Gender Differences in Prevalence, Risk, and Clinical Correlates of Alcoholism Comorbidity in Bipolar Disorder
Abstract
OBJECTIVE: The prevalence of lifetime alcohol abuse and/or dependence (alcoholism) in patients with bipolar disorder has been reported to be higher than in all other axis I psychiatric diagnoses. This study examined gender-specific relationships between alcoholism and bipolar illness, which have previously received little systematic study. METHOD: The prevalence of lifetime alcoholism in 267 outpatients enrolled in the Stanley Foundation Bipolar Network was evaluated by using the Structured Clinical Interview for DSM-IV. Alcoholism and its relationship to retrospectively assessed measures of the course of bipolar illness were evaluated by patient-rated and clinician-administered questionnaires. RESULTS: As in the general population, more men (49%, 57 of 116) than women with bipolar disorder (29%, 44 of 151) met the criteria for lifetime alcoholism. However, the risk of having alcoholism was greater for women with bipolar disorder (odds ratio=7.35) than for men with bipolar disorder (odds ratio=2.77), compared with the general population. Alcoholism was associated with a history of polysubstance use in women with bipolar disorder and with a family history of alcoholism in men with bipolar disorder. CONCLUSIONS: This study suggests that there are gender differences in the prevalence, risk, and clinical correlates of alcoholism in bipolar illness. Although this study is limited by the retrospective assessment of illness variables, the magnitude of these gender-specific differences is substantial and warrants further prospective study.
Alcoholism occurs among male patients in about a quarter of the cases, but it is to be regarded as the consequence of debaucheries committed in excitement, not as a cause.
—Kraepelin 1921 (1)
As originally identified in Kraepelin’s work, the common co-occurrence of bipolar disorder and alcoholism has long been recognized (2–5). More recent epidemiological data have confirmed this co-occurrence. The Epidemiologic Catchment Area (ECA) Study reported a 60.7% lifetime prevalence for substance abuse or dependence in persons with bipolar I disorder, with alcohol the most commonly abused substance (6). Further analysis of ECA data showed that persons with bipolar I disorder and bipolar II disorder had the highest lifetime prevalence of alcohol abuse or dependence (46.2% and 39.2%, respectively), followed by persons with schizophrenia (33.7%), panic disorder (28.7%), and unipolar depression (16.5%). The general population prevalence was 13.8%. In addition, patients with alcoholism (alcohol abuse and/or dependence) had an odds ratio of 6.2 for co-occurring mania; except for other drug abuse and/or dependence diagnoses, mania represented the most prevalent of all comorbid axis I diagnoses (7). Similarly, in the National Comorbidity Survey, both men and women with a lifetime diagnosis of alcohol dependence had significantly increased odds of having a co-occurring lifetime diagnosis of mania (odds ratio=12.03 and 5.3, respectively) (8). Thus, by prevalence data alone, this comorbidity or co-occurrence represents an enormous public health problem.
From the standpoint of gender, Kraepelin’s initial observation of a greater prevalence of alcoholism in men with bipolar disorder, compared with women with bipolar disorder, has been confirmed in more recent epidemiological studies (6–9) and in a community registry study of outpatients with bipolar disorder (10). However, this relationship is not unique to patients with bipolar disorder, as prevalence studies of alcoholism in the general population have reported similar findings (11, 12). For example, the ECA Study reported lifetime alcoholism prevalences of 23.8% for men and 4.6% for women (13). The current study sought to further investigate the rates and correlates of a lifetime history of alcoholism in men and women with bipolar disorder.
Method
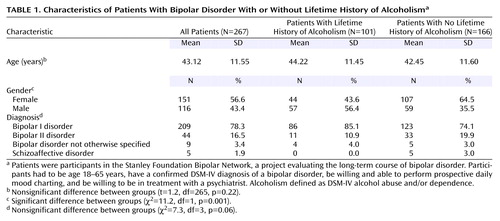
The Stanley Foundation Bipolar Network was created to evaluate the long-term course of bipolar disorder through prospective clinical research, including development of novel drugs (14). Written informed consent for participating in the Stanley Foundation Bipolar Network was obtained after the study had been fully explained to each subject. Patients with bipolar disorder were recruited from academic settings, community mental health outpatient clinics, physician referral, and local advertisement. Participants entered the Stanley Foundation Bipolar Network if they met the following inclusion criteria: 1) age 18–65 years, 2) confirmed DSM-IV diagnosis of bipolar disorder, 3) willingness and ability to perform prospective daily mood charting, and 4) willingness to be in treatment with a psychiatrist. Patients were not paid for their participation. Bipolar disorder patients with active substance use (i.e., heavy use associated with significant morbidity), as determined by the clinical judgment of the research clinician, were excluded from the study. Of the 267 patients in the study, eight (2.9%) (three women, five men) met the criteria for current alcohol abuse that was judged not to be clinically significant enough to warrant separate substance abuse treatment. No patients met the criteria for current alcohol dependence. The participating sites at the time this analysis was done (University of California Los Angeles; University of Texas Southwestern, Dallas; University of Cincinnati; National Institute of Mental Health (NIMH); and University Medical Center, Utrecht, the Netherlands) evaluated the lifetime prevalence of alcoholism in 267 outpatients with bipolar illness (209 with bipolar I disorder, 44 with bipolar II disorder, nine with bipolar disorder not otherwise specified, and five with schizoaffective disorder).
Recruitment into the Stanley Foundation Bipolar Network involved an extensive baseline evaluation (14). The Structured Clinical Interview for DSM-IV (SCID) (15) was completed to establish the diagnosis of bipolar disorder, subtype pattern, age at onset, presence or absence of lifetime alcohol abuse or dependence, and other comorbid axis I diagnoses (16). The SCID was administered by clinical research assistants who had received training locally and at the NIMH site. This training was supervised by one of the principal investigators (G.S.L.). Interrater reliability for the diagnosis of bipolar disorder was established, with an overall kappa score of 0.92 (14).
The evaluation also included completion of structured patient-rated and clinician-administered questionnaires to determine demographic (e.g., current level of occupational functioning, marital status, race, and education level) and historical illness variables (i.e., age of symptom onset, prior episodes and hospitalizations for depression and mania, history of a cycling disorder [rapid cycling, defined by DSM-IV criteria; ultrarapid cycling, defined as four or more mood episodes in a month; and ultradian cycling, defined as four or more discrete mood shifts in a week], suicide attempts, history of abuse, and family history) (17). Although alcohol abuse and/or dependence was identified by the SCID, data on the pattern and quantity of alcohol intake, current length of sobriety, and total duration of alcohol use (years) were not always available in a reliable fashion and, thus, were not analyzed for this study.
The prevalence of a lifetime history of alcoholism by diagnostic subtype and gender was analyzed with Fisher’s exact test. Odds ratios for risk of alcoholism were calculated separately for men and women by using the ECA Study general population rate of alcoholism for comparison. The ECA Study has published rates of alcoholism by age, gender, and race; therefore, we were able to generate odds ratios that were appropriately weighted for these variables. The Breslow-Day statistic was used to test for significant difference between the risk for alcoholism in women with bipolar disorder and the risk in men with bipolar disorder.
Differences in demographic and course-of-illness variables between bipolar disorder patients with a lifetime history of alcoholism and those without a lifetime history of alcoholism were examined by using Fisher’s exact test and the t test for independent samples. Hochberg’s adjustment was used for multiple comparisons (18), as the Bonferroni correction could have been too conservative. A binary logistic regression analysis was then conducted separately with the data for men and for women to assess which factors were most strongly associated with a lifetime history of alcoholism. Only those variables with significant associations before correction in the univariate analyses were included in the regression analyses. The categorical variables were calculated as the percentage of subjects who reported these historical variables. The corresponding chi-square values, odds ratios, and 95% confidence intervals (CIs) are reported.
Results
Prevalence and Risk of Comorbid Alcoholism
As presented in Table 1, 101 (38%) of 267 patients met the criteria for a lifetime history of alcoholism and 166 (62%) did not. No significant difference in age was found between those with and without a lifetime history of alcoholism (t=1.2, df=265, p=0.22). Of all the bipolar subtype groups, patients with bipolar I disorder had the highest rate of alcoholism, but the difference was not significant (χ2=7.3, df=3, p=0.06). No significant differences in current level of occupational functioning, marital status, race, and education level were found between those with and without a lifetime history of alcoholism.
Fewer women with bipolar disorder (29.1%, 44 of 151) had a lifetime history of alcoholism, compared to men with bipolar disorder (49.1%, 57 of 116) (p=0.001, Fisher’s exact test). However, women with bipolar disorder had a much greater likelihood of alcoholism, compared with the general female population (13) (odds ratio=7.35, 95% CI=3.32–16.26, χ2=1293.1, df=1, p<0.0001), than did men with bipolar disorder, compared to the general male population (13) (odds ratio=2.77, 95% CI=1.59–4.81, χ2=37.7, df=1, p<0.0001). The difference in the odds ratios was statistically significant (Breslow Day χ2=4.0, df=1, p<0.05) (Figure 1).
Clinical Characteristics Associated With Comorbid Alcoholism
Data for the women and men with bipolar disorder were analyzed separately to assess for gender differences in clinical characteristics associated with alcoholism. The univariate analyses are presented in Table 2. The characteristics that were more prevalent in women with bipolar disorder and a lifetime history of alcoholism than in women with bipolar disorder without alcoholism were polysubstance use, family history of alcoholism, history of verbal abuse, four or more prior episodes of depression, and social phobia. These variables were subsequently evaluated by binary logistic regression. In the logistic regression analysis, the results for polysubstance use (odds ratio=9.09, 95% CI=3.26–25.37, Wald χ2=17.8, df=1, p<0.001) remained significant and the results for social phobia (odds ratio=2.95, 95% CI=0.91–9.54, Wald χ2=3.3, df=1, p=0.07) approached significance.
The clinical characteristics that were more prevalent in men with comorbid bipolar disorder and a lifetime history of alcoholism than in men with bipolar disorder without alcoholism were family history of alcoholism, family history of drug abuse and/or dependence, family history of bipolar disorder, history of physical abuse, and past suicide attempt. In the subsequent binary logistic regression, the results for family history of alcoholism (odds ratio=8.76, 95% CI= 2.36–32.54, Wald χ2=10.5, df=1, p<0.001) remained significant, and the results for family history of bipolar disorder (odds ratio=2.71, 95% CI=0.98–7.5, Wald χ2=3.7, df=1, p=0.054) and history of physical abuse (odds ratio=3.41, 95% CI=0.99–11.79, Wald χ2=3.8, df=1, p=0.053) approached significance.
Discussion
This study supports previous observations of a high rate of alcoholism in bipolar disorder. The 38% prevalence of a lifetime history of alcoholism in this study is consistent with previous reports on clinical subjects (2–5) but is most likely an underestimate, for several reasons. First, this study was not based on data from a community sample but rather from patients with bipolar disorder who were seeking treatment in subspecialty clinics, and patients with comorbid alcoholism may be less likely to be in treatment with an added research focus. Furthermore, patients with bipolar disorder and active substance abuse or dependence that required separate treatment (i.e., heavy substance use with significant morbidity) were excluded. Although gender differences in the likelihood of receiving treatment were not systematically analyzed in our study, previous research has found that men were more likely than women to receive treatment for alcoholism (19). Previous studies of hospitalized patients with mania have clearly implicated alcoholism as being significantly related to treatment noncompliance (20, 21). Thus, alcoholism can be viewed as a contributory factor to illness severity, lack of effort to seek treatment for bipolar disorder, referral to a substance abuse program (particularly for men), and/or research ineligibility.
Despite these limitations, our data on lifetime prevalence of alcoholism and bipolar subtype are remarkably consistent with those of the ECA Study and a nontreatment voluntary community registry study of outpatients with bipolar disorder. All three studies reported a higher prevalence of alcoholism in patients with bipolar I disorder than in those with bipolar II disorder (ECA Study: 46.2% and 39.2%, respectively; outpatient registry study: 49.3% and 38.9%, respectively; our study: 41.1% and 25.0%, respectively). Although the patients in this study did not constitute an outpatient epidemiological sample, and, furthermore, this study excluded patients with bipolar disorder who required treatment for substance dependence, the underestimate appears to not have affected significantly the prevalence difference between the bipolar I and bipolar II subtypes.
Although women with bipolar disorder had a lower prevalence of lifetime alcoholism than men with bipolar disorder, women with bipolar disorder appear to be particularly vulnerable to alcoholism, compared to the general female population (odds ratio=7.35). This higher odds ratio in women is consistent with a previous estimate reported by Hendrick et al. (22). In this smaller study, 20% of the women and 48% of the men with bipolar disorder met the criteria for alcohol use disorder. Although the findings were not reported as odds ratios weighted for the particular group of subjects, this study used the same general population estimate (13) and reported similar findings of a greater risk for alcoholism in women with bipolar disorder than in men with bipolar disorder (fourfold versus twofold, respectively).
These data do not allow conclusions about whether and why men and women with bipolar disorder change alcohol intake patterns over the course of their illness. The relationship between mood states and alcohol intake patterns has not been well studied. Several, but not all, studies done more than 25 years ago have suggested that excessive drinking predominated in the manic phase (23–26). However, these uncontrolled observations overrepresented men or otherwise did not specify gender. Although hospitalization for mania characterizes one of the most severe forms of bipolar disorder, female-predominant patterns of bipolar disorder (i.e., rapid cycling, dysphoric mania, bipolar II disorder) (27, 28) have not been evaluated for their relationship to alcohol use.
Despite a lower prevalence of alcoholism in women than in men with bipolar disorder, some data have suggested serious consequences of alcohol use in women. The National Longitudinal Alcohol Epidemiology Survey (29) found that women had a higher rate of binge drinking and acute intoxication than men and were less likely to receive treatment for alcoholism (19). The latter observation may be related to physician bias (30). Animal data have suggested gender differences in alcohol-induced adrenal axis activation; female rats and castrated male rats implanted with an estradiol patch had greater alcohol-induced increases in ACTH and cortisol (a neuroendocrine axis hormone implicated in depression) than noncastrated males (31). The influence of gonadal steroids and their role in female-predominant subtypes of bipolar illness (such as rapid cycling and dysphoric mania) were extensively reviewed by Leibenluft in 1996 (32); however, the clinical implications of these steroids in the context of alcoholism in patients with bipolar disorder are not known. Other medical consequences of gender differences in alcoholism were comprehensively reviewed by Brady and Randall in 1999 (33).
Men and women with bipolar disorder and alcoholism appear to have different clinical patterns of bipolar illness. There appears to be greater “genetic loading” for men with bipolar disorder and alcoholism, who have a more robust family history of bipolar disorder, alcoholism, and drug abuse and/or dependence, compared to men with bipolar disorder without alcoholism. An area of investigation that continues to be important is the extent to which bipolar and alcohol use disorders are genetically separate but co-occurring with some degree of shared risk, genetically distinct but overlapping, or phenotypically linked (3–5, 34).
Women with bipolar disorder and alcoholism, on the other hand, did not appear to have as great of a family loading, compared to men with bipolar disorder and alcoholism. Rather, alcoholism in these women was associated with comorbidity (depressive episodes and social phobia). Although this association only approached significance, this pattern is potentially clinically relevant. The association of alcoholism and depressive morbidity in women is not specific to bipolar disorder but has also been described in unipolar depression and in primary alcoholism (35, 36). In a previous prospective study assessing risk of heavy alcohol use (five drinks at one time), women with a history of depression had a 2.6-fold increased relative risk for heavy drinking compared to women without such history (35). In addition, recent data have suggested that patients with social phobia complicated by alcohol abuse (past or current) had a much higher rate of bipolar II disorder (a bipolar subtype more common in women) than patients with social phobia who did not have comorbid alcohol abuse (37). Furthermore, patients with subclinical social phobia (fear of a specific social situation, but not to a degree resulting in avoidance or impairment) may be at greatest risk for developing alcohol use disorders (38). Whatever the causal mechanisms of these associations, our data suggest a greater vulnerability to alcoholism in women with bipolar disorder. Further evaluation of the interface between depressive or social phobic morbidity and alcohol use as an attempt to self-medicate is warranted.
This study had several limitations. The main limitation was the retrospective ascertainment of course-of-illness measures and the potential bias introduced by patients’ self-reports. The data were largely based on patient information; prospective validation of the study results is needed. Certainly, Berkson’s bias (39) may contribute to inaccurate or excessive endorsement. However, the main findings of the study were gender differences in alcoholism history, for which men’s and women’s self-reports would be equally likely to be affected by potential biases.
Despite these limitations, to our knowledge, this study included the largest cohort of persons with bipolar disorder who have had a retrospective assessment evaluating the relationship between alcoholism and course of bipolar illness. In addition, the study subjects were drawn from diverse geographic areas, including four American sites and one European site. The number of subjects was large enough to identify clinical factors associated with alcoholism in bipolar disorder and to evaluate these factors by gender.
In conclusion, the study findings suggest important gender differences in prevalence (higher in men than women), risk of alcoholism compared to the general population (greater in women than in men), and clinical correlates associated with alcoholism in bipolar disorder. The magnitude of the gender-specific differences is substantial. Because women with bipolar disorder are at an unusually high risk for alcoholism, mental health professionals should carefully assess alcohol use on an ongoing basis in this group, particularly in the context of bipolar depressive-predominant illness or comorbid social phobia.
Further prospective examination of the relationship between gender, bipolar depressive morbidity, treatment response, and alcoholism may be an important step in understanding the neurobiology of the two disorders. It has been reported that a lag time of up to 10 years exists between patients’ self-report of symptom onset and the first treatment intervention for bipolar disorder (14, 40). Early recognition of bipolar illness may not only allow early appropriate treatment of the mood components of the illness but may also lead to a decreased vulnerability to alcoholism. Education regarding this risk should be incorporated into standard treatment programs. Future studies should assess the effect of acute and prophylactic mood stabilization on alcohol use and relapse prevention.
 |
 |
Presented in part at the 2nd European Stanley Foundation Conference on Bipolar Disorder, Amsterdam, Sept. 21, 2000. Received July 20, 2001; revision received April 5, 2002; accepted Oct. 14, 2002. From the Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, School of Medicine; the Department of Biological Psychiatry, University of Cincinnati School of Medicine, Cincinnati; the Department of Psychiatry, University of Texas Southwestern, Dallas; the Biological Psychiatry Branch, NIMH, Bethesda, Md.; Altrecht Institute for Mental Health Care, University Medical Center, Utrecht, the Netherlands; Psychiatrische Klinik de Ludwig-Maximilian Universität, Munich; and Universitatsklinik für Psychiatrie der Universität Freiburg, Freiburg, Germany. Address reprint requests to Dr. Frye, UCLA Bipolar Research Program, 300 UCLA Medical Plaza, Suite 1544, Los Angeles, CA 90095; [email protected] (e-mail). Supported by the Theodore and Vada Stanley Foundation.
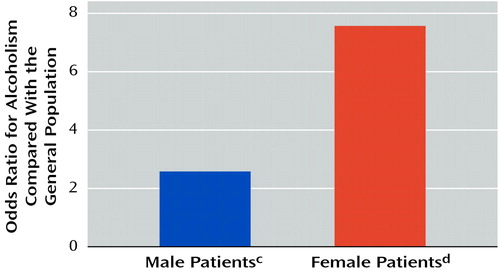
Figure 1. Odds Ratios for Alcoholism in Female and Male Patients With Bipolar Disordera, Compared With the General Populationb
aPatients were participants in the Stanley Foundation Bipolar Network, a project evaluating the long-term course of bipolar disorder. Participants had to be age 18–65 years, have a confirmed DSM-IV diagnosis of a bipolar disorder, be willing and able to perform prospective daily mood charting, and be willing to be in treatment with a psychiatrist. Alcoholism was defined as DSM-IV alcohol abuse and/or dependence.
bGeneral population estimates of alcoholism prevalence, based on results of the Epidemiologic Catchment Area Study (13), weighted by age and race and split by gender to match the group of bipolar disorder patients in the study.
cPrevalence of alcoholism=49.1% (57 of 116 subjects) (odds ratio=2.77, 95% CI=1.59–4.81, χ2=37.7, df=1, p<0.0001, odds ratio significantly different from odds ratio for female patients [Breslow Day χ2=4.0, df=1, p<0.05]).
dPrevalence of alcoholism=29.1% (44 of 151 subjects) (odds ratio=7.35, 95% CI=3.32–16.26, χ2=1293.1, df=1, p<0.0001, odds ratio significantly different from the odds ratio for male patients [Breslow Day χ2=4.0, df=1, p<0.05]).
1. Kraepelin E: Manic-Depressive Insanity and Paranoia (1921). Translated by Barclay RM. Salem, NH, Ayer, 1976Google Scholar
2. Brady KT, Lydiard RB: Bipolar affective disorder and substance abuse. J Clin Psychopharmacol 1992; 12:17S-22SCrossref, Medline, Google Scholar
3. Tohen M, Greenfield SF, Weiss R, Zarate CA, Vagge LM: The effect of comorbid substance use disorders on the course of bipolar disorder: a review. Harv Rev Psychiatry 1988; 6:133-141Crossref, Google Scholar
4. Strakowski SM, DelBello MP, Fleck DE, Arndt S: The impact of substance abuse on the course of bipolar disorder. Biol Psychiatry 2000; 48:477-485Crossref, Medline, Google Scholar
5. Salloum IM, Thase ME: Impact of substance abuse on the course and treatment of bipolar disorder. Bipolar Disord 2000; 2:269-280Crossref, Medline, Google Scholar
6. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK: Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) Study. JAMA 1990; 264:2511-2518Crossref, Medline, Google Scholar
7. Helzer JE, Pryzbeck TR: The co-occurrence of alcoholism with other psychiatric disorders in the general population and its impact on treatment. J Stud Alcohol 1988; 49:219-224Crossref, Medline, Google Scholar
8. Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC: Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry 1997; 54:313-321Crossref, Medline, Google Scholar
9. Ravelli A, Bijl RV, van Zessen G: Comorbidity of psychiatric disorders in the Netherlands: results of the Netherlands Mental Health Survey and Incidence Study (NEMESIS). Tijdschrift voor Psychiatrie 1988; 40:531-544Google Scholar
10. Chengappa KNR, Levine J, Gershon S, Kupfer DJ: Lifetime prevalence of substance or alcohol abuse and dependence among subjects with bipolar I and II disorders in a voluntary registry. Bipolar Disord 2000; 1:191-195Crossref, Google Scholar
11. Dawson DA, Archer L: Gender differences in alcohol consumption: effects of measurement. Br J Addict 1992; 87:119-123Crossref, Medline, Google Scholar
12. Grant BF: Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: results of the National Longitudinal Alcohol Epidemiological Survey. J Stud Alcohol 1997; 58:464-473Crossref, Medline, Google Scholar
13. Robins LN, Regier DA (eds): Psychiatric Disorders in America: The Epidemiological Catchment Area Study. New York, Free Press, 1991, p 84Google Scholar
14. Leverich GS, Nolen WA, Rush AJ, McElroy SL, Keck PE, Denicoff KD, Suppes T, Altshuler LL, Kupka R, Kramlinger KG, Post RM: The Stanley Foundation Bipolar Treatment Outcome Network, I: longitudinal methodology. J Affect Disord 2001; 67:33-44Crossref, Medline, Google Scholar
15. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
16. McElroy SL, Altshuler LL, Suppes T, Keck PE Jr, Frye MA, Denicoff KD, Nolen WA, Kupka RW, Leverich GS, Rochussen JR, Rush AJ, Post RM: Axis I psychiatric comorbidity and its relationship to historical illness variables in 288 patients with bipolar disorder. Am J Psychiatry 2001; 158:420-426Link, Google Scholar
17. Suppes T, Leverich GS, Keck PE, Nolen WA, Denicoff KD, Altshuler LL, McElroy SL, Rush AJ, Kupka R, Frye MA, Bickel M, Post RM: The Stanley Foundation Bipolar Treatment Outcome Network, II: demographic and illness characteristics of the first 261 patients. J Affect Disord 2001, 67:45-59Google Scholar
18. Hochberg Y: A sharper Bonferroni procedure for multiple tests of significance. Biometrika 1988; 74:800-802Crossref, Google Scholar
19. Dawson DA: Gender differences in the probability of alcohol treatment. J Subst Abuse 1996; 8:211-225Crossref, Medline, Google Scholar
20. Aagaard J, Vestergaard P: Predictors of outcome in prophylactic lithium treatment: a 2-year prospective study. J Affect Disorder 1990; 18:259-266Crossref, Medline, Google Scholar
21. Strakowski SM, Sax KW, McElroy SL, Keck PE, Hawkins JM: Course of psychiatric and substance abuse syndromes co-occurring with bipolar disorder after a first psychiatric hospitalization. J Clin Psychiatry 1998; 59:465-471Crossref, Medline, Google Scholar
22. Hendrick V, Altshuler LL, Gitlin MJ, Delrahim S, Hammen C: Gender and bipolar illness. J Clin Psychiatry 2000; 61:393-396Crossref, Medline, Google Scholar
23. Mayfield DG, Coleman LL: Alcohol use and affective disorder. Dis Nerv Syst 1968; 29:467-474Medline, Google Scholar
24. Reich LH, Davies RK, Himmelhoch JM: Excessive alcohol use in manic-depressive illness. Am J Psychiatry 1974; 131:83-86Link, Google Scholar
25. Freed EX: Alcohol abuse by manic patients. Psychol Rep 1969; 25:280Crossref, Medline, Google Scholar
26. Cassidy WL, Flanagan NB, Spellman M, Cohen ME: Clinical observations in manic-depressive disease: a study of 100 manic-depressive patients and 50 medically sick controls. JAMA 1957; 164:1535-1546Crossref, Medline, Google Scholar
27. Goodwin FK, Jamison KR: Manic-Depressive Illness. New York, Oxford University Press, 1990Google Scholar
28. McElroy SL, Keck PE Jr, Pope HG Jr, Hudson JI, Faedda GL, Swann AC: Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. Am J Psychiatry 1992; 149:1633-1644Link, Google Scholar
29. Dawson DA, Grant BF, Chou PS: Gender differences in alcohol intake, in Stress, Gender, and Alcohol Seeking Behavior. Edited by Hunt WA, Zakhari S. Bethesda, Md, National Institute on Alcohol and Alcohol abuse, 1995, pp 125-137Google Scholar
30. Dawson NV, Dadheech G, Speroff T, Smith RL, Schubert DSP: The effect of patient gender on the prevalence and recognition of alcoholism on a general medicine inpatient service. J Gen Intern Med 1992; 7:38-45Crossref, Medline, Google Scholar
31. Ogilvie KM, Rivier C: Gender difference in hypothalamic-pituitary-adrenal axis response to alcohol in rat: activation role of gonadal steroids. Brain Res 1997; 22:19-28Crossref, Google Scholar
32. Leibenluft E: Women with bipolar illness: clinical and research issues. Am J Psychiatry 1996; 153:163-173Link, Google Scholar
33. Brady KT, Randall CL: Gender differences in substance use disorders. Addict Disord 1999; 22:241-252Google Scholar
34. Winokur G, Turvey C, Akiskal H, Coryell W, Solomon D, Leon A, Mueller T, Endicott J, Maser J, Keller M: Alcoholism and drug abuse in three groups—bipolar I, unipolars and acquaintances. J Affect Disord 1998; 50:81-89Crossref, Medline, Google Scholar
35. Dixit AR, Crum RM: Prospective study of depression and the risk of heavy alcohol use in women. Am J Psychiatry 2000; 157:751-758Link, Google Scholar
36. Spak L, Spak F, Allebeck P: Alcoholism and depression in a Swedish female population: co-morbidity and risk factors. Acta Psychiatr Scand 2000; 102:44-51Crossref, Medline, Google Scholar
37. Perugi G, Frare F, Madaro D, Maremmani I, Akiskal H: Alcohol abuse in social phobic patients: is there a bipolar connection? J Affect Disord 2002; 68:33-39Crossref, Medline, Google Scholar
38. Crum RM, Pratt LA: Risk of heavy drinking and alcohol use disorders in social phobia: a prospective analysis. Am J Psychiatry 2001; 158:1693-1700Link, Google Scholar
39. Berkson J: Limitations of the application of fourfold table analysis to hospital data. Biometric Bulletin 1946; 2:47-53Crossref, Medline, Google Scholar
40. Lish JD, Dime-Meenan S, Whybrow P, Price RA, Hirschfeld RM: The National Depressive and Manic-Depressive Association (DMDA) survey of bipolar members. J Affect Disord 1994; 31:281-294Crossref, Medline, Google Scholar



