Dopaminergic Abnormalities in Select Thalamic Nuclei in Schizophrenia: Involvement of the Intracellular Signal Integrating Proteins Calcyon and Spinophilin
Abstract
OBJECTIVE: While both thalamic abnormalities and dopaminergic dysregulation have been separately implicated in the pathophysiology of schizophrenia, little is known about the possible dysfunction of molecules associated with dopaminergic neurotransmission in the thalamus in this illness. In this study, the authors studied this question by measuring in postmortem brain the expression of molecules associated with dopaminergic neurotransmission. METHOD: Using in situ hybridization and receptor autoradiography, the authors determined in schizophrenia and comparison subjects 1) thalamic expression of the transcripts encoding the five dopamine receptors; 2) binding to the dopamine D1, D2, and D3 receptors; 3) monoaminergic innervation as assessed by binding to the vesicular monoamine transporter; and 4) transcripts encoding three dopamine receptor-associated intracellular proteins (calcyon, spinophilin, and DARPP-32) that mediate integration of dopaminergic signaling with other neurotransmitter systems. RESULTS: Both calcyon and spinophilin transcripts were significantly elevated in schizophrenia subjects. Monoaminergic innervation, as well as dopamine receptor transcripts and binding sites, were unaffected in this illness. CONCLUSIONS: These data indicate that there are dopaminergic abnormalities in the thalamus in schizophrenia but that they are at the level of intracellular integration of dopamine signaling with other neurotransmitter systems, likely including glutamate, in thalamic neurons.
The dopamine hypothesis of schizophrenia is based largely on pharmacological evidence, including the observations that dopaminergic agonists can induce psychosis in healthy subjects or exacerbate psychotic symptoms in schizophrenia, and that D2-like receptor antagonists effectively reduce psychotic symptoms. Subsequently, numerous investigators have examined myriad aspects of the dopamine system in schizophrenia (1–3). Dopamine is packaged into secretory vesicles by the vesicular monoamine transporter (VMAT) and released into the synaptic cleft, where it can activate one of five receptor subtypes (D1–D5). Dopamine receptors are classified as either “D1-like” (D1 and D5) or “D2-like” (D2, D3, and D4) on the basis of their molecular structure and pharmacology; they couple to specific G proteins to regulate second messenger molecules (4).
D1-like receptors primarily couple to Gs proteins to stimulate cAMP formation (5), but this also leads to increased levels of inositol phosphates (6) and intracellular calcium (7). One of the D1 receptor-linked cAMP signaling pathways involves cAMP-dependent protein kinase, which phosphorylates DARPP-32 (dopamine- and cAMP-regulated phosphoprotein), a potent inhibitor of protein phosphatase-1 (8). The DARPP-32/protein phosphatase-1 cascade controls the phosphorylation states of myriad downstream effectors and therefore exerts a powerful effect on neuronal function (8). Another recently identified transmembrane protein, calcyon, interacts with the D1 receptor and mediates intracellular calcium mobilization, permitting “crosstalk” between expressed D1 receptors and Gq/11-protein-coupled receptors such as metabotropic glutamate receptors and certain muscarinic cholinergic receptors (9).
D2-like receptors couple to either Gi or Go proteins to inhibit adenylyl cyclase and decrease cAMP production (4), modulate intracellular calcium levels and arachidonic acid release, and increase outward potassium currents (10). Yeast two-hybrid techniques have shown that the D2 receptor binds to the protein spinophilin, which is enriched in dendritic spines and interacts with actin and protein phosphatase-1 (11). This D2/spinophilin interaction may be important for building an intracellular signaling complex that modulates interactions between D2 and neighboring receptors, especially the AMPA subtype of glutamate receptor, as well as their downstream effectors (11, 12). While most studies of dopaminergic abnormalities in schizophrenia have focused on the dopamine receptors, there has been recent interest in potential abnormalities of some of the intracellular proteins associated with the transduction of dopamine-mediated signaling (13, 14).
Superimposed on dopaminergic dysregulation in schizophrenia is the neuroanatomical circuitry that underlies this illness. While earlier hypotheses viewed schizophrenia as a disorder involving discrete brain regions, recent models often conceptualize schizophrenia as a disorder of the functional integrity of distributed circuitry (15, 16). The predominant neuroanatomical structures hypothesized to be abnormal in schizophrenia include the prefrontal cortex and other cortical and subcortical limbic regions. A critical neuroanatomical structure that links all these areas is the thalamus. The thalamus consists of discrete, topographically organized nuclei with reciprocal projections to and from limbic, sensory, and motor regions of the cortex. Further, much information reaching the cortex from subcortical areas converges on the thalamus before being distributed to cortical regions. While earlier views tended to relegate thalamic function to a simple relay station, thalamic nuclei play a pivotal role in gating and processing information sent to the cerebral cortex. Fibers from brain regions implicated in schizophrenia, including the prefrontal and cingulate cortices, hippocampus, and amygdala, terminate in several nuclei of the dorsal thalamus (e.g., dorsomedial, anterior, lateral dorsal, and central medial nuclei [17]). The fundamental plan of thalamic circuitry is straightforward: afferent and efferent neurotransmission of all dorsal thalamic nuclei is mediated by glutamate and, in turn, regulated by inhibitory GABAergic effects exerted by neurons of the reticular nucleus and by intrinsic interneurons of the thalamus. Monoaminergic and cholinergic afferents modulate neurotransmission in both the dorsal thalamus and reticular nucleus (17).
There is growing appreciation of thalamic abnormalities in schizophrenia. Multiple studies have detected structural and functional thalamic pathology in this illness. Postmortem and structural imaging studies have reported decreases in thalamic cell number and volume, while functional in vivo images studies have reported decreased thalamic metabolism as well as alterations in corticothalamic connectivity (18–26) (see reference 27 for review).
Both thalamic and dopaminergic dysfunction have been separately implicated in the pathophysiology of schizophrenia, but possible abnormalities of the dopamine system in the thalamus in this illness have been largely unexplored. While one study in the 1980s reported increased concentrations of dopamine in the thalamus of patients with schizophrenia (28), no studies to date have examined the expression of dopamine receptors or associated intracellular proteins in the thalamus in schizophrenia. Therefore, in this project we examined a number of molecules associated with dopaminergic neurotransmission in several thalamic nuclei in schizophrenia. We have determined the expression of dopamine receptor transcripts and receptor binding, binding to the vesicular monoamine transporter as a marker of afferent innervation, and transcripts encoding three intracellular proteins with a role in the integration of dopaminergic signaling at the intraneuronal level.
Method
Subjects
Thirteen subjects with schizophrenia and eight nonpsychiatrically ill individuals from the Mount Sinai Medical Center Brain Bank were studied (Table 1). The cases in the present study are the same as those utilized in our previous reports on glutamate receptor and transporter expression in the thalamus in schizophrenia (29–33). Cases were classified as having schizophrenia if the presence of schizophrenic symptoms could be documented before age 40; the medical records contained evidence of psychotic symptoms and ≥10 years of psychiatric hospitalization with a diagnosis of schizophrenia; the DSM-III-R diagnosis of schizophrenia was agreed upon by two experienced clinicians; and neuropathological examination did not reveal Alzheimer’s disease or other degenerative disorders. Subjects with a history of alcoholism or substance abuse were excluded. Normal comparison subjects had no history of any psychiatric or neurologic disorders and no discernible neuropathologic lesions. Next of kin consent was obtained for each subject. There were no significant between-group differences in age (t=1.6, df=19, p=0.12), postmortem interval (t=1.2, df=19, p=0.25), tissue pH (t=2.3, df=19, p=0.15), sex distribution (χ2=1.7, df=1, p=0.19), side of brain studied (χ2=0.91, df=1, p=0.34), or cigarette smoking history (χ2=1.1, df=1, p>0.20). At the time of death, six persons with schizophrenia were receiving typical antipsychotics, five had a mean drug-free period of 5.8 weeks (SD=3.4), and one had been drug free for many years.
Tissue Preparation
Brains were prepared by slicing one hemisphere into 1-cm coronal slabs, which were snap frozen. Blocks containing thalamus were cryostat-sectioned (15 μm), and thaw-mounted onto poly-L-lysine-subbed microscope slides, dried, and stored at –80°C. Two slides per subject were prepared for in situ hybridization for each probe. For receptor autoradiography, three slides per subject were used for each radioligand, two slides for total binding and the third to define nonspecific labeling.
In Situ Hybridization
We performed in situ hybridization with riboprobes to determine the expression of transcripts encoding the five dopamine receptors and calcyon, spinophilin, and DARPP-32. For D1– D5 receptors, we used probes that we have previously described and validated (34–38). For the three intracellular proteins, we prepared new subclones. We amplified unique regions of calcyon (NCBI Genebank accession number AF225903, nucleotide coding region 120–509), DARPP-32 (AF233349, 12-274), and spinophilin (AF016252, 2423-2905) from a human brain cDNA library (EdgeBiosystems, Gaithersburg, Md.). Amplified cDNA segments were extracted (QIAquick Gel Extraction Kit, Qiagen, Valencia, Calif.), subcloned (Zero Blunt TOPO PCR cloning kit, Invitrogen, Carlsbad, Calif.), and confirmed by nucleotide sequencing (Thermo Sequenase Radiolabeled Termination Cycle Sequencing Kit, USB, Cleveland). Linearized subclones were used to synthesize [35S]UTP-labeled riboprobes for in situ hybridization, as we have described extensively in the past (34–38). To ensure the specificity of in situ hybridization signals, adjacent sections were incubated with sense strand probes for each of the dopamine receptors or associated intracellular protein and processed in parallel with antisense-labeled slides as described previously (34–38). One set of these slides was exposed to Kodak film for 7–60 days, and a second set was dipped in Kodak NTB-2 emulsion and stored at 4°C for 21–90 days before developing (39).
Receptor Audioradiography
Three slides (two for total and one for nonspecific binding) were used for each subject for each radioligand. Slides were removed from –80°C storage and warmed at room temperature for 15 minutes. For the dopamine receptors, slides underwent a binding prewash at room temperature for 30 minutes. Slides were rinsed in deionized water and dried under a stream of cool air. Slides were then incubated for 2 hours in buffer containing radioligands at concentrations three times Kd, as determined for each ligand in this lab. Nonspecific binding was determined by adding an unlabeled compound to the ligand buffer mixture. After incubation, excess buffer was tapped off onto a paper towel, and slides were rinsed in fresh buffer twice at 4°C for 2 minutes. Slides were then briefly rinsed in deionized water at 4°C and dried under a stream of cool air. Slides were apposed to Amersham [3H]Hyperfilm.
For D1 receptor autoradiography, both pre- and postbinding washes were in 50 mM Tris-HCl (pH 7.4), 120 mM NaCl, 5 mM KCl, and 1 mM MgCl2 buffer. The radioligand was 5 mM [3H] SCH23390 in the pre/postincubation buffer to which 5 μM ketanserin and 0.1% ascorbic acid were added. Nonspecific binding was determined using the same solution, to which 1 μM SCH23390 was added. For D2 receptors, pre- and postbinding washes were in 50 mM Tris-HCl (pH 7.4), 120 mM NaCl, and 100 μM pNHGG. Binding to the D2 receptor was performed with 3 nM [3H] raclopride in the same buffer, with the addition of 50 μM 7-OH-DPAT. Nonspecific binding was determined in the presence of 1 μM spiperone. D3 receptor binding was performed in 50 mM Tris-HCl (pH 7.4) and 1 mM EDTA. For visualization of D3 receptors, 1 nM [3H] PD128907 was used, with nonspecific binding determined in the presence of 1 μM 7-OH-DPAT.
VMAT Audioradiography
Binding to VMAT2 was assayed by quantitative autoradiography of [3H]methoxytetrabenazine (MTBZ) binding as described previously (40). In brief, sections were prewashed for 5 min at 25°C in potassium phosphate buffer (137 mM KCl, 3 mM NaCl, 8 mM K2HPO4, 1.5 mM NaH2PO2, 1 mM EDTA; pH 8.0) followed by incubation in the same buffer containing 20 nM [3H]MTBZ for 30 minutes at 25°C. Slides were subsequently rinsed twice for 2 minutes each in buffer at 4°C, followed by a brief dip in distilled water at 4°C to remove excess buffer salts. Nonsaturable binding was assessed in adjacent tissue sections incubated in [3H]MTBZ together with 10 μM unlabeled tetrabenazine. Under these conditions, saturable MTBZ binding to VMAT2 displays a Kd of 3.9 nM in rat brain sections (40). Sections were air-dried and apposed to tritium-sensitive X-ray film for 2 weeks.
Data Analysis
The boundaries of thalamic nuclei were determined in each section based upon cellular and white matter patterns as defined by cresyl violet and gold chloride staining of sections from each subject, as we have previously described (29, 33). Thalamic nuclei were identified on the basis of descriptions of thalamic architecture (17). The following nuclei were identified for each subject: anterior, dorsomedial, ventral anterior, central medial, ventral, and reticular. In this study, the ventral nucleus corresponds to a grouping of several nuclei of the ventral tier. Images were digitized from films and analyzed by quantitative densitometry. For in situ hybridization images, gray scale values were obtained from each of the six thalamic nuclei, corrected for tissue background, and converted to optical density. These values are linear with concentration over the gray scale values found in this study. For receptor binding images, gray scale values for each nucleus were corrected for nonspecific binding and converted to optical density. Values from two sections for each subject were averaged and used for statistical analysis. Statistical analysis was performed for each probe by analysis of variance, with diagnosis and nuclei as the independent variables. The Kolmogorov-Smirnov test was used to ensure normality of all data, and Pearson product-moment correlations were used to determine relationships between continuously distributed variables. For all tests, alpha=0.05.
Results
Normal Distribution of Dopamine Receptor Transcripts in Human Thalamus
All five dopamine receptor transcripts were identified in thalamic nuclei, although at low levels of expression (Figure 1). Both D1 and D2 receptor mRNAs were present, but at considerably lower levels than in striatal structures. D3, D4, and D5 receptor mRNAs were also present at low levels of expression, comparable to the very low levels of these three transcripts in the caudate and putamen.
There was significant heterogeneity of expression for each transcript across different thalamic nuclei. D1 receptor mRNA differed across nuclei (F=14.3, df=5, 95, p<0.000001); post hoc analyses indicated that this effect was due to higher levels of D1 mRNA expression in the dorsomedial, anterior, central medial, and ventral anterior nuclei than in the reticular and ventral nuclei. D2 receptor mRNA (F=15.9, df=5, 95, p<0.000001) and D5 receptor mRNA (F=18.8, df=5, 95, p<0.000001) also differed across nuclei, with relatively higher levels in the anterior and central medial nuclei compared with the ventral tier and reticular nuclei. There was also heterogeneity of D3 receptor mRNA expression (F=10.1, df=5, 95, p<0.000001), which was primarily associated with high levels of D3 transcripts in the anterior nucleus and very low levels in the reticular nucleus. Finally, D4 receptor mRNA expression varied across nuclei (F=9.31, df=5, 95, p<0.000001), with very low levels of expression in the ventral tier nuclei compared with other nuclei studied.
Dopamine Receptor Binding in Human Thalamus
Binding to the D1, D2, and D3 receptors was determined by receptor autoradiography (Figure 2). Similar to the dopamine receptor transcripts, significant main effects were identified for both D2 and D3 binding sites across thalamic nuclei. There was a main effect of nucleus for D2 receptor binding (F=4.1, df=5, 95, p<0.005) that was due to higher D2 receptor binding in the dorsomedial nucleus than in the other five nuclei. There was also a main effect of nucleus for D3 receptor binding (F=7.1, df=5, 95, p<0.00005). This variability of D3 receptor binding was associated with decreased binding in the reticular nucleus compared with other nuclei as well as the ventral nuclei having lower D3 receptor binding than the anterior and central medial nuclei. D1 binding did not significantly vary across these nuclei.
VMAT2 Binding in Human Thalamus
As a marker of presynaptic dopaminergic innervation, we investigated binding to the vesicular monoamine transporter, VMAT2 (Figure 3). Modest levels of VMAT2 binding were seen in all thalamic nuclei, with particular enrichment in the dorsomedial, central medial, and anterior nuclei. There was significant regional heterogeneity (F=14.8, df=5, 95, p<0.000001), with enrichment in the more medial nuclei.
Normal Distribution of Transcripts Encoding Calcyon, DARPP-32, and Spinophilin in the Human Thalamus
Finally, we examined the expression of the transcripts encoding three intracellular molecules associated with dopamine receptor-mediated neurotransmission: calcyon, spinophilin, and DARPP-32 (Figure 4). As in the case of other dopaminergic molecules, there was significant regional heterogeneity of the expression of these three transcripts across thalamic nuclei. In the case of calcyon, there was a main effect of nucleus (F=22.2, df=5, 95, p<0.000001) that was associated with higher levels of calcyon mRNA being expressed in the anterior, dorsomedial, central medial, and ventral anterior nuclei compared with the ventral tier and reticular nuclei. Spinophilin (F=12.5, df=5, 95, p<0.000001) and DARPP-32 (F=44.5, df=5, 95, p<0.000001) transcripts were also heterogeneously expressed across the thalamus, with relatively lower levels expressed in the ventral tier and reticular nuclei compared with the more medial thalamic nuclei studied.
Dopaminergic Abnormalities in the Thalamus in Schizophrenia
There were no significant main effects of diagnosis nor any significant diagnosis-by-nucleus interactions for any of the dopamine receptor transcripts (D1–D5) (Figure 5). Receptor autoradiography studies showed that there were no significant main effects of diagnosis or significant diagnosis-by-region interactions for the D1–D3 binding sites (Figure 6). Similarly, there was no difference in expression of VMAT2 binding between persons with schizophrenia and the comparison group (Figure 7). We did find a main effect of diagnosis for transcripts encoding two of the three dopamine receptor-interacting proteins (Figure 8). There was a main effect of diagnosis for calcyon expression (F=8.65, df=1, 19, p<0.01), which was associated with a nearly two-fold increase of calcyon transcript expression in the thalamus in schizophrenia relative to comparison subjects (Figure 8). We calculated total antipsychotic exposure for the 6 months preceding death, expressed as chlorpromazine equivalents for the subjects with schizophrenia, and found that calcyon mRNA levels in the central medial and dorsomedial nuclei negatively correlated with antipsychotic exposure (central medial nucleus: r=–0.89, df=11, p=0.007; dorsomedial nucleus: r=–0.79, df=11, p=0.03), but no other correlations were noted. Calcyon mRNA expression was not significantly correlated with tissue pH. We also found a main effect of diagnosis for spinophilin (F=4.6, df=1, 19, p<0.05) that was due to an elevation of spinophilin mRNA in all thalamic nuclei in schizophrenia relative to comparison subjects (Figure 8). This is similar to the pattern seen for calcyon, although the magnitude of the differences between comparison and schizophrenia subjects was not as great. There were no correlations between expression of this transcript and antipsychotic exposure or tissue pH. Finally, DARPP-32 was not significantly different in schizophrenia (Figure 8). There were no significant diagnosis-by-region interactions for calcyon, spinophilin, or DARPP-32.
Discussion
These data indicate that dopaminergic neurotransmission in the thalamus is potentially impaired in schizophrenia. It is interesting that the expression of dopamine receptors is spared in the thalamus in schizophrenia, as is presynaptic innervation as determined by binding to the vesicular monamine transporter. On the other hand, expression of both calcyon and spinophilin, intracellular molecules associated with the integration of dopaminergic neurotransmission with signaling from other neurotransmitter systems in the dendritic spine, are grossly abnormal. These data suggest that dopamine signaling is altered in the thalamus in schizophrenia, but at the level of intracellular signal integration.
Relatively little is known about the normal neurochemical anatomy of the dopamine system in the thalamus. Studies on rodents have revealed only sparse dopaminergic innervation to a few thalamic nuclei, including the dorsomedial nucleus (41–44). This is typically associated with low levels of dopamine receptors (45, 46) with several exceptions: increased densities of D2 receptors have been noted in the anterior and lateral dorsal nuclei, and high densities of D4 receptors were reported in the reticular nucleus (47–49). Studies on rodents and primates have also typically revealed low levels of transcripts encoding dopamine receptor subtypes in thalamus, although these earlier studies tend to have focused on other structures and only note thalamic expression in passing (39, 50–53). One study focused on human brain D2 and D3 dopamine receptor expression noted specific nuclear distributions in the thalamus (54). Our dopamine receptor data are consistent with most of these earlier reports: transcripts for all five of the dopamine receptors were detected in human thalamus but at very low levels of expression. Similarly, D1, D2, and D3 receptor binding were all detected in the thalamus. It is interesting that both D2 and D3 binding were enriched in more medial, limbic-associated nuclei, which is consistent with earlier work showing relatively increased expression of D2 and D3 transcripts and binding in the anterior and dorsomedial nuclei of the human thalamus (54). Furthermore, our studies of VMAT2 binding and the three intracellular protein transcripts revealed a similar pattern of expression, with enriched levels expressed in medial and anterior nuclei. Taken together, these data support a role for dopaminergic modulation of human thalamic function that is more extensive in limbic-related nuclei.
While a large body of work has examined dopaminergic abnormalities in the striatum and prefrontal cortex in schizophrenia, relatively little attention has been focused on alterations of molecules associated with this neurotransmitter in the thalamus. A few studies have suggested that dopamine levels are elevated in the thalamus in schizophrenia. Using high-performance liquid chromatography, one group found a substantial elevation of dopamine but not norepinephrine levels in the thalamus in schizophrenia (28, 55). These authors presented detailed maps for the thalami from selected comparison and schizophrenia patients, highlighting “dopamine hot spots” where dopamine/norepinephrine ratios were especially high (28). The thalami of comparison subjects in this work contained very little endogenous dopamine, but there was significant enhancement of dopamine/norepinephrine ratios in many regions of the thalami in schizophrenia subjects. The pattern of “dopamine hot spots” widely varied between the thalami from each of the patients, however, making these data difficult to interpret. Using a similar assay, this group found significant differences in thalamic dopamine levels within a group of rats that all were handled, housed, etc. under identical conditions, suggesting that technical matters may have confounded this experiment (56).
This group speculated that the observed increase in thalamic dopamine content might be due to increased dopaminergic innervation of the thalamus in schizophrenia (28). We have examined afferent monoaminergic innervation to the thalamus by measuring binding to VMAT2 as measured by MTBZ. VMAT2 is the transporter responsible for neuronal translocation of neurotransmitter monoamines from cytoplasm to synaptic vesicles and is expressed in neurons employing the neurotransmitter dopamine, norepinephrine, serotonin, or histamine (57–59). Unlike alternative markers of synaptic terminals, the expressed concentration of VMAT2 appears to reflect the density and integrity of presynaptic innervation and is relatively unaffected by counterregulatory and compensatory changes accompanying drug treatment. The vast majority of data supporting this interpretation are derived from study of the nigrostriatal dopaminergic projection. Experimental animals treated with a range of drugs affecting dopaminergic neurotransmission (including D2 dopamine receptor agonists, D2 antagonists, dopamine reuptake inhibitors, and dopamine metabolism inhibitors) do not significantly alter the expressed levels of VMAT2 in the striatum (59, 60) or in the cerebral cortex (61). Conversely, interventions associated with the irreversible lesions of nigrostriatal neurons or terminals (40, 62) result in reduced striatal VMAT2 binding levels, reflecting lesion severity (40). We interpret our present MTBZ data as a reflection of the distribution and density of presynaptic monoaminergic terminals in the thalamus. Given the striking parallels among VMAT2, D2, and D3 binding sites and calcyon and DARPP-32 transcripts, we suspect that VMAT2 is to a large extent reflecting dopaminergic innervation in the thalamus. Our data suggest that there is no change in monoaminergic innervation to the thalamus in schizophrenia.
Similarly, there were no changes found for either dopamine receptor transcripts or binding sites in schizophrenia subjects. On the other hand, there were significant changes in the expression of two intracellular proteins that are enriched in the dendritic spine and link dopamine receptors to other effector pathways. The most dramatic change was noted for the transcript that encodes calcyon. Calcyon is a recently identified protein found to interact with the D1 receptor, which may facilitate D1 receptor-mediated increases in intracellular calcium. Yeast two-hybrid and coimmunoprecipitation studies have shown that calcyon binds intracellular C-terminal residues of the D1 receptor, and immunocytochemical studies showed that this protein is widely expressed throughout the brain (9). By binding a C-terminal region of the D1 receptor, calcyon appears to mediate calcium mobilization by permitting “crosstalk” between D1-coupled Gs proteins and Gq/11 protein-coupled receptors, including metabotropic glutamate and muscarinic cholinergic receptors (9). D1 receptor stimulation alone does not affect calcium levels, but application of a D1 agonist after stimulating P2Y purinergic or M1 muscarinic receptors triggered a large increase of intracellular calcium (9). Calcyon has also been shown to regulate the affinity state of the D1 receptor (63). Therefore, calcyon may serve several functions in the neuron, including regulating D1 receptor affinity for agonists as well as the integration of D1-mediated signaling with Gq11 protein- coupled receptors. A recent study found that levels of expression of calcyon protein (expressed both per total protein and per neuronal DNA) were significantly increased in the dorsolateral prefrontal cortex of patients with schizophrenia (13). These results in the prefrontal cortex are consistent with our present data of a significant elevation of calcyon mRNA in the thalamus in schizophrenia.
Spinophilin was also significantly increased in the thalamus in schizophrenia. Yeast two-hybrid has been used to demonstrate an interaction between the third intracytoplasmic loop of the D2 receptor and spinophilin, a protein enriched in dendritic spines and known to bind PP-1 and F-actin (64, 65). Spinophilin is also widely expressed in the brain but is particularly enriched in the hippocampus, striatum, and thalamus (64). Spinophilin may function, in part, as a protein phosphatase-1-targeting protein to concentrate protein phosphatase-1 in dendritic spines, where it would be in close proximity to postsynaptic targets such as N-methyl-d-aspartic acid (NMDA) and AMPA glutamate receptors and calcium/calmodulin-dependent kinase II (CamKII) (12, 64). Spinophilin may also impact synaptic transmission by regulating dendritic spine number and density (64, 66) by targeting protein phosphatase-1 to the spine microfilament network, which is dynamically modified by protein phosphatase-1 activity (67). Data from a spinophilin knockout mouse support both of these possibilities, as these animals show both changes in glutamatergic transmission and spine density (66). Although spinophilin has been found to interact with the D2 receptor in vitro, this interaction has not yet been established in vivo (11). Since spinophilin has several binding sites, including a site to bind F-actin, protein phosphatase-1, and D2, as well as a PDZ domain, it may serve as a scaffold-adapter protein for G-protein coupled receptors, which in turn could link receptors to the cytoskeleton and coordinate their associated signaling pathways. A recent study that examined levels of expression of spinophilin in the dorsolateral prefrontal cortex in schizophrenia did not reveal any significant differences (13). Our present data indicating increased spinophilin mRNA in the thalamus in schizophrenia suggest that brain region-specific alterations in levels of expression of spinophilin may occur in schizophrenia.
DARPP-32 levels were unchanged in schizophrenia. D1-type dopamine receptors primarily couple to Gs–G proteins to stimulate cAMP formation, which activates cAMP-dependent protein kinase A. Protein kinase can phosphorylate numerous intracellular proteins, including the phosphoprotein DARPP-32, which plays a central role in neuronal function, particularly in brain regions that receive rich dopaminergic input (8). Many neurotransmitters, including dopamine, modulate the phosphorylation and/or dephosphorylation state of DARPP-32 (68). The phosphorylated form of DARPP-32 is a potent inhibitor of protein phosphatase-1, the predominant phosphatase used in neurons, which is particularly enriched in the postsynaptic density (69). The DARPP-32/protein phosphatase-1 cascade controls the phosphorylation states and activity of myriad downstream effectors and therefore exerts a powerful effect on neuronal function (8).
A previous study revealed that DARPP-32 expression was significantly reduced in the dorsolateral prefrontal cortex in schizophrenia (14). The same study also revealed that DARPP-32 was not reduced in patients with Alzheimer’s disease who had been treated with haloperidol, suggesting that the decrease in DARPP-32 observed in schizophrenia is unlikely to be the result of antipsychotic treatment alone. Our finding of no change of DARPP-32 mRNA in schizophrenia is perhaps not unexpected. DARPP-32 is likely not transcriptually regulated in schizophrenia. Instead, changes in this molecule are likely translational or particularly at the level of phosphorylation state.
Our study has several limitations. These data are from elderly patients with schizophrenia, so generalization of our findings to younger patients should be made with caution. Another potential confounding variable that must be considered in this study, as in any study of postmortem brain in schizophrenia, is the possibility that any findings are due to past antipsychotic exposure. It is unlikely that our significant results are due to drug effects. Several studies in the macaque have examined the effects of haloperidol on expression of both calcyon and spinophilin, although only in the cortex. Chronic antipsychotic treatment did not alter expression of calcyon in the prefrontal cortex (13). On the other hand, spinophilin levels are decreased in the cortex following haloperidol treatment (13, 70), opposite to our findings in the thalamus in schizophrenia. In addition, we did not detect any positive correlations between expression of these transcripts and antipsychotic exposure. While we cannot completely rule out the possibility that our findings are associated with antipsychotic treatment, these data strongly suggest otherwise.
Many studies of neurotransmitter-mediated abnormalities in schizophrenia have focused on cell membrane expressed molecules, particularly neurotransmitter receptors. Our results suggest that there are dopaminergic abnormalities in the thalamus in schizophrenia but that they occur at the level of intracellular proteins that modulate dopamine receptor-related intracellular signaling events. These proteins, calcyon and spinophilin, link dopamine receptor-signaling pathways to other neurotransmitter receptors (11, 12, 71, 72), so our findings suggest that in schizophrenia, the intracellular integration of dopamine signaling with other neurotransmitter systems in the dendritic spine is abnormal in the thalamus. Specifically, while calcyon and spinophilin appear to mediate intracellular interactions between dopamine and other neurotransmitters, both have been shown to promote dopamine-glutamate synergies (12, 71, 72). Our current data, along with our previously published observations of altered expression of NMDA receptors (29) and NMDA receptor-interacting intracellular molecules like PSD95 (33) in these same subjects, suggest that the neurochemical disturbances in thalamic circuits in schizophrenia may involve abnormalities of the molecules that integrate glutamatergic and dopaminergic signaling pathways in thalamic neurons.
 |
Received May 27, 2003; revisions received April 22 and July 28, 2004; accepted Sept. 9, 2004. From the University of Michigan Mental Health Research Institute and the Departments of Psychiatry, Radiology, and Neurology; and the Department of Psychiatry, Mount Sinai School of Medicine, New York. Address correspondence and reprint requests to Dr. Clinton, Mental Health Research Institute, University of Michigan, 205 Zina Pitcher Place, Ann Arbor, MI 48109-0720; [email protected] (e-mail). Supported by a grant from the Mental Illness Research Association.
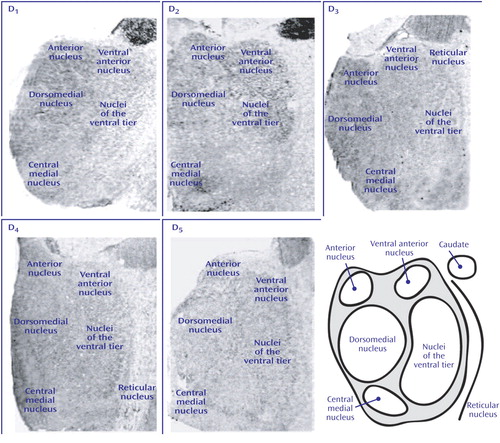
Figure 1. Dopamine Receptor mRNA Expression in Human Thalamusa
aAll five transcripts are detected, but at low levels of expression. Note the much higher labeling for D1 and D2 mRNA in the tail of the caudate nucleus.
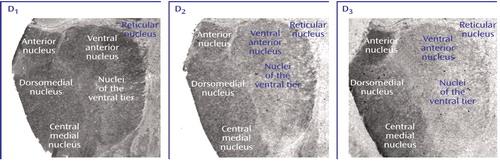
Figure 2. Dopamine Receptor Binding in Human Thalamusa
aNote the relatively homogeneous distribution of D1 receptor binding across thalamic nuclei and the relative enrichment of both D2 and D3 binding in the more medial nuclei (anterior, dorsomedial, central medial). D1 binding determined with [3H]SCH23390, D2 with [3H]raclopride in the presence of 7-OH-DPAT, and D3 with [3H]PD128907.
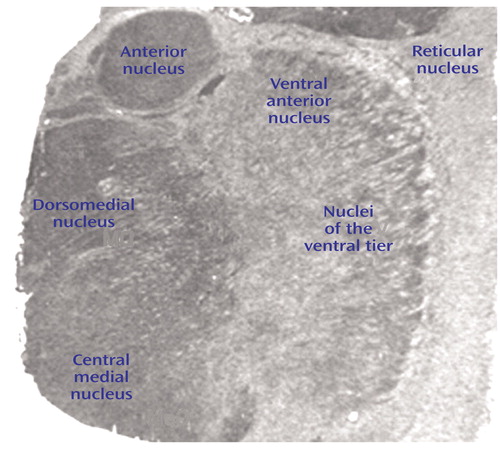
Figure 3. VMAT2 Binding in Human Thalamusa
aNote the strong enrichment in the more medial nuclei (anterior, dorsomedial, central medial), similar to the pattern seen for D2 and D3 receptor binding.
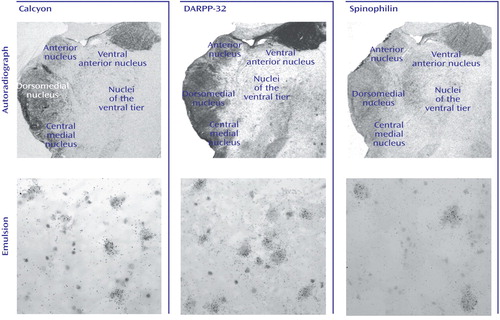
Figure 4. Intracellular Protein Transcript Expression in Human Thalamusa
aTop row shows film autoradiographs of sections treated with antisense strand riboprobes for each transcript. As in the case of other molecules associated with dopaminergic signaling in the thalamus, note the relative enrichment of transcripts encoding these intracellular proteins in more medial nuclei associated with limbic function (anterior, dorsomedial, central medial). Bottom row shows bright-field micrographs of emulsion-dipped slides labeled for each transcript, demonstrating cellular localization within the dorsomedial nucleus for each probe. Calcyon and DARPP-32 transcripts were present in both large glutamatergic relay neurons and small GABAergic interneurons. Spinophilin transcripts, on the other hand, were only expressed in the large relay cells.
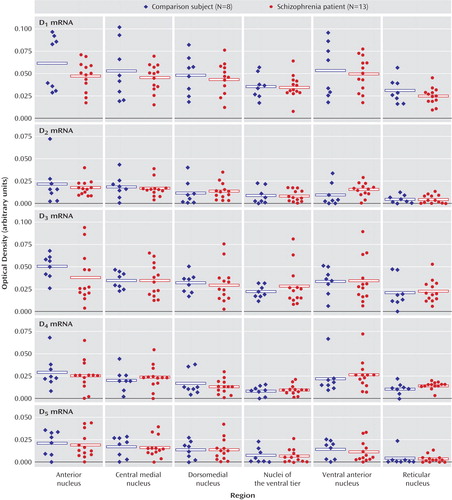
Figure 5. Dopamine Receptor Transcript Expression in Six Thalamic Nuclei in Schizophrenia and Comparison Subjectsa
aThere were no differences in the expression of these five transcripts between the persons with schizophrenia and comparison subjects.
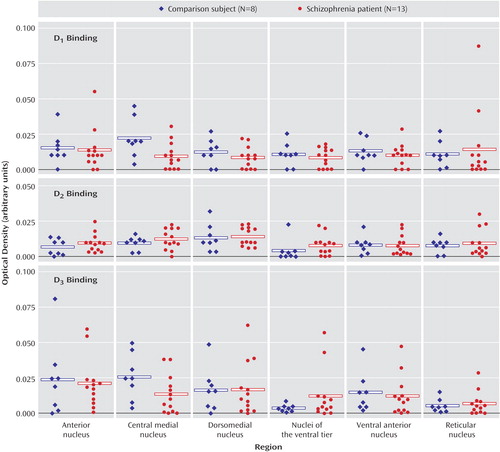
Figure 6. Dopamine Receptor Binding in Thalamus in Schizophrenia and Comparison Subjectsa
aThere were no differences in levels of these three binding sites between the persons with schizophrenia and the comparison group.
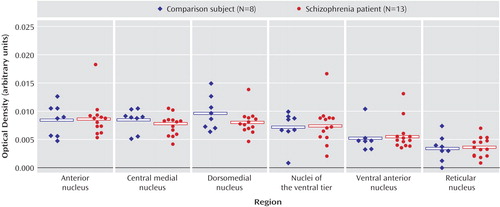
Figure 7. VMAT2 Binding in Thalamus in Schizophrenia and Comparison Subjectsa
aThere were no differences in levels of vesicular monoamine transporter in schizophrenia versus control subjects.
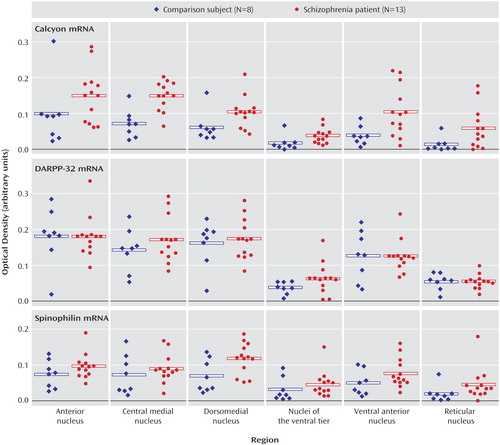
Figure 8. Intracellular Protein Transcript Expression in Thalamus in Schizophrenia and Comparison Subjectsa
aBoth calcyon and spinophilin transcripts were significantly increased in schizophrenia.
1. Meador-Woodruff JH: Novel D2-like dopamine receptors in schizophrenic brain, in Search for the Causes of Schizophrenia, vol 4. Edited by Gattaz WF, Hafner H. New York, Springer, 1999, pp 251–260Google Scholar
2. Lewis DA, Lieberman JA: Catching up on schizophrenia: natural history and neurobiology. Neuron 2000; 28:325–334Crossref, Medline, Google Scholar
3. Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R: Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry 1999; 46:56–72Crossref, Medline, Google Scholar
4. Mansour A, Meador-Woodruff JH, Lopez JF, Watson SJ: Biochemical anatomy: insights into the cell biology and pharmacology of the dopamine and serotonin systems in the brain, in Textbook of Psychopharmacology, 2nd ed. Edited by Schatzberg AF, Nemeroff CB. Washington, DC, American Psychiatric Press, 1998, pp 55–73Google Scholar
5. Zhou QY, Grandy DK, Thambi L, Kushner JA, Van Tol HH, Cone R, Pribnow D, Salon J, Bunzow JR, Civelli O: Cloning and expression of human and rat D1 dopamine receptors. Nature 1990; 347:76–80Crossref, Medline, Google Scholar
6. Undie AS, Friedman E: Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther 1990; 253:987–992Medline, Google Scholar
7. Lin CW, Miller TR, Witte DG, Bianchi BR, Stashko M, Manelli AM, Frail DE: Characterization of cloned human dopamine D1 receptor-mediated calcium release in 293 cells. Mol Pharmacol 1995; 47:131–139Medline, Google Scholar
8. Greengard P, Nairn AC, Girault JA, Ouimet CC, Snyder GL, Fisone G, Allen PB, Fienberg A, Nishi A: The DARPP-32/protein phosphatase-1 cascade: a model for signal integration. Brain Res Brain Res Rev 1998; 26:274–284Crossref, Medline, Google Scholar
9. Lezcano N, Mrzljak L, Eubanks S, Levenson R, Goldman-Rakic P, Bergson C: Dual signaling regulated by calcyon, a D1 dopamine receptor interacting protein. Science 2000; 287:1660–1664Crossref, Medline, Google Scholar
10. Missale C, Nash SR, Robinson SW, Jaber M, Caron MG: Dopamine receptors: from structure to function. Physiol Rev 1998; 78:189–225Crossref, Medline, Google Scholar
11. Smith FD, Oxford GS, Milgram SL: Association of the D2 dopamine receptor third cytoplasmic loop with spinophilin, a protein phosphatase-1-interacting protein. J Biol Chem 1999; 274:19894–19900Crossref, Medline, Google Scholar
12. Yan Z, Hsieh-Wilson L, Feng J, Tomizawa K, Allen PB, Fienberg AA, Nairn AC, Greengard P: Protein phosphatase 1 modulation of neostriatal AMPA channels: regulation by DARPP-32 and spinophilin. Nat Neurosci 1999; 2:13–17Crossref, Medline, Google Scholar
13. Koh PO, Bergson C, Undie AS, Goldman-Rakic PS, Lidow MS: Up-regulation of the D1 dopamine receptor-interacting protein, calcyon, in patients with schizophrenia. Arch Gen Psychiatry 2003; 60:311–319Crossref, Medline, Google Scholar
14. Albert KA, Hemmings HC Jr, Adamo AI, Potkin SG, Akbarian S, Sandman CA, Cotman CW, Bunney WE Jr, Greengard P: Evidence for decreased DARPP-32 in the prefrontal cortex of patients with schizophrenia. Arch Gen Psychiatry 2002; 59:705–712Crossref, Medline, Google Scholar
15. Carpenter WT Jr, Buchanan RW: Schizophrenia. N Engl J Med 1994; 330:681–690Crossref, Medline, Google Scholar
16. Carlsson A: Neurocircuitries and neurotransmitter interactions in schizophrenia. Int Clin Psychopharmacol 1995; 10(suppl 3):21–28Google Scholar
17. Steriade M, Jones EG, McCormick DA: Thalamus: Organization and Function. Amsterdam, Elsevier, 1997Google Scholar
18. Andreasen NC, Arndt S, Swayze V Jr, Cizadlo T, Flaum M, O’Leary D, Ehrhardt JC, Yuh WT: Thalamic abnormalities in schizophrenia visualized through magnetic resonance image averaging. Science 1994; 266:294–298Crossref, Medline, Google Scholar
19. Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr: PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 1996; 153:191–199Link, Google Scholar
20. Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, Siever LJ: Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 2001; 58:133–140Crossref, Medline, Google Scholar
21. Gilbert AR, Rosenberg DR, Harenski K, Spencer S, Sweeney JA, Keshavan MS: Thalamic volumes in patients with first-episode schizophrenia. Am J Psychiatry 2001; 158:618–624Link, Google Scholar
22. Pakkenberg B: Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 1990; 47:1023–1028Crossref, Medline, Google Scholar
23. Popken GJ, Bunney WE Jr, Potkin SG, Jones EG: Subnucleus-specific loss of neurons in medial thalamus of schizophrenics. Proc Natl Acad Sci USA 2000; 97:9276–9280Crossref, Medline, Google Scholar
24. Hazlett EA, Buchsbaum MS, Byne W, Wei T-C, Spiegel-Cohen J, Geneve C, Kinderlehrer R, Haznedar MM, Shihabuddin L, Siever LJ: Three-dimensional analysis with MRI and PET of the size, shape, and function of the thalamus in the schizophrenia spectrum. Am J Psychiatry 1999; 156:1190–1199Abstract, Google Scholar
25. Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ: A functional neuroanatomy of hallucinations in schizophrenia. Nature 1995; 378:176–179Crossref, Medline, Google Scholar
26. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
27. Clinton SM, Meador-Woodruff JH: Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res 2004; 69:237–253Crossref, Medline, Google Scholar
28. Oke AF, Adams RN: Elevated thalamic dopamine: possible link to sensory dysfunctions in schizophrenia. Schizophr Bull 1987; 13:589–604Crossref, Medline, Google Scholar
29. Ibrahim HM, Hogg AJ Jr, Healy DJ, Haroutunian V, Davis KL, Meador-Woodruff JH: Ionotropic glutamate receptor binding and subunit mRNA expression in thalamic nuclei in schizophrenia. Am J Psychiatry 2000; 157:1811–1823Link, Google Scholar
30. Richardson-Burns SM, Haroutunian V, Davis KL, Watson SJ, Meador-Woodruff JH: Metabotropic glutamate receptor mRNA expression in the schizophrenic thalamus. Biol Psychiatry 2000; 47:22–28Crossref, Medline, Google Scholar
31. Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH: Vesicular glutamate transporter transcript expression in the thalamus in schizophrenia. Neuroreport 2001; 12:2885–2887Crossref, Medline, Google Scholar
32. Smith RE, Haroutunian V, Davis KL, Meador-Woodruff JH: Expression of excitatory amino acid transporter transcripts in the thalamus of subjects with schizophrenia. Am J Psychiatry 2001; 158:1393–1399Link, Google Scholar
33. Clinton SM, Haroutunian V, Davis KL, Meador-Woodruff JH: Altered transcript expression of NMDA receptor-associated postsynaptic proteins in the thalamus of subjects with schizophrenia. Am J Psychiatry 2003; 160:1100–1109Link, Google Scholar
34. Meador-Woodruff JH, Damask SP, Watson SJ Jr: Differential expression of autoreceptors in the ascending dopamine systems of the human brain. Proc Natl Acad Sci USA 1994; 91:8297–8301Crossref, Medline, Google Scholar
35. Meador-Woodruff JH, Grandy DK, Van Tol HH, Damask SP, Little KY, Civelli O, Watson SJ Jr: Dopamine receptor gene expression in the human medial temporal lobe. Neuropsychopharmacology 1994; 10:239–248Crossref, Medline, Google Scholar
36. Meador-Woodruff JH, Little KY, Damask SP, Watson SJ: Effects of cocaine on D3 and D4 receptor expression in the human striatum. Biol Psychiatry 1995; 38:263–266Crossref, Medline, Google Scholar
37. Meador-Woodruff JH, Damask SP, Wang J, Haroutunian V, Davis KL, Watson SJ: Dopamine receptor mRNA expression in human striatum and neocortex. Neuropsychopharmacology 1996; 15:17–29Crossref, Medline, Google Scholar
38. Meador-Woodruff JH, Haroutunian V, Powchik P, Davidson M, Davis KL, Watson SJ: Dopamine receptor transcript expression in striatum and prefrontal and occipital cortex: focal abnormalities in orbitofrontal cortex in schizophrenia. Arch Gen Psychiatry 1997; 54:1089–1095Crossref, Medline, Google Scholar
39. Meador-Woodruff JH, Mansour A, Bunzow JR, Van Tol HH, Watson SJ Jr, Civelli O: Distribution of D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci USA 1989; 86:7625–7628Crossref, Medline, Google Scholar
40. Vander Borght TM, Sima AA, Kilbourn MR, Desmond TJ, Kuhl DE, Frey KA: [3H]Methoxytetrabenazine: a high specific activity ligand for estimating monoaminergic neuronal integrity. Neuroscience 1995; 68:955–962Crossref, Medline, Google Scholar
41. Lindvall O, Bjorklund A, Nobin A, Stenevi U: The adrenergic innervation of the rat thalamus as revealed by the glyoxylic acid fluorescence method. J Comp Neurol 1974; 154:317–347Crossref, Medline, Google Scholar
42. Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D: Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain, I: tyrosine hydroxylase in the mesand diencephalon. Med Biol 1976; 54:427–453Medline, Google Scholar
43. Groenewegen HJ: Organization of the afferent connections of the mediodorsal thalamic nucleus in the rat, related to the mediodorsal-prefrontal topography. Neuroscience 1988; 24:379–431Crossref, Medline, Google Scholar
44. Papadopoulos GC, Parnavelas JG: Distribution and synaptic organization of dopaminergic axons in the lateral geniculate nucleus of the rat. J Comp Neurol 1990; 294:356–361Crossref, Medline, Google Scholar
45. Young KA, Wilcox RE: Characterization of D2 receptors and dopamine levels in the thalamus of the rat. Life Sci 1991; 48:1845–1852Crossref, Medline, Google Scholar
46. Levesque D, Diaz J, Pilon C, Martres MP, Giros B, Souil E, Schott D, Morgat JL, Schwartz JC, Sokoloff P: Identification, characterization, and localization of the dopamine D3 receptor in rat brain using 7-[3H]hydroxy-N,N-di-n-propyl-2-aminotetralin. Proc Natl Acad Sci USA 1992; 89:8155–8159Crossref, Medline, Google Scholar
47. Bouthenet ML, Martres MP, Sales N, Schwartz JC: A detailed mapping of dopamine D-2 receptors in rat central nervous system by autoradiography with [125I]iodosulpride. Neuroscience 1987; 20:117–155Crossref, Medline, Google Scholar
48. Sales N, Martres MP, Bouthenet ML, Schwartz JC: Ontogeny of dopaminergic D-2 receptors in the rat nervous system: characterization and detailed autoradiographic mapping with [125I]iodosulpride. Neuroscience 1989; 28:673–700Crossref, Medline, Google Scholar
49. Mrzljak L, Bergson C, Pappy M, Huff R, Levenson R, Goldman-Rakic PS: Localization of dopamine D4 receptors in GABAergic neurons of the primate brain. Nature 1996; 381:245–248Crossref, Medline, Google Scholar
50. Mansour A, Meador-Woodruff JH, Bunzow JR, Civelli O, Akil H, Watson SJ: Localization of dopamine D2 receptor mRNA and D1 and D2 receptor binding in the rat brain and pituitary: an in situ hybridization-receptor autoradiographic analysis. J Neurosci 1990; 10:2587–2600Crossref, Medline, Google Scholar
51. Tiberi M, Jarvie KR, Silvia C, Falardeau P, Gingrich JA, Godinot N, Bertrand L, Yang-Feng TL, Fremeau RT Jr, Caron MG: Cloning, molecular characterization, and chromosomal assignment of a gene encoding a second D1 dopamine receptor subtype: differential expression pattern in rat brain compared with the D1A receptor. Proc Natl Acad Sci USA 1991; 88:7491–7495Crossref, Medline, Google Scholar
52. Weiner DM, Levey AI, Sunahara RK, Niznik HB, O’Dowd BF, Seeman P, Brann MR: D1 and D2 dopamine receptor mRNA in rat brain. Proc Natl Acad Sci USA 1991; 88:1859–1863Crossref, Medline, Google Scholar
53. Meador-Woodruff JH, Mansour A, Grandy DK, Damask SP, Civelli O, Watson SJ Jr: Distribution of D5 dopamine receptor mRNA in rat brain. Neurosci Lett 1992; 145:209–212Crossref, Medline, Google Scholar
54. Gurevich EV, Joyce JN: Distribution of dopamine D3 receptor expressing neurons in the human forebrain: comparison with D2 receptor expressing neurons. Neuropsychopharmacology 1999; 20:60–80Crossref, Medline, Google Scholar
55. Oke AF, Adams RN, Winblad B, von Knorring L: Elevated dopamine/norepinephrine ratios in thalami of schizophrenic brains. Biol Psychiatry 1988; 24:79–82Crossref, Medline, Google Scholar
56. Oke A, Solnick J, Adams RN: Catecholamine distribution patterns in rat thalamus. Brain Res 1983; 269:180–183Crossref, Medline, Google Scholar
57. Scherman D, Boschi G, Rips R, Henry JP: The regionalization of [3H]dihydrotetrabenazine binding sites in the mouse brain and its relationship to the distribution of monoamines and their metabolites. Brain Res 1986; 370:176–181Crossref, Medline, Google Scholar
58. Weihe E, Schafer MK, Erickson JD, Eiden LE: Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J Mol Neurosci 1994; 5:149–164Crossref, Medline, Google Scholar
59. Peter D, Liu Y, Sternini C, de Giorgio R, Brecha N, Edwards RH: Differential expression of two vesicular monoamine transporters. J Neurosci 1995; 15:6179–6188Crossref, Medline, Google Scholar
60. Vander Borght T, Kilbourn M, Desmond T, Kuhl D, Frey K: The vesicular monoamine transporter is not regulated by dopaminergic drug treatments. Eur J Pharmacol 1995; 294:577–583Crossref, Medline, Google Scholar
61. Kemmerer ES, Desmond TJ, Albin RL, Kilbourn MR, Frey KA: Treatment effects on nigrostriatal projection integrity in partial 6-OHDA lesions: comparison of L-DOPA and pramipexole. Exp Neurol 2003; 183:81–86Crossref, Medline, Google Scholar
62. Frey K, Kilbourn M, Robinson T: Reduced striatal vesicular monoamine transporters after neurotoxic but not after behaviorally-sensitizing doses of methamphetamine. Eur J Pharmacol 1997; 334:273–279Crossref, Medline, Google Scholar
63. Lidow MS, Roberts A, Zhang L, Koh PO, Lezcano N, Bergson C: Receptor crosstalk protein, calcyon, regulates affinity state of dopamine D1 receptors. Eur J Pharmacol 2001; 427:187–193Crossref, Medline, Google Scholar
64. Allen PB, Ouimet CC, Greengard P: Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA 1997; 94:9956–9961Crossref, Medline, Google Scholar
65. Satoh A, Nakanishi H, Obaishi H, Wada M, Takahashi K, Satoh K, Hirao K, Nishioka H, Hata Y, Mizoguchi A, Takai Y: Neurabin-II/spinophilin: an actin filament-binding protein with one pdz domain localized at cadherin-based cell-cell adhesion sites. J Biol Chem 1998; 273:3470–3475Crossref, Medline, Google Scholar
66. Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P: Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA 2000; 97:9287–9292Crossref, Medline, Google Scholar
67. Fernandez A, Brautigan DL, Mumby M, Lamb NJ: Protein phosphatase type-1, not type-2A, modulates actin microfilament integrity and myosin light chain phosphorylation in living nonmuscle cells. J Cell Biol 1990; 111:103–112Crossref, Medline, Google Scholar
68. Greengard P, Allen PB, Nairn AC: Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 1999; 23:435–447Crossref, Medline, Google Scholar
69. Ouimet CC, da Cruz e Silva EF, Greengard P: The alpha and gamma 1 isoforms of protein phosphatase 1 are highly and specifically concentrated in dendritic spines. Proc Natl Acad Sci USA 1995; 92:3396–3400Crossref, Medline, Google Scholar
70. Lidow MS, Song ZM, Castner SA, Allen PB, Greengard P, Goldman-Rakic PS: Antipsychotic treatment induces alterations in dendrite- and spine-associated proteins in dopamine-rich areas of the primate cerebral cortex. Biol Psychiatry 2001; 49:1–12Crossref, Medline, Google Scholar
71. Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P: Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J Neurosci 2000; 20:4480–4488Crossref, Medline, Google Scholar
72. Leveque JC, Macias W, Rajadhyaksha A, Carlson RR, Barczak A, Kang S, Li XM, Coyle JT, Huganir RL, Heckers S, Konradi C: Intracellular modulation of NMDA receptor function by antipsychotic drugs. J Neurosci 2000; 20:4011–4020Crossref, Medline, Google Scholar



