Correlates of 1-Year Prospective Outcome in Bipolar Disorder: Results From the Stanley Foundation Bipolar Network
Abstract
OBJECTIVE: The purpose of the study was to examine potential correlates of outcome in patients treated for bipolar disorder. METHOD: During a 1-year period, 258 patients with DSM-IV bipolar disorder or schizoaffective disorder were rated with the prospective NIMH-Life Chart Method, which characterizes each day in terms of the severity of manic and depressive symptoms on the basis of patients’ mood-related impairment in their usual educational, social, or occupational roles. Mean ratings for the severity of mania, depression, and overall bipolar illness and the number of manic, depressive, and overall illness episodes were calculated. Potential risk factors were assessed at the start of the study, and multivariate linear regression analysis was used to determine the correlates of the six 1-year outcome measures. RESULTS: Three of the six outcome measures were largely independent of each other and were used in the analysis. The mean rating for severity of mania was associated with comorbid substance abuse, history of more than 10 prior manic episodes, and poor occupational functioning at study entry. The mean rating for severity of depression was associated with a history of more than 10 prior depressive episodes and poor occupational functioning at study entry. The total number of overall illness episodes was associated with a positive family history of drug abuse, a history of prior rapid cycling, and poor occupational functioning. In addition, the mean rating for severity of mania and the total number of overall illness episodes were both initially associated with a history of childhood abuse, but these relationships were lost with the addition of other illness variables to the analysis. CONCLUSIONS: Clinicians who treat patients with bipolar disorder should consider a family history of drug abuse, a history of childhood abuse, prior course of illness, comorbid substance abuse, and occupational functioning in determining prognosis and setting goals for further treatment.
Several naturalistic studies have found that absence of relapse over 1 (or more) year(s) of treatment is seen in only a minority of patients with bipolar disorder (1–5). These results appear to contradict the results of several randomized, controlled trials of the prophylactic efficacy of lithium, carbamazepine, and valproate, in which the response rates were usually 50% or higher (6–8). This discrepancy can be explained partly by the characteristics of patients recruited for randomized, controlled trials, which usually exclude patients with significant comorbid physical or psychiatric conditions and acute suicidal ideation. In addition, outcome criteria in these randomized, controlled trials involved either new episodes or the need for additional treatment because of symptoms of emerging episodes. However, many patients have considerable symptoms without fulfilling the criteria for a mood episode.
Several naturalistic studies (9–22) have examined correlates or predictors of the course of illness, but the findings have been inconsistent. The following summary describes the major statistically significant findings from these studies.
Many studies examined gender, but only four studies found significant gender effects. In two studies, women had more depressed episodes than manic episodes (9, 10). In addition, female gender was associated with more episodes and a greater likelihood of rapid cycling (11) and with poorer psychosocial adjustment (12).
Age at onset was related to outcome in two studies. In one study, poorer functioning was found in patients with onset before age 40 years (13). In the other study, less psychopathology was found in patients with younger age at onset (12).
Two studies found an effect of type of bipolar disorder. Compared to bipolar II disorder, bipolar I disorder was associated with more hospitalizations (14) and with less rapid cycling (11).
A more difficult prior course of illness was found to be associated with poorer outcome in six studies: a higher number of prior illness episodes was related to more future episodes (9) and poorer psychosocial adjustment (12); a higher number of prior depressive episodes was related to rapid cycling (11) and poorer functioning (13); and a higher number of prior admissions was related to lower scores on the Global Assessment Scale (GAS) (15). A higher number of cycles was associated with less time ill in one study (9) but with more affective morbidity in another study (16).
In five studies, psychiatric comorbidity was associated with poorer outcome. Comorbid alcoholism was related to poorer psychosocial adjustment in two studies (14, 15); alcoholism after the development of bipolar disorder, but not prior to it, was found to be associated with more episodes (17); comorbid drug abuse was related to slower recovery (18) and a lower GAS score (15); and comorbid panic disorder was related to a higher likelihood of rapid cycling (11).
Finally, severe stressful life events have been found to be associated with slower recovery (19) and higher relapse rates (20).
We recently evaluated morbidity over 1 prospective year in the first 258 patients in the Stanley Foundation Bipolar Network (21). Within the Bipolar Network, we attempted to select a representative sample of outpatients with bipolar illness with considerable morbidity and comorbidity (22, 23). Thus, we included very ill patients, of whom 27% were ill most of the year, 40% were intermittently ill, and only 33% had minimal or no symptoms. We sought to examine potential correlates of outcome in the first prospective year of treatment in these patients.
Method
Patients
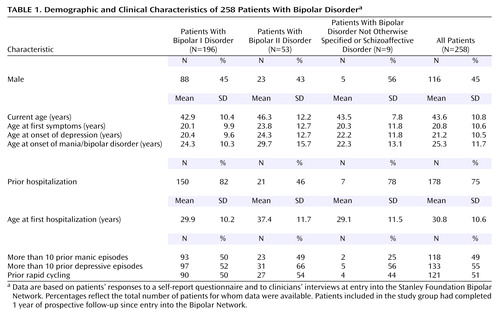
All patients were recruited through one of the five original Stanley Foundation Bipolar Network sites (22). They met the DSM-IV criteria for bipolar I disorder (N=196), bipolar II disorder (N=53), or bipolar disorder not otherwise specified or schizoaffective disorder (N=9), as assessed by the Structured Clinical Interview for DSM-IV (SCID). Only patients with current comorbid substance use disorders that required adjunctive treatment in another setting were excluded. The study group of 258 patients overlaps substantially with that of other studies that used data from the Bipolar Network (23–27) but was limited by the requirement for completion of 1 year of prospective follow-up. After complete description of the study to the subjects, written informed consent was obtained.
Clinicians rated patients with the prospective NIMH-Life Chart Method, characterizing each day in terms of the severity of manic and depressive symptoms on the basis of mood-related functional impairment in patients’ usual educational, social, or occupational roles. Severity ratings ranged from 0 (normal) through 2.5 (minimally ill), 5 (low moderate), and 7.5 (high moderate) to 10 (severely ill). Clinicians assessed patients approximately every 2–4 weeks using all available information, including information from patient interviews and the patient’s self-rated prospective NIMH-Life Chart Method, to determine the daily ratings. All raters in the Bipolar Network participated in training on the prospective NIMH-Life Chart Method and showed good reliability (kappa=0.82) (28). The prospective NIMH-Life Chart Method ratings have been validated in relation to cross-sectional rating scales (29, 30).
At study entry, patients completed a questionnaire on demographic characteristics and provided their own retrospective report of illness variables as well as a history of prior psychosocial adversities (23). In addition, clinicians completed a questionnaire based on their knowledge of the patient’s prior illness variables.
Outcome Variables
For this study, prospective 1-year outcome was based on the clinicians’ prospective NIMH-Life Chart Method ratings and was calculated in two ways. The first outcome measure was the mean severity of illness over the year, which included mean severity of mania, depression, and overall bipolar illness. The average daily scores ranged from 0 (normal) to 10 (severe) for each pole (mania or depression) separately or the maximum of both poles (overall). Days on which patients switched mood states at least once were assigned the most severe depression score and the most severe mania score. A second set of outcome measures was the number of episodes, which included manic, depressive, and overall episodes. The number of episodes was calculated by a computer algorithm based on the so-called NIMH Leapfrog method as previously described by Denicoff et al. (3). In short, manic episodes were counted if they included a minimum of 1 day with a score indicating at least low moderate mania, and depressive episodes were counted if they included a minimum of at least 1 day of severe depression or 2 days of low moderate depression. In addition, an episode was considered ended when a switch in mood polarity (from mania to depression or vice versa) occurred, after at least 2 weeks of complete euthymia, or when the euthymic interval lasted less than 2 weeks but at least 1 day longer than the longest duration of the adjacent episode of the same polarity.
Correlates of Outcome
We assessed as independent variables six sets of potential risk factors that have been found in prior studies: 1) demographic characteristics (age and gender); 2) family history in first-degree relatives of bipolar disorder, unipolar depression, and alcohol or drug abuse as reported by the proband; 3) serious physical, verbal, or sexual abuse as a child or adult; 4) course of illness (age at onset of first symptoms, first depression, and first hypomania or mania; prior number of hypomanic or manic and depressive episodes; prior history of cycling; and prior hospitalization [yes/no]) and mood state during the first week after entry into the Bipolar Network; 5) comorbidity (history of alcohol or drug abuse); and 6) social variables at entry into the study (marital status, occupational functioning, and level of income). All potential correlates were assessed at entry into the Bipolar Network, i.e., before obtaining the data on the prospective outcomes.
Analytic Procedure
The relationships between the six outcome variables (mean severity of mania, depression, and overall bipolar illness and the number of manic, depressive, and overall illness episodes) were first examined for their relative independence, and three relatively independent outcomes were left for further analysis (see Results). This step was taken to reduce the complexity of the overall analysis while preserving the intricate quality of the illness in the analytic model. Next, we performed a univariate analysis with Pearson’s product-moment correlation. The Hochberg adjustment was used with each outcome separately to minimize the number of potentially spurious correlations in the data. Finally, a multivariate linear regression analysis of the risk factors was performed.
The univariate analysis was included for several reasons. First, the lack of consistency in studies of bipolar disorder risk factors means there is a need to determine when and how certain risk factors are relevant. Univariate results help to elucidate the manner in which factors that are eliminated from a multivariate model fit into the data that are being examined. Second, because various studies collect data on different factors, the univariate results may provide additional information to facilitate comparisons with other studies in the literature. Finally, clinicians may have information on only a limited number of factors in considering the likely course of patients. If we reported only those factors in the multivariate model, then clinicians would have no way to consider other factors that were excluded from the model only because of significant overlap with other variables in the model.
Linear regression assumes that risk factors will be relatively uncorrelated. Using highly correlated risk factors can increase the variance in regression estimates and make such estimates unstable (the problem of multicollinearity). Another assumption is that variables are continuous in nature. Nevertheless, several of the factors in our analysis are dichotomous, which is allowed in regression analyses, provided that such variables have properties similar to those of interval scale measurements.
Multivariate linear regressions for each of the three outcomes were performed in an attempt to adjust for problems with multiple comparisons and multicollinearity and to determine how much of the variation in outcome could be explained. In this analysis, any potential variable that was significantly correlated with at least one outcome measure in the univariate analysis, as suggested by Hochberg’s adjustment (31) for multiple comparisons, was included in order to facilitate discussion of how the various risk factors affected the various outcomes differently. The six sets of potential risk factors were organized hierarchically but also rather arbitrarily in a chronological order in an attempt to examine how vulnerability factors (i.e., family history), psychosocial stressors experienced as a child and as an adult, and then illness-related variables might successively influence outcome.
After a first run of the multiple regression analysis, some of the risk factors that were found to be highly related to others and some that were not found to be significant in any stage of the hierarchical regression for any outcome were eliminated from the subsequent multiple regression analyses to preserve parsimony within the models.
Results
The patients’ demographic data and illness characteristics are summarized in Table 1. The patients received a mean of 4.0 (SD=2.0) different psychotropic medications during the prospective year, including one or more mood stabilizers (N=252, 98%), antipsychotics (N=65, 25%), antidepressants (N=152, 59%), and/or benzodiazepines (N=129, 50%).
The mean severity of overall bipolar illness was highly related to the mean severity of depression (r=0.93, df=256, p<0.001) but not to the mean severity of mania (r=0.45, df=256, p<0.001). The mean severity of depression and the mean severity of mania were not strongly associated (r=0.14, df=256, p=0.03), so both were used in the multivariate linear regression analysis. The overall number of episodes was highly related to the number of depressive episodes (r=0.89, df=256, p<0.001) and manic episodes (r=0.96, df=256, p<0.001), and the numbers of depressive and manic episodes were also highly related (r=0.73, df=256, p<0.001), so only the overall number of episodes was used in the multivariate analysis. None of the severity measures had large associations with the number-of-episodes measures.
Table 2 shows the results of the univariate analysis of all variables for the three sets of outcome measures. Gender, age, and family history of mood disorder (and family history of either bipolar disorder or depression) were not found to be associated with any outcome. A family history of any substance use disorder, especially of drug abuse, was related to the number of episodes. The self-reported experience of physical and verbal abuse as a child, but not as an adult, was related to the number of episodes, but only childhood physical abuse was related to a higher mean severity of mania.
Regarding illness variables, earlier age at onset of first symptoms and at onset of depression and mania, prior experience of more than 10 manic or more than 10 depressive episodes, and prior rapid cycling were related to a higher number of episodes. Correlations with mean severity of mania or depression were less strong, except for the correlation of more than 10 prior depressive episodes with mean severity of depression. A positive history of comorbid alcoholism and drug abuse in the proband was associated with higher mean severity of mania and a higher number of episodes. Mood state at entry into the Bipolar Network was associated with the compatible outcome measures. Finally, self-reported limited occupational functioning at network entry was strongly associated with poor outcome across all three measures.
Family history of any substance use disorder was related to family history of drug abuse (r=0.44, df=245, p<0.001), and the strongest association with outcome was found for drug abuse. Thus, only drug abuse was used in the subsequent multivariate analysis. Physical, sexual, and verbal abuse as a child were only moderately correlated (range of r=0.27 to 0.49, df=239 to 241, p<0.001), and sexual abuse did not remain in the model when the other types of abuse were included. Therefore, we included physical and verbal abuse in the multivariate analysis because of their differential univariate associations with the outcome measures. Age at onset of mania (indicating formal onset of bipolar disorder) was included instead of other specific measures for first appearance of symptoms or depression because of the large correlations (ranging from 0.68 to 0.95, df=214 to 229, p<0.001) among these variables. As comorbidity with alcohol use disorder and drug abuse were strongly correlated (r=0.77, df=243, p<0.001), we included comorbidity of any substance abuse disorder in the multivariate analysis.
Table 3 shows the results of the multivariate analysis. In the hierarchical model for mean severity of mania, family history was not significant. Physical abuse, but not verbal abuse, was initially associated with higher mean severity of mania. However, its significance disappeared when prior course of illness variables were added. The presence of a comorbid substance use disorder was significantly associated with increased mania severity, and this relation remained significant after adding other prior illness variables (model 4). Finally, a history of more than 10 prior manic episodes and poor occupational functioning at entry into the Bipolar Network were associated with mania severity (model 5).
Outcome measured by depression severity was associated with the prior experience of more than 10 depressive episodes and poor occupational functioning at entry into the Bipolar Network.
In the model for the total number of episodes, a positive family history of drug abuse was significant. This variable remained significant when prior illness variables were added and other outcome measures were considered. While reported childhood verbal abuse and an earlier age at onset of mania were significantly associated with number of episodes, both relationships were lost after the addition of prior illness variables. Finally, a history of prior rapid cycling and poor occupational functioning at entry into the Bipolar Network were associated with more episodes; only rapid cycling remained significant after adjustment for the other outcome measures.
Discussion
In this naturalistic study, several groups of temporally distinct variables (family history of drug abuse, reported negative life events in childhood, and several retrospective illness variables) were found to be statistically significant correlates of 1-year outcome, as assessed prospectively with daily life charts. Before further discussion of the key findings, two major limitations of this study should be noted. First, we cannot rule out that some findings in the univariate analysis are chance findings (even after the Hochberg correction), given that multiple comparisons were made. We studied a large number of variables, and some of them were highly interrelated. Although they represented domains that had previously been found to be related to outcome in other studies (see literature review at the beginning of the article), we did not test any of them on the basis of our a priori hypothesis. Second, we must reemphasize that correlations do not imply causal relationships. Although the models of risk factors in the multivariate analysis were based on their chronological order, assuming that they might act as risk factors following each other in time, other models and other patterns of ordering the variables would also have been possible. For instance, child abuse as a risk factor was presented as if it had its effect in children who were also affected by a family history of drug abuse, but child abuse could also have been a result of substance use disorders in the parents.
The overall amount of variance accounted for was limited in the first three groups of variables (models 1–3 in Table 3). For example, the combination of 1) family history of drug abuse, 2) a self-reported history of child abuse, and 3) age at onset of mania and history of comorbid substance use disorder accounted for at most only 15% of the variance, i.e., in the prediction of total number of episodes. Only when 4) prior illness variables and 5) clinical state at entry into the Bipolar Network were added did the amount of variance accounted for increase to 31%–42% for any of the three outcome measures.
A history of more than 10 manic episodes and manic symptoms at entry into the Bipolar Network were associated with a higher mean severity of mania, more than 10 depressive episodes and depressive symptoms at study entry were associated with higher mean severity of depression, and prior rapid cycling and cycling at study entry were associated with a greater number of episodes. These findings indicate that self-reports of prior course of illness as assessed retrospectively at study entry and ratings of morbidity at study entry tend to be replicated by prospective clinician ratings over the following year. This pattern has also been seen in previous studies (9, 16), which indicates some syndromal continuity or stability of bipolar illness over time.
Earlier age at illness onset was not found to be strongly associated with outcome. Earlier age at onset had only an initial relationship with the number of episodes, and this relationship did not remain significant when variables relating to the prior course of illness were added. This finding is consistent with a previous report (13), but other studies either did not find an effect (11, 19) or had contrasting results (12).
It is rather surprising that a positive family history of mood disorders—either bipolar disorder or depression—was not found to be associated with any of the outcome measures. This finding suggests that the genetic risk for mood disorders may not have a strong effect on the severity of the disorder. However, a limitation of our study is that family history was scored as present when one or more family members were identified by the proband as having a positive history of mood disorders; the total number of first-degree relatives was not taken into account.
In contrast, a positive family history of drug abuse (and to a lesser extent also alcohol abuse) was associated with a greater number of episodes. In addition, a history of prior substance abuse (in the proband) was associated with a higher severity of mania. Although other explanations (including a chance finding) cannot be ruled out, we assume these findings suggest that genetic or familial vulnerability for substance use disorders, as well as their actual development in the proband, constitutes a risk factor for poorer outcome in patients with bipolar disorder, especially in relation to episode frequency and severity of mania. Even after exclusion of patients with severe current substance abuse, our findings are consistent with previous reports that bipolar disorders and substance use disorders are related (17) and that they contribute to the worsening of each other’s outcome (32).
It is interesting to note that patients’ self-reports of childhood physical abuse were found to be associated with a higher mean severity of mania and that reports of childhood verbal abuse were associated with more episodes. Nevertheless, the associations were not very strong and were not maintained after the addition of illness variables, such as age at onset, comorbid substance abuse (for overall number of episodes), and prior course of illness (for mania). These findings may suggest that childhood abuse is especially related to an earlier onset and a more severe course in the beginning of the disorder, which subsequently is associated with more adverse outcomes. In the present study, the experience of abuse as an adult was not associated with outcome, which is in contrast with previous studies in which recent stressful life events were related to a greater risk of relapse (20, 33) or slower recovery (19). One explanation is that we did not adequately assess such experiences or their relationship to outcome. An alternative explanation is that early traumatic events may have a greater effect than later ones. We suggest that this aspect deserves further study.
Almost 80% of the patients in our study had bipolar I disorder. Moreover, the current study was performed in a naturalistic setting with highly motivated patients, all of whom were being treated in academic settings and many of whom were participating in treatment studies. Therefore, the generalizability to other populations of patients with bipolar disorders, especially bipolar II disorder, and to those with better responses to treatment is limited. Another point is that we could not examine the potential effects of treatment adherence as correlates of outcome (12, 34). However, similar to previous findings that a history of rapid cycling is a predictor of poor response to treatment with lithium, carbamazepine, or other mood stabilizers (35–37), our findings suggest that rapid cycling is a predictor of poor outcome in general.
In conclusion, the data indicate syndromal continuity between retrospective self-reported illness characteristics and clinician-rated 1-year prospective outcomes in patients with bipolar disorder. The findings suggest that physicians who treat patients with bipolar disorder should consider a family history of drug abuse, reported childhood abuse, prior adverse course of illness, comorbid substance abuse, and prior poor occupational functioning as potential correlates of a more difficult prospective course. It would appear useful for clinicians to ask about these risk factors when treating new patients with bipolar disorder. In addition to having prognostic implications, these issues may also help to determine prognosis and to set goals for further and more specific treatments, e.g., pharmacotherapy and psychotherapy focusing on, when applicable, reduction of comorbid substance abuse or moderating the effects of early traumatization. Which such tailored treatments are appropriate and whether such treatments are successful should be further studied. From an additional methodological perspective, these variables should also be assessed in medication studies, as their covert and unaccounted-for presence may have substantial effects on response to treatments. Finally, it would appear useful for these variables to be considered in the development of treatment guidelines for patients with bipolar disorders.
 |
 |
 |
Received July 8, 2002; revisions received Dec. 27, 2002 and July 29, 2003; accepted Nov. 18, 2003. From Altrecht Institute for Mental Health Care, Utrecht, The Netherlands; University Hospital Groningen, Groningen, The Netherlands; National Institute of Mental Health, Bethesda, Md.; the Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles; University of Texas–Southwestern Medical Center, Dallas; and the Department of Psychiatry, University of Cincinnati College of Medicine, Cincinnati. Address reprint requests to Dr. Nolen, University Hospital Groningen, P.O. Box 30.001, 9700 RB Groningen, the Netherlands; [email protected] (e-mail). Supported by the Stanley Medical Research Institute.
1. Goldberg JF, Harrow M, Grossman LS: Course and outcome in bipolar affective disorder: a longitudinal follow-up study. Am J Psychiatry 1995; 152:379–384Link, Google Scholar
2. Gitlin MJ, Swendsen J, Heller TL, Hammen C: Relapse and impairment in bipolar disorder. Am J Psychiatry 1995; 152:1635–1640Link, Google Scholar
3. Denicoff KD, Smith-Jackson EE, Disney ER, Ali SO, Leverich GS, Post RM: Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997; 58:470–478Crossref, Medline, Google Scholar
4. Maj M, Pirozzi R, Magliano L, Bartoli L: Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry 1998; 155:30–35Link, Google Scholar
5. Licht RW, Vestergaard P, Rasmussen NA, Jepsen K, Brodersen A, Hansen PE: A lithium clinic for bipolar patients: 2-year outcome of the first 148 patients. Acta Psychiatr Scand 2001; 104:387–390Crossref, Medline, Google Scholar
6. Greil W, Ludwig-Mayerhofer W, Erazo N, Schochlin C, Schmidt S, Engel RR, Czernik A, Giedke H, Muller-Oerlinghausen B, Osterheider M, Rudolf GA, Sauer H, Tegeler J, Wetterling T: Lithium versus carbamazepine in the maintenance treatment of bipolar disorders—a randomised study. J Affect Disord 1997; 43:151–161Crossref, Medline, Google Scholar
7. Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, Pope HG Jr, Chou JC, Keck PE Jr, Rhodes LJ, Swann AC, Hirschfeld RM, Wozniak PJ (Divalproex Maintenance Study Group): A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Arch Gen Psychiatry 2000; 57:481–489Crossref, Medline, Google Scholar
8. Hartong EGTM, Moleman P, Hoogduin CAL, Broekman TG, Nolen WA (LitCar Group): Prophylactic efficacy of lithium versus carbamazepine in treatment-naive bipolar patients. J Clin Psychiatry 2003; 64:144–151Crossref, Medline, Google Scholar
9. Roy-Byrne P, Post RM, Uhde TW, Porcu T, Davis D: The longitudinal course of recurrent affective illness: life chart data from research patients at the NIMH. Acta Psychiatr Scand Suppl 1985; 317:1–34Crossref, Medline, Google Scholar
10. Robb JC, Young LT, Cooke RG, Joffe RT: Gender differences in patients with bipolar disorder influence outcome in the Medical Outcomes Survey (SF-20) subscale scores. J Affect Disord 1998; 49:189–193Crossref, Medline, Google Scholar
11. Coryell W, Endicott J, Keller M: Rapidly cycling affective disorder: demographics, diagnosis, family history, and course. Arch Gen Psychiatry 1992; 49:126–131Crossref, Medline, Google Scholar
12. Tsai SM, Chen C, Kuo C, Lee J, Lee H, Strakowski SM: 15-year outcome of treated bipolar disorder. J Affect Disord 2001; 63:215–220Crossref, Medline, Google Scholar
13. Meeks S: Bipolar disorder in the latter half of life: symptom presentation, global functioning and age of onset. J Affect Disord 1999; 52:161–167Crossref, Medline, Google Scholar
14. Coryell W, Keller M, Endicott J, Andreasen N, Clayton P, Hirschfeld R: Bipolar II illness: course and outcome over a five-year period. Psychol Med 1989; 19:129–141Crossref, Medline, Google Scholar
15. O’Connell RA, Mayo JA, Flatow L, Cuthbertson B, O’Brien BE: Outcome of bipolar disorder on long-term treatment with lithium. Br J Psychiatry 1991; 159:123–129Crossref, Medline, Google Scholar
16. Turvey CL, Coryell WH, Solomon DA, Leon AC, Endicott J, Keller MB, Akiskal H: Long-term prognosis of bipolar I disorder. Acta Psychiatr Scand 1999; 99:110–119Crossref, Medline, Google Scholar
17. Winokur G, Coryell W, Akiskal HS, Endicott J, Keller M, Mueller T: Manic-depressive (bipolar) disorder: the course in light of a prospective ten-year follow-up of 131 patients. Acta Psychiatr Scand 1994; 89:102–110Crossref, Medline, Google Scholar
18. Strakowski SM, Keck PE Jr, McElroy SL, West SA, Sax KW, Hawkins JM, Kmetz GF, Upadhyaya VH, Tugrul KC, Bourne ML: Twelve-month outcome after a first hospitalization for affective psychosis. Arch Gen Psychiatry 1998; 55:49–55Crossref, Medline, Google Scholar
19. Johnson SL, Miller I: Negative life events and time to recovery from episodes of bipolar disorder. J Abnorm Psychol 1997; 106:449–457Crossref, Medline, Google Scholar
20. Ellicott A, Hammen C, Gitlin M, Brown G, Jamison K: Life events and the course of bipolar disorder. Am J Psychiatry 1990; 147:1194–1198Link, Google Scholar
21. Post RM, Denicoff KD, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Rush AJ, Keck PE Jr, McElroy SL, Luckenbaugh DA, Pollio C, Kupka R, Nolen WA: Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry 2003; 64:680–690Crossref, Medline, Google Scholar
22. Leverich GS, Nolen WA, Rush AJ, McElroy SL, Keck PE Jr, Denicoff KD, Suppes T, Altshuler LL, Kupka R, Kramlinger KG, Post RM: The Stanley Foundation Bipolar Treatment Outcome Network, I: longitudinal methodology. J Affect Disord 2001; 76:33–44Crossref, Google Scholar
23. McElroy SL, Altshuler LL, Suppes T, Keck PE Jr, Frye MA, Denicoff KD, Nolen WA, Kupka RW, Leverich GS, Rochussen JR, Rush AJ, Post RM: Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry 2001; 158:420–426Link, Google Scholar
24. Kupka RW, Nolen WA, Altshuler LL, Denicoff KD, Frye MA, Leverich GS, Keck PE Jr, McElroy SL, Rush AJ, Suppes T, Post RM: The Stanley Foundation Bipolar Network, 2: preliminary summary of demographics, course of illness and response to novel treatments. Br J Psychiatry Suppl 2001; 41:S177-S183Google Scholar
25. Leverich GS, McElroy SL, Suppes T, Keck PE Jr, Denicoff KD, Nolen WA, Altshuler LL, Rush AJ, Kupka R, Frye M, Autio R, Post RM: Early physical or sexual abuse and the course of bipolar illness. Biol Psychiatry 2002; 51:288–297Crossref, Medline, Google Scholar
26. Suppes T, Leverich GS, Keck PE, Nolen WA, Denicoff KD, Altshuler LL, McElroy SL, Rush AJ, Kupka R, Frye MA, Bickel M, Post RM: The Stanley Foundation Bipolar Treatment Outcome Network, II: demographics and illness characteristics of the first 261 patients. J Affect Disord 2001; 67:45–54Crossref, Medline, Google Scholar
27. Frye MA, Altshuler LL, McElroy SL, Suppes T, Keck PE, Denicoff K, Nolen WA, Kupka R, Leverich GS, Pollio C, Grunze H, Walden Post RM: Gender differences in prevalence, risk, and clinical correlates of alcoholism comorbidity in bipolar disorder. Am J Psychiatry 2003; 160:883–889Link, Google Scholar
28. Denicoff KD, Leverich GS, Nolen WA, Rush AJ, McElroy SL, Keck PE, Suppes T, Altshuler LL, Kupka R, Frye MA, Hatef J, Brotman MA, Post RM: Validation of the prospective NIMH-Life-Chart Method (NIMH-LCM-p) for longitudinal assessment of bipolar illness. Psychol Med 2000; 30:1391–1397Crossref, Medline, Google Scholar
29. Denicoff KD, Smith-Jackson EE, Disney ER, Suddath RL, Leverich GS, Post RM: Preliminary evidence of the reliability and validity of the prospective life-chart methodology (LCM-p). J Psychiatr Res 1997; 31:593–603Crossref, Medline, Google Scholar
30. Meaden PM, Daniels RE, Zajecka J: Construct validity of life chart functioning scales for use in naturalistic studies of bipolar disorder. J Psychiatr Res 2000; 34:187–192Crossref, Medline, Google Scholar
31. Hochberg Y: A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988; 75:800–802Crossref, Google Scholar
32. Strakowski SM, DelBello MP, Fleck DE, Arndt S: The impact of substance abuse on the course of bipolar disorder. Biol Psychiatry 2000; 48:477–485Crossref, Medline, Google Scholar
33. Perlick DA, Rosenheck RR, Clarkin JF, Raue P, Sirey J: Impact of family burden and patient symptom status on clinical outcome in bipolar affective disorder. J Nerv Ment Dis 2001; 189:31–37Crossref, Medline, Google Scholar
34. Keck PE Jr, McElroy SL, Strakowski SM, West SA, Sax KW, Hawkins JM, Bourne ML, Haggard P: 12-month outcome of patients with bipolar disorder following hospitalization for a manic or mixed episode. Am J Psychiatry 1998; 155:646–652Link, Google Scholar
35. Post RM, Frye MA, Denicoff KD, Leverich GS, Dunn RT, Osuch EA, Speer AM, Obrocea G, Jajodia K: Emerging trends in the treatment of rapid cycling bipolar disorder: a selected review. Bipolar Disord 2000; 2:305–315Crossref, Medline, Google Scholar
36. Sachs GS, Thase ME: Bipolar disorder therapeutics: maintenance treatment. Biol Psychiatry 2000; 48:573–581Crossref, Medline, Google Scholar
37. Strakowski SM, DelBello MP, Adler CM: Comparative efficacy and tolerability of drug treatments for bipolar disorder. CNS Drugs 2001; 15:701–718Crossref, Medline, Google Scholar



