorder exhibit moderate impairment across a few cognitive domains (4, 10–16). The study of patients with schizotypal personality disorder offers a unique opportunity to disentangle the pathophysiological mechanisms implicated in schizophrenia without the confounds of institutionalization, neuroleptic exposure, and other consequences of illness seen in schizophrenia. Thus, the study of patients with schizotypal personality disorder provides the opportunity to identify the mechanisms that are central to disease susceptibility rather than a consequence of it.
Working memory (referred to by Baddeley [17] as a “system that is necessary for the storage and manipulation of information,” a paradigm originally proposed in Baddeley and Hitch [18]) has been widely accepted as one of the key areas of cognitive impairment in schizophrenia patients (19, 20) (for review of working memory and schizophrenia see Goldman-Rakic [21]). However, there are several other key areas of cognitive impairment in schizophrenia spectrum disorders, such as attention (12, 22), executive function (23), or processing speed (24). While we should keep in mind that the possibility of a generalized deficit in schizophrenia may exist, there is the possibility that dysfunction in a key area may underlie dysfunctions in other related areas. It is logical to hypothesize that if an area of cognitive impairment represents such a “core” deficit, its effects should be observed across the entire schizophrenia spectrum but not in populations with psychopathology outside of the schizophrenia spectrum.
The goals of this study, which used the largest sample of patients with clinically diagnosed schizotypal personality disorder to date, were to 1) investigate whether these patients would demonstrate cognitive impairment relative to healthy comparison subjects, 2) examine whether this cognitive impairment would be specific to schizophrenia spectrum disorders, and 3) assess whether any of these cognitive domains represent a “core” area of neuropsychological dysfunction in the spectrum. In addition, we explored the relationship between these cognitive measures and the schizotypal symptom clusters (25), since there is evidence that cognitive deficits are related to deficits in social and other functional skills (3, 26).
Method
Subjects
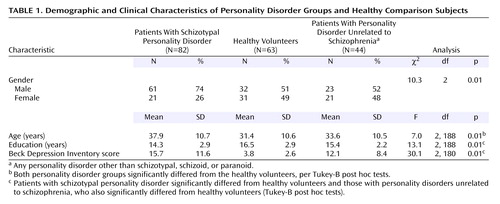
The study group included 1) 82 subjects who met criteria for DSM-III-R schizotypal personality disorder, 2) 44 subjects who met criteria for a personality disorder unrelated to schizophrenia (i.e., a personality disorder other than schizotypal, schizoid, or paranoid) and met no more than two DSM-III-R schizotypal personality disorder criteria, and 3) 63 healthy comparison subjects, all recruited by advertisement and word of mouth. Clinical and demographic characteristics of the study group are displayed in Table 1. All were studied as outpatients. (We have previously published data on 48 of the subjects with schizotypal personality disorder, 22 of those with personality disorders unrelated to schizophrenia, and 32 of the healthy volunteers [10].)
Written informed consent was obtained according to institutional guidelines. Subjects then underwent a medical evaluation (laboratory analyses and physical examination) and were excluded if there was evidence of systemic medical illness or neurological abnormalities, a history of significant head trauma (with loss of consciousness), or positive toxicology screen results. All subjects were evaluated by doctoral-level clinical psychologists with the Structured Clinical Interview for DSM-III-R (27) for axis I disorders and the Structured Interview for DSM-III-R Personality, Revised (28) for axis II disorders (kappa=0.73 for schizotypal personality disorder diagnosis [range=0.68–0.84 for each criterion]). Diagnoses were reached in a consensus meeting with an expert diagnostician (J.M.S.) in which the clinical interviewer presented information gathered from all sources. Patients were excluded if they met criteria for a psychotic disorder or bipolar I disorder, had met lifetime criteria for substance dependence or abuse in the preceding 6 months, or were currently taking psychotropic medications. Healthy comparison subjects had no history of axis I or axis II disorders and had no first-degree relative with an axis I disorder. The Beck Depression Inventory (29) was also given to all subjects on the day of testing in order to assess the effect, if any, of depressive symptoms on performance.
Consistent with the nature of personality disorders and reports from other centers (16), patients who met criteria for schizotypal personality disorder also met criteria for other axis II diagnoses, including paranoid personality disorder (N=41 [50%]), avoidant personality disorder (N=26 [32%]), and borderline personality disorder (N=22 [27%]). The personality disorders unrelated to schizophrenia mainly were borderline personality disorder (N=21 [48%]), obsessive-compulsive personality disorder (N=8 [18%]), or avoidant personality disorder (N=7 [16%]). (We report here only personality disorder diagnoses with group prevalence greater than 15%.) Patients with schizotypal personality disorder met criteria for a mean of 1.81 other personality disorders (SD=1.50), whereas the patients with personality disorders unrelated to schizophrenia met criteria for a mean of 1.40 other personality disorders (SD=0.79).
Cognitive Testing
Eleven cognitive measures of five general cognitive domains were included for this report (Table 2). From each measure one task was selected on which abnormal performance had been consistently demonstrated in numerous studies of schizophrenia patients as well as some previous studies of first-degree relatives and clinical subjects with schizotypal personality disorder.
Working memory assessments
The Dot test is a test of visuospatial working memory developed by Keefe et al. (30, 31). Subjects are presented a dot at a specific position on a standard size paper and then asked to reproduce it at the same location on a separate sheet after different periods of delay (no delay and after 10, 20, and 30 seconds). Performance is measured as the distance in cm between the drawn dot and the actual dot (distance error). The distance error at the 30-second delay (longest memory load of all three delays) minus the distance error at no delay is the dependent variable.
The Paced Auditory Serial Addition Test is a test of verbal (auditory) working memory that has been well described and validated (32). Briefly, subjects listen to a tape recorded voice presenting a series of numbers (50 numbers at a rate of one digit per 2 seconds) and are asked to add each adjacent pair of numbers and respond by verbalizing the sum. The total number of correct responses is the dependent variable.
Episodic memory assessments
The California Verbal Learning Test involves five presentations of a list of 16 words (four words from each of four semantic categories), with recall after each presentation (33). The total number of words recalled (trials 1 through 5) is the dependent variable.
The Wechsler Memory Scale (WMS) visual reproduction test is a test of memory for nonverbal stimuli. Four line drawings are presented one at a time for a 10-second exposure period. After the drawing is removed, the subject is asked to immediately draw the figure from memory. Performance is scored according to standardized criteria (34). The raw score is the dependent variable.
Delayed recall assessments
The California Verbal Learning Test contains multiple recall measures, with the number of free recalled words after a 20-minute delay the dependent variable used in this report.
As part of the WMS visual reproduction test, subjects are asked to draw the four items from memory after a 30-minute delay, with no prompts or cues provided. The raw score of the visual reproduction of the four figures after this delay is the dependent variable.
Processing speed assessments
The Trailmaking Test has two conditions that combine to assess verbal/spatial perception and psychomotor speed (35). In part A the subject must connect numbers presented on a standard sheet of paper in ascending order (1–2–3). In part B the subject must alternate connecting numbers and letters (for example, 1-A, 2-B, 3-C, etc.). The amount of time it takes to complete part A and part B are the two dependent variables.
The Stroop Color and Word Test measures the subject’s ability to shift perceptual sets in response to changing demand characteristics and to avoid interference from irrelevant aspects of the stimulus situation (36). The subject is asked first to read the names of colors written in black ink (first condition), then is asked to name the color of noncolor words written in colored ink (second condition). The third condition elicits what is called the Stroop interference effect, where the subject must name the color of the ink in which the names of various colors are written while ignoring the word. The dependent measure is the latency for naming all of the colors correctly in this third condition minus the score of the second condition.
Overall intellectual function assessments
The WAIS-R vocabulary and block design tests are standardized, well-validated measures assessing overall intelligence. These two measures were administered in order to estimate both verbal and nonverbal intellectual functions (37). The dependent measures are the age-corrected scaled scores.
Data Analysis
Creation of normative standards
A regression-based approach was used to create normative standards. Age, education, and gender were regressed on each of the dependent variables in the healthy volunteer group. Scores were standardized on the basis of these regression results (see Heaton et al. [38] for a description of this procedure). In addition, the adjusted scores were then found to be uncorrelated with the demographic variables, suggesting that the standardization had actually controlled for the effect of these factors on performance. The same procedure was applied to the schizotypal personality disorder group, using these same norms to create standardized scores (i.e., z scores). The creation of normative standards across measures is used so that comparisons between cognitive measures can be made and represents a standard way of reporting results across a large cognitive battery (19).
Analyses between diagnostic groups
A repeated-measures analysis of variance (ANOVA) was conducted on the standardized scores, using the 11 measures as the dependent variables and group membership as the independent variable followed up by a Tukey-B post hoc test to reveal which groups were different. While age, education, and gender were controlled in the creation of normative standards for each test, the Beck depression score was controlled by an analysis of covariance.
Evaluation of cognitive domains implicated in schizophrenia spectrum disorders
A simultaneous entry for all 11 measures using the schizotypal personality disorder and the healthy comparison group (dependent measure) was conducted in order to examine which measures accounted for the variance. This procedure was repeated using both personality disorder groups as well as using the healthy volunteers and the group with personality disorders unrelated to schizophrenia. Moreover, in order to determine whether the Paced Auditory Serial Addition Test accounted for unique variance over and above the other cognitive measures, all the measures (except the Paced Auditory Serial Addition Test) were entered as a block in a regression analysis followed by the Paced Auditory Serial Addition Test (this analysis was performed in the patients with schizotypal personality disorder and healthy volunteers).
Correlational analyses
In order to investigate whether a particular group of symptoms was associated with cognitive impairment, DSM-III-R schizotypal personality disorder symptoms were grouped into three factors: interpersonal, paranoid, or cognitive/perceptual on the basis of previous work (25) and were correlated with the 11 cognitive measures for the total personality disorder cohort (N=126). For each of the five cognitive domains, we are reporting correlations that are significant at the p<0.005 level in the total patient cohort (so that they would remain significant if they were Bonferroni corrected for multiple correlations).
Results
Analyses of Clinical and Demographic Variables
Subjects in all three groups were studied in their 30s and had an average of a 2-year college education. The three groups were significantly different in age and education. These differences were controlled for statistically by the creation of standardized scores. Regarding the gender distribution, more patients with schizotypal personality disorder were men compared with the other two groups and, as expected, patients with schizotypal personality disorder demonstrated higher Beck Depression Inventory scores relative to comparison subjects and patients with personality disorders unrelated to schizophrenia (Table 1). (Similar results were obtained when the sample was restricted to the previously unreported subjects.)
Analyses Between Diagnostic Groups
In order to evaluate our primary hypothesis that subjects with schizotypal personality disorder would demonstrate significant cognitive impairment specific to the schizophrenia spectrum, an ANOVA using the 11 standardized scores was conducted. An overall significant effect of diagnosis was found, with schizotypal personality disorder subjects demonstrating overall impairment relative to both healthy volunteers and those with personality disorders unrelated to schizophrenia (Figure 1). Healthy volunteers and patients with personality disorders unrelated to schizophrenia were not different per post hoc simple contrast (similar results were obtained when the sample was restricted to the previously unreported subjects). Entering the Beck Depression Inventory score as a covariate did not change these results (effect of Beck score: F=0.3, df=1, 177, p=0.75; effect of diagnosis: F=5.9, df=2, 177, p<0.005). An ANOVA with post hoc tests was then conducted in order to investigate which of the 11 measures contributed to the overall effect of diagnosis. Patients with schizotypal personality disorder demonstrated impairment when compared with both healthy volunteers and those with personality disorders unrelated to schizophrenia for the following individual measures (listed in order of effect size): Paced Auditory Serial Addition Test, WMS visual reproduction test (immediate recall), California Verbal Learning Test (total words recalled), Dot test, and California Verbal Learning Test (delayed recall). Moreover, patients with schizotypal personality disorder were impaired compared with healthy volunteers for the WMS visual reproduction test delayed recall condition and Trailmaking Test part A. (No statistically significant differences were observed for the Trailmaking Test part B, Stroop interference test, or WAIS vocabulary or block design; entering Beck Depression Inventory score as a covariate did not change the results.)
Regression Analysis
A simultaneous entry regression analysis conducted utilizing all 11 standardized measures in the schizotypal personality disorder group and healthy volunteers (dependent measure) was highly significant (F=4.78, df=11, 133, p<0.0001; R2=0.28). A follow-up stepwise regression analysis revealed that the measure that entered the model first was the Paced Auditory Serial Addition Test score (explaining 15% of variance), while the WMS visual reproduction test immediate recall, California Verbal Learning Test total words recalled, and vocabulary scores entered the model next and accounted for an additional 10% (individually 5%, 3%, and 2%, respectively) (overall model: F=11.9, df=4, 144, p<0.001; R2=0.25). In order to investigate whether the Paced Auditory Serial Addition Test contributed unique variance to the model we conducted a regression analysis two ways. First, we entered all 10 variables as a block (i.e., all variables except for the Paced Auditory Serial Addition Test) in the model, followed by the Paced Auditory Serial Addition Test. Second, we entered the Paced Auditory Serial Addition Test first and then the remaining variables in the second block. Regardless of whether the Paced Auditory Serial Addition Test was entered first or last it accounted for unique variance in the model (R2=0.08 when last; R2=0.15 when first, overall model R2=0.28). Indeed, the Paced Auditory Serial Addition Test score as a covariate in the ANOVA above abolished the group differences across the three groups (Figure 2). The Paced Auditory Serial Addition Test also accounted for most of the variance (8%) in the discrimination of the schizotypal personality disorder and no schizophrenia groups, while the only other variable that entered the model was the California Verbal Learning Test total words recalled (4%) (F=8.3, df=2, 125, p<0.001). (No variable entered the model when the regression analysis looked at the healthy volunteers and those with personality disorders unrelated to schizophrenia.)
Correlational Analysis
In order to investigate whether any of the schizotypal personality disorder symptom dimensions were associated with cognitive impairment, the 11 individual tests were correlated with each of the three symptom clusters. These correlations performed for the entire sample of patients revealed that only the interpersonal factor had a significant inverse correlation (i.e., greater isolation associated with poorer performance) with cognitive measures: Paced Auditory Serial Addition Test, WMS visual reproduction test immediate recall, and California Verbal Learning Test delayed recall scores (all r≥0.26, df=124, p<0.005). Similar results were obtained between the Paced Auditory Serial Addition Test and the interpersonal factor when the study group was restricted to the schizotypal personality disorder subjects (r=–0.29, df=80, p<0.05), whereas no statistically significant association was seen between Paced Auditory Serial Addition Test performance and schizotypal symptoms in patients with personality disorders unrelated to schizophrenia. (Again the results are the same when the sample is restricted to the previously unpublished subjects.) The correlational matrix across all 11 measures within the schizotypal personality disorder group, the group of interest, is presented in Table 3. We have highlighted those that would remain significant after multiple comparisons (p<0.001). The directionality of the correlations remains the same if the entire sample is used.
Discussion
We have demonstrated, in the largest group of clinically identified subjects with schizotypal personality disorder to date, that patients with schizotypal personality disorder demonstrate modest cognitive impairment on a variety of cognitive measures. The areas of impairment are specific to domains of working memory, episodic memory, and delayed recall but not in processing speed, susceptibility to interference, or overall intellectual function. Thus, a generalized deficit in performance was clearly not detected. This impairment was specific to the schizotypal personality disorder group, since patients with psychopathology unrelated to schizophrenia did not demonstrate cognitive impairment relative to the healthy group but did differ from the schizotypal personality disorder group. Moreover, we have demonstrated that a measure of auditory working memory (the Paced Auditory Serial Addition Test) accounts for the observed group differences. These results are similar to the ones previously reported with a partially overlapping sample and do not change if we limit the current study group to previously unreported subjects. While patients with schizotypal personality disorder demonstrated impairment relative to healthy subjects in other domains, the Paced Auditory Serial Addition Test was the measure accounting for most of the variance. Indeed, the Paced Auditory Serial Addition Test accounted for unique variance over and above all the other cognitive measures combined. In addition, the same auditory working memory task accounted for most of the variance in the difference between the schizotypal personality disorder subjects and those with personality disorders unrelated to schizophrenia. These results support the notion that working memory represents a core neuropsychological deficit in the schizophrenia spectrum that may have cascading effects leading to impairment in several other cognitive areas (39). Our results are consistent with a recent study (40) in which we reported that subjects with schizotypal personality disorder exhibited impairment relative to healthy subjects on a working memory task that required online maintenance of context. The auditory working memory task presented in this report (the Paced Auditory Serial Addition Test) is similar in that it places demands on context processing, since subjects have to actively update and discard information. While it is widely accepted that the dorsolateral prefrontal cortex is a key area associated with working memory (as reviewed by Levy and Goldman-Rakic [41]) and also an area of dysfunction in the schizophrenia spectrum (42), previous work has focused on trying to characterize the specific brain regions within the dorsolateral prefrontal cortex that are associated with different modalities of working memory (43, 44).
It is of interest that the cognitive task on which the patients with schizotypal personality disorder exhibited the most impairment (the Paced Auditory Serial Addition Test) was significantly associated with the interpersonal factor of the schizotypal symptoms (comprising the following four symptoms: odd/eccentric behavior/appearance, no close friends/confidants, odd speech, and inappropriate/constricted affect). This finding is consistent with findings previously reported in studies of patients with schizophrenia. In those studies, working memory deficits (as well as deficits in learning and vigilance) have been reported to be correlated with social functioning deficits. The association between working memory impairment and social deficit provides modest support for the hypothesis that deficits in information processing and the inability to titrate effort to situational demands could possibly lead to increases in social isolation, a core characteristic of the schizophrenia spectrum disorders (8, 9, 45). However, it is also possible that in early life poor social skills may impede interactions that improve cognitive performance.
Limitations of the study may include the fact that the healthy volunteers were screened to exclude any history of axis I or axis II disorders—leading this group to be “supernormal.” This concern is partly obviated by the fact that we do have a nonschizophrenia personality disorder cohort and the finding that there was no overall group difference in IQs. Moreover, there are always drawbacks in defining a comparison group. We wanted to have a group that was not contaminated with psychopathology and in addition be free of schizophrenia spectrum pathology. In addition, studies in schizophrenia spectrum disorders by definition need rigorous screening of healthy volunteers, since there have been reports of group differences among normal healthy volunteer groups in sensorimotor gating based on psychosis proneness items (46). Another limitation may include the unmatched study group sizes, which means that differences between the subjects with personality disorders unrelated to schizophrenia and the healthy subjects cannot be detected with the same power as the differences between the patients with schizotypal personality disorder and healthy volunteers. This concern is partly obviated by the essential trivial magnitude of differences between healthy volunteers and those with personality disorders unrelated to schizophrenia, wherein even a very substantial sample size would not have found statistically significant differences. Even though we did not find any differences in cognitive function between the patients with personality disorders unrelated to schizophrenia and the healthy volunteer group on the 11 measures selected to compare impairment between schizotypal personality disorder subjects and healthy volunteers, it is possible that these two groups are different in other measures. While there is no agreed upon “perfect” battery to test for impairment in cognitive domains, we have included tests that have relatively consistently differentiated schizophrenia and healthy subjects.
In summary, we have found that patients with schizotypal personality disorder demonstrate moderate cognitive impairment, supporting the view that working memory may be a core deficit in schizophrenia spectrum disorders. The similar yet more circumscribed and specific impairments observed in patients with schizotypal personality disorder compared with schizophrenia patients make them a particularly attractive population for studies designed to clarify the etiology and design paradigms to evaluate potential treatment of this impairment.
 |
 |
 |
Received Aug. 2, 2004; revision received Nov. 15, 2004; accepted Dec. 3, 2004. From the Department of Psychiatry, Mt. Sinai School of Medicine; the VA Medical Center, Bronx, N.Y.; and the VA Mental Illness Research, Education and Clinical Center (VISN 3), New York. Address correspondence and reprint requests to Ms. Mitropoulou, Clinical Research Center, Box 1027, Mt. Sinai School of Medicine, One Gustave Levy Place, New York, NY 10029; [email protected] (e-mail). Supported by NIMH grants to Dr. Siever (RO1 MH-5606) and Dr. Harvey (R01 MH-63116); a Merit Award (Dr. Siever) and the VISN3 MIRECC award from the Department of Veterans Affairs; and a grant from the NIH Center for Research Resources (MO1 RR-00071) awarded to Mt. Sinai School of Medicine. The authors have no financial conflict to disclose pertaining to this report. The authors thank Richard Keefe, Ph.D., for the visuospatial working memory assessment (Dot test) used in our center.
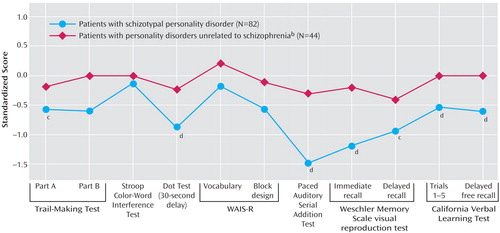
Figure 1. Neuropsychological Performance Profiles of Personality Disorder Groupsa
aRegression-based approach for normative standards with age, education, and gender controlled; performance of healthy volunteers (N=63) has been set to zero (SD=1). Repeated-measures analysis of variance showed an overall significant effect of diagnosis (F=6.9, df=2, 186, p<0.001); with post hoc tests revealing measures on which those with schizotypal personality disorder significantly differed from healthy volunteers (c) and both healthy volunteers and those with personality disorders unrelated to schizophrenia (d).
bAny personality disorder other than schizotypal, schizoid, or paranoid.
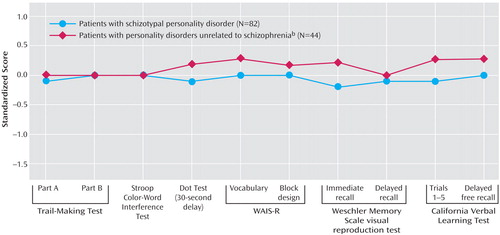
Figure 2. Neuropsychological Performance Profiles of Personality Disorder Groups After Paced Auditory Serial Addition Test Performance Controlleda
aRegression-based approach for normative standards with age, education, gender, and Paced Auditory Serial Addition Test score controlled; performance of healthy volunteers (N=63) has been set to zero (SD=1). Repeated-measures analysis of variance showed no significant effect of diagnosis.
bAny personality disorder other than schizotypal, schizoid, or paranoid.
1. Goldberg TE, Gold JM: Neurocognitive deficit in schizophrenia, in Schizophrenia. Edited by Hirsch SR, Weinberger DR. Oxford, UK, Blackwell Science, 1995, pp 146–162Google Scholar
2. Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Link, Google Scholar
3. Green MF, Kern RS, Braff DL, Mintz J: Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull 2000; 26:119–136Crossref, Medline, Google Scholar
4. Trestman RL, Keefe RS, Mitropoulou V, Harvey PD, deVegvar ML, Lees-Roitman S, Davidson M, Aronson A, Silverman J, Siever LJ: Cognitive function and biological correlates of cognitive performance in schizotypal personality disorder. Psychiatry Res 1995; 59:127–136Crossref, Medline, Google Scholar
5. Kendler KS, Masterson CC, Ungaro R, Davis KL: A family history study of schizophrenia-related personality disorders. Am J Psychiatry 1984; 141:424–427Link, Google Scholar
6. Kendler KS: Diagnostic approaches to schizotypal personality disorder: a historical perspective. Schizophr Bull 1985; 11:538–553Crossref, Medline, Google Scholar
7. Torgersen S: Relationship of schizotypal personality disorder to schizophrenia: genetics. Schizophr Bull 1985; 11:554–563Crossref, Medline, Google Scholar
8. Siever LJ, Davis KL: A psychobiological perspective on the personality disorders. Am J Psychiatry 1991; 148:1647–1658Link, Google Scholar
9. Siever LJ, Davis KL: The pathophysiology of schizophrenia disorders: perspectives from the spectrum. Am J Psychiatry 2004; 161:398–413Link, Google Scholar
10. Mitropoulou V, Harvey PD, Maldari LA, Moriarty PJ, New AS, Silverman JM, Siever LJ: Neuropsychological performance in schizotypal personality disorder: evidence regarding diagnostic specificity. Biol Psychiatry 2002; 52:1175–1182Crossref, Medline, Google Scholar
11. Lenzenweger MF, Gold JM: Auditory working memory and verbal recall memory in schizotypy. Schizophr Res 2000; 42:101–110Crossref, Medline, Google Scholar
12. Lees Roitman SE, Cornblatt BA, Bergman A, Obuchowski M, Mitropoulou V, Keefe RSE, Silverman JM, Siever LJ: Attentional functioning in schizotypal personality disorder. Am J Psychiatry 1997; 154:655–660; correction, 154:1180Link, Google Scholar
13. Cadenhead KS, Perry W, Shafer K, Braff DL: Cognitive functions in schizotypal personality disorder. Schizophr Res 1999; 37:123–132Crossref, Medline, Google Scholar
14. Moritz S, Mass R: Reduced cognitive inhibition in schizotypy. Br J Clin Psychol 1997; 36:365–376Crossref, Medline, Google Scholar
15. Voglmaier MM, Seidman LJ, Salisbury D, McCarley RW: Neuropsychological dysfunction in schizotypal personality disorder: a profile analysis. Biol Psychiatry 1997; 41:530–540Crossref, Medline, Google Scholar
16. Voglmaier MM, Seidman LJ, Niznikiewicz MA, Dickey CC, Shenton ME, McCarley RW: Verbal and nonverbal neuropsychological test performance in subjects with schizotypal personality disorder. Am J Psychiatry 2000; 157:787–793Link, Google Scholar
17. Baddeley A: Working memory. Science 1992; 255:556–559Crossref, Medline, Google Scholar
18. Baddeley A, Hitch G: Working memory, in The Psychology of Learning and Motivation: Advances in Research and Theory, vol 8. Edited by Bower GH. New York, Academic Press, 1974, pp 47–89Google Scholar
19. Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
20. Park S, Holzman PS: Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry 1992; 49:975–982Crossref, Medline, Google Scholar
21. Goldman-Rakic PS: Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci 1994; 6:348–357Crossref, Medline, Google Scholar
22. Lenzenweger MF: Reaction time slowing during high-load, sustained-attention task performance in relation to psychometrically identified schizotypy. J Abnorm Psychol 2001; 110:290–296Crossref, Medline, Google Scholar
23. Braff DL, Heaton R, Kuck J, Cullum M, Moranville J, Grant I, Zisook S: The generalized pattern of neuropsychological deficits in outpatients with chronic schizophrenia with heterogeneous Wisconsin Card Sorting Test results. Arch Gen Psychiatry 1991; 48:891–898Crossref, Medline, Google Scholar
24. Brebion G, Amador X, Smith MJ, Gorman JM: Memory impairment and schizophrenia: the role of processing speed. Schizophr Res 1998; 30:31–39Crossref, Medline, Google Scholar
25. Bergman AJ, Harvey PD, Mitropoulou V, Aronson A, Marder D, Silverman J, Trestman R, Siever LJ: The factor structure of schizotypal symptoms in a clinical population. Schizophr Bull 1996; 22:501–509Crossref, Medline, Google Scholar
26. McGurk SR, Meltzer HY: The role of cognition in vocational functioning in schizophrenia. Schizophr Res 2000; 45:175–184Crossref, Medline, Google Scholar
27. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R, Version 1.0 (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
28. Pfohl B, Blum N, Zimmerman M, Stangl D: Structured Interview for DSM-III-R Personality, Revised (SIDP-R). Iowa City, University of Iowa College of Medicine, Department of Psychiatry, 1989Google Scholar
29. Beck AT, Beamesderfer A: Assessment of depression: the depression inventory. Mod Probl Pharmacopsychiatry 1974; 7:151–169Crossref, Medline, Google Scholar
30. Keefe RS, Roitman SE, Harvey PD, Blum CS, DuPre RL, Prieto DM, Davidson M, Davis KL: A pen-and-paper human analogue of a monkey prefrontal cortex activation task: spatial working memory in patients with schizophrenia. Schizophr Res 1995; 17:25–33Crossref, Medline, Google Scholar
31. Keefe RS, Lees-Roitman SE, Dupre RL: Performance of patients with schizophrenia on a pen and paper visuospatial working memory task with short delay. Schizophr Res 1997; 26:9–14Crossref, Medline, Google Scholar
32. Stuss DT, Stethem LL, Pelchat G: Three tests of attention and rapid information processing: an extension. Clin Neuropsychol 1988; 2:246–250Crossref, Google Scholar
33. Delis DC, Kramer JH, Kaplan E, Ober BA: California Verbal Learning Test Manual—Research Edition. San Diego, Psychological Corp, 1987Google Scholar
34. Wechsler D: Wechsler Memory Scale—Revised. New York, Psychological Corp/Harcourt Brace Jovanovich, 1987Google Scholar
35. Reitan RM: Validity of the Trail Making Test as an indicator of organic brain damage. Percept Mot Skills 1958; 8:271–276Crossref, Google Scholar
36. Stroop JR: Studies of interference in serial verbal reactions. J Exp Psychol 1935; 18:643–662Crossref, Google Scholar
37. Wechsler D: WAIS-R Manual. New York, Psychological Corp, 1981Google Scholar
38. Heaton RK, Grant I, Matthews CG: Comprehensive Norms for an Expanded Halstead-Reitan Battery: Demographic Corrections, Research Findings, and Clinical Applications. Odessa, Fla, Psychological Assessment Resources, 1991Google Scholar
39. Silver H, Feldman P, Bilker W, Gur RC: Working memory deficit as a core neuropsychological dysfunction in schizophrenia. Am J Psychiatry 2003; 160:1809–1816Link, Google Scholar
40. Barch DM, Mitropoulou V, Harvey PD, New AS, Silverman JM, Siever LJ: Context-processing deficits in schizotypal personality disorder. J Abnorm Psychol 2004; 113:556–568Crossref, Medline, Google Scholar
41. Levy R, Goldman-Rakic PS: Segregation of working memory functions within the dorsolateral prefrontal cortex. Exp Brain Res 2000; 133:23–32Crossref, Medline, Google Scholar
42. Weinberger DR, Berman KF, Suddath R, Torrey EF: Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am J Psychiatry 1992; 149:890–897Link, Google Scholar
43. Braver TS, Bongiolatti SR: The role of frontopolar cortex in subgoal processing during working memory. Neuroimage 2002; 15:523–536Crossref, Medline, Google Scholar
44. Huguelet P, Zanello A, Nicastro R: A study of visual and auditory verbal working memory in schizophrenic patients compared to healthy subjects. Eur Arch Psychiatry Clin Neurosci 2000; 250:79–85Crossref, Medline, Google Scholar
45. Cornblatt BA, Keilp JG: Impaired attention, genetics, and the pathophysiology of schizophrenia. Schizophr Bull 1994; 20:31–46; correction, 20:248Crossref, Medline, Google Scholar
46. Swerdlow NR, Filion D, Geyer MA, Braff DL: “Normal” personality correlates of sensorimotor, cognitive, and visuospatial gating. Biol Psychiatry 1995; 37:286–299Crossref, Medline, Google Scholar



