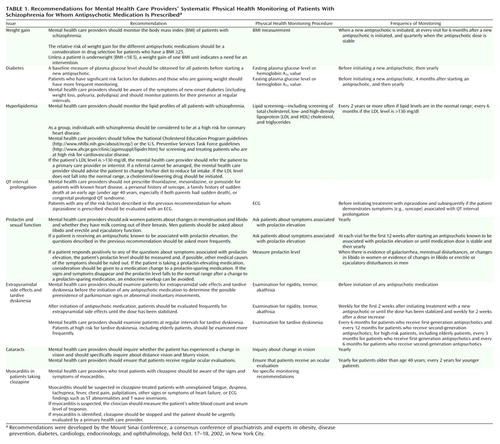
Physical Health Monitoring of Patients With Schizophrenia
Abstract
OBJECTIVE: Schizophrenia is associated with several chronic physical illnesses and a shorter life expectancy, compared with life expectancy in the general population. One approach to improving the health of patients with schizophrenia is to improve the monitoring of physical health that occurs in psychiatric settings. The authors discuss a consensus panel’s recommendations for improving the physical health monitoring of patients with schizophrenia who are treated in outpatient settings. METHOD: A consensus meeting including psychiatric and other medical experts assembled on October 17–18, 2002, to evaluate the existing literature and to develop recommendations for physical health monitoring of patients with schizophrenia. Conference participants reviewed the literature in the following areas: 1) weight gain and obesity; 2) diabetes; 3) hyperlipidemia; 4) prolongation of the QT interval on the ECG; 5) prolactin elevation and related sexual side effects; 6) extrapyramidal side effects, akathisia, and tardive dyskinesia; 7) cataracts; and 8) myocarditis. Experts for each topic area formulated monitoring recommendations that were discussed by all of the participants until a consensus was reached. RESULTS: Consensus recommendations included regular monitoring of body mass index, plasma glucose level, lipid profiles, and signs of prolactin elevation or sexual dysfunction. Information from monitoring should guide the selection of antipsychotic agents. Specific recommendations were made for cardiac monitoring of patients who receive medications associated with QT interval prolongation, including thioridazine, mesoridazine, and ziprasidone, and for monitoring for signs of myocarditis in patients treated with clozapine. Patients who receive both first- and second-generation antipsychotic medications should be examined for extrapyramidal symptoms and tardive dyskinesia. Patients with schizophrenia should receive regular visual examinations. CONCLUSIONS: The conference participants recommended that mental health care providers perform physical health monitoring that typically occurs in primary care settings for their patients who do not receive physical health monitoring in those settings. This change in usual practice is recommended on the basis of the conference participants’ belief that this additional monitoring will result in the earlier detection of common, serious risk factors that could, without detection and intervention, contribute to impaired health of patients with schizophrenia.
Individuals with schizophrenia have a 20% shorter life expectancy than the population at large (1) and a greater vulnerability to several illnesses, including diabetes, coronary heart disease, hypertension, and emphysema. One set of explanations for these vulnerabilities points to the lifestyles of people with serious mental illnesses, which are often associated with poor dietary habits, obesity, high rates of smoking, and the use of alcohol and street drugs. Moreover, as described later in this article, some antipsychotic medications used to treat schizophrenia have been associated with weight gain, the onset of diabetes, increases in levels of plasma lipids, and abnormal findings on ECGs. In addition to their potential for exacerbating the physical health problems that are common in schizophrenia, antipsychotics have been associated with other side effects that may affect health, including prolactin elevation, cataract formation, movement disorders, and sexual dysfunction. Because of the heightened health risks associated with schizophrenia and the medications used in its treatment, physical health monitoring as a means of health promotion is especially important in this patient population.
The Mount Sinai Conference, held October 17–18, 2002, at the Mount Sinai School of Medicine in New York City, was organized by individuals who shared the belief that the health needs of people with schizophrenia who take antipsychotic medications typically are not adequately addressed by clinicians in specialty mental health programs or in primary care settings. The goal of the conference was to develop recommendations for the systematic health monitoring of individuals with schizophrenia for whom antipsychotic medication is prescribed. The conference organizers—Susan M. Essock, Ph.D., Alexander L. Miller, M.D., and Stephen R. Marder, M.D.—believed that a major obstacle to improving the quality of physical health monitoring for patients with schizophrenia is the lack of a consensus regarding which health parameters should be monitored and when they should be monitored. The organizers also believed that the work product from the conference should be a set of recommendations that could be implemented in nearly any public psychiatry setting. For this reason, information regarding the cost of health care interventions was considered in developing the consensus recommendations. (For example, among the diagnostic tests considered in these recommendations, an ECG has an estimated cost of $23; a slit-lamp eye examination, $23; tests for plasma prolactin level, $27; glucose level, $5; hemoglobin A1c level, $13; triglyceride level, $8; total cholesterol level, $6; and high-density lipoprotein (HDL) cholesterol level, $11.)
The conference participants developed recommendations for monitoring the physical health of patients who take antipsychotic medication and focused on interventions that could be initiated in routine psychiatric settings. Interventions that would ordinarily involve consultation with another medical specialist were not considered nor was the evaluation of comorbidities, including smoking, street drug abuse, and alcohol abuse or dependence, which are major contributors to the health risks of this population. We did not consider how recommendations for monitoring might differ for children. Rather, the focus was on physical health monitoring that should occur in the course of treating an adult with schizophrenia who takes an antipsychotic medication and on whether and how such monitoring should vary as a function of the particular antipsychotics that are prescribed.
The quality of available evidence for the association of specific antipsychotics with particular side effects varied considerably. We reviewed the literature on each side effect and grouped studies by the quality of evidence they provided. Clear evidence from multiple randomized, controlled trials was considered level-1 evidence; data from cohort studies, outcomes research, or low-quality randomized, controlled studies were considered level-2 evidence; and data from case-control studies were considered level-3 evidence.
The conference had the following format. For each side effect, one of the conference participants conducted a literature review before the meeting and summarized this review at the conference. One or more experts in the topic area under consideration commented on the evidence and formulated monitoring recommendations based on the evidence. The recommendations were then discussed by all of the participants until a consensus was reached. A previous consensus conference focused on major components of treatment algorithms for schizophrenia, including side effect monitoring, but stopped short of providing specific recommendations for which physical conditions to monitor and how often they should be monitored (2). The current conference differed substantially in key respects. The first conference produced recommendations for side effect monitoring, whereas this conference focused more broadly on health monitoring, with an emphasis on aspects of health that the literature suggests may be negatively affected by various antipsychotic medications. The first conference relied on the opinions of a group of schizophrenia experts. The current conference developed a consensus through discussions that included experts in obesity, disease prevention, diabetes, cardiology, endocrinology, and ophthalmology.
The conference was supported by several sponsors, including the Department of Psychiatry of Mount Sinai School of Medicine; the University of Texas Health Science Center at San Antonio; the Mental Illness Research, Education, and Clinical Centers of the U.S. Department of Veterans Affairs (VA) Veterans’ Integrated Service Networks 3 and 22; the VA Mental Health Quality Enhancement Research Initiative; and the VA National Center for Health Promotion and Disease Prevention. The organizers specifically excluded support from the pharmaceutical industry, and industry employees were not included in the conference. In addition, all session participants disclosed potential conflicts of interest at the time of the conference. (The disclosures are reported at the end of this article.)
Weight Gain and Obesity
Individuals with schizophrenia are more likely than members of the general population to be overweight or obese. Moreover, some antipsychotics are associated with weight gain.
Background
Obesity can have serious effects on health and life expectancy through a number of disease processes, including hypertension, coronary artery disease, osteoarthritis, type II diabetes mellitus, and stroke. Individuals with schizophrenia are more likely to be overweight or obese than the population at large (3, 4). Using a body mass index (BMI) of 27 or higher as a definition of obesity, Allison et al. (3) found that 42% of a group of individuals with schizophrenia, compared to 27% of the general population, met this criterion. This difference was largely due to the high proportion of women with schizophrenia who were obese. Obesity has been particularly severe for young women with schizophrenia. Combined with smoking, which has a high prevalence in patients with schizophrenia, obesity further increases patients’ risk for cardiovascular morbidity and mortality. In addition, excessive weight and obesity can have important effects on an individual’s adjustment in the community, adherence to prescribed medication, ability to participate in rehabilitation efforts, and self-image.
Previous reviews have documented that treatment with first- and second-generation antipsychotics can contribute to weight gain (5–7). Further, the reviews indicate marked differences among the antipsychotics in their relationship with obesity. A meta-analysis by Allison and Casey (7) provided an estimate of mean weight gain in patients who received standard doses of antipsychotics over a 10-week period. The mean increases were 4.45 kg with clozapine, 4.15 kg with olanzapine, 2.92 kg with sertindole, 2.10 kg with risperidone, and 0.04 kg with ziprasidone. Several randomized, controlled trials provided level-1 evidence for differences among the antipsychotics in their weight gain liabilities (8–12).
The conference participants agreed that there was sufficient evidence to suggest that weight should be monitored for all individuals with schizophrenia. They also agreed that BMI, computed by using the formula, kg/m2, or by using a BMI table, is the most widely accepted monitoring standard for determining the need for intervention. (A table for computing BMI is available at http://www.niddk.nih.gov/health/nutrit/pubs/statobes.htm#other.) Following World Health Organization and U.S. National Institutes of Health recommendations, a person with a BMI of 25.0 to 29.9 is considered overweight and one with a BMI of 30 or higher is considered obese; a person with a BMI of 18.5 to 24.9 is considered of normal weight (13, 14). Individuals of Native American or Asian descent may be overweight at lower BMIs.
The patient’s waist size also is a useful risk indicator. A waist size of 35 inches or more for women and 40 inches or more for men is associated with increased health risks, including increased risks of high blood pressure, type II diabetes, dyslipidemia, and metabolic syndrome (15). For women, having a waist size of 35 inches or more was associated with these risk factors regardless of their BMI category (normal, overweight, or obese). For men, having a high-risk weight measurement incurred somewhat less health risk if their BMI was in the normal range (15).
Clinicians should focus on preventing initial weight gain and obesity, because subsequent weight loss is very difficult to achieve and existing interventions to promote weight loss are often ineffective. Clinicians should sensitize patients and their caregivers to the health risks associated with excess weight and should encourage patients to self-monitor their weight. A family history of obesity or diabetes should further raise the provider’s and patient’s awareness of the potential adverse health risks of weight gain.
The conference participants agreed that the patient’s BMI should be recorded before medication initiation or change and at every visit for the first 6 months after medication initiation or change. When the patient’s weight stabilizes, the patient should be weighed (and the BMI recorded) at least quarterly and more often if the patient is overweight. Patients who are seen at intervals of more than 1 month should be instructed to self-monitor and to notify the prescribing physician if they gain more than the number of pounds corresponding to an increase in one BMI unit. A gain of one BMI unit in a normal-weight or overweight patient should lead the clinician to consider an intervention. Interventions may include nutritional counseling (for both the patient and caregiver or food preparer), initiation of a personal exercise program, use of medications that promote weight loss, and/or a change of antipsychotic medication to one associated with less weight gain (16, 17). More extensive behavioral analysis with appropriate intervention may be necessary to address underlying issues regarding weight gain.
The conference participants had different opinions about recommendations for or against certain antipsychotics for patients who are vulnerable to weight gain. There was agreement that the data supported a continuum of weight gain liability among the second-generation antipsychotics. Ziprasidone is associated with minimal risk, risperidone with medium risk, and olanzapine and clozapine with the greatest risk. Data on quetiapine have been variable but suggest that its weight gain liability is likely to be similar to that of risperidone (18).
Quality of Evidence
Quality of evidence for differential effects of specific antipsychotics on weight: level 1.
Recommendations
| 1. | Mental health providers should monitor and chart the BMI of every patient with schizophrenia, regardless of the antipsychotic medication prescribed.
| ||||||||||||||||||||||||||||
| 2. | The relative risk of weight gain for the different antipsychotic medications should be a consideration in drug selection for patients who have a BMI of 25 or higher. | ||||||||||||||||||||||||||||
| 3. | Unless a patient is underweight (BMI <18.5), a weight gain of one BMI unit indicates a need for an intervention. Mental health providers should also initiate an intervention if the patient’s waist circumference is 35 inches or greater for a woman and 40 inches or greater for a man. | ||||||||||||||||||||||||||||
| 4. | Interventions may include closer monitoring of weight, engagement in a weight management program, use of an adjunctive treatment to reduce weight, or changes in a patient’s antipsychotic medication. If a patient is taking a medication that is associated with a higher risk for weight gain, the mental health care provider should consider switching the medication to one with less weight gain liability. | ||||||||||||||||||||||||||||
Diabetes
Previous studies have provided evidence that patients who are treated with certain antipsychotic medications are more likely to develop type II diabetes mellitus.
Background
Patients with schizophrenia may be at a higher risk for developing diabetes than the population at large (19). The concern is related to the development of type II diabetes mellitus, which represents about 90% of the cases of diabetes. The high prevalence of diabetes among people with schizophrenia could be related to the high prevalence of obesity, as 90% of individuals with type II diabetes are obese (20). Several reports have raised concerns that some of the second-generation antipsychotics may further increase the risk of type II diabetes. Most of the data in this area consist of case reports (reviewed in references 20 and 21). Clozapine and olanzapine are the agents most commonly associated with diabetes. One study followed patients who were taking clozapine for 5 years and found that 36.6% eventually received a diagnosis of type II diabetes (22). Plasma glucose levels were measured in a 14-week randomized, double-blind trial that compared the effects of clozapine, haloperidol, olanzapine, and risperidone (23). Significant increases in glucose levels were found with clozapine and haloperidol at 8 weeks (but not at 14 weeks) and with olanzapine at 14 weeks (but not at 8 weeks). A study that used a large VA database found significantly higher risks for a diagnosis of diabetes among patients who were taking clozapine (odds ratio=1.25), olanzapine (odds ratio=1.11), and quetiapine (odds ratio=1.31), but not risperidone (odds ratio=1.05), compared with patients who were taking first-generation antipsychotics (21). The effect was strongest for patients who were younger than age 40 years. For younger individuals, the odds ratios were higher and the increased risk was statistically significant for all four of the second-generation agents studied.
Using the United Kingdom General Practice Research Database, Koro and coworkers (24) found 451 cases of diabetes among 19,637 individuals with schizophrenia. Patients who were taking olanzapine had a significantly higher risk of diabetes, compared with patients who were nonusers of antipsychotics (odds ratio=5.8, 95% confidence interval [CI]=2.0–16.7) and compared with patients who were taking first-generation antipsychotics (odds ratio=4.2, 95% CI=1.5–12.2). Risperidone was not associated with a significantly increased risk of diabetes. For patients taking risperidone, compared to nonusers, the odds ratio was 2.2 (95% CI=0.9–5.2); for patients taking risperidone, compared to patients taking first-generation antipsychotics, the odds ratio was 1.6 (95% CI=0.7–3.8). Patients who were taking clozapine were not included in this study.
Findings of an association between clozapine and diabetes are contradicted by a study that used data from drug benefit programs in New Jersey (25). In this study, patients who received clozapine did not have a higher risk of diabetes (odds ratio=0.98), compared with patients who did not receive that medication, whereas chlorpromazine (odds ratio=1.31) and perphenazine (odds ratio=1.34) were associated with a significantly higher risk. Also, a study sponsored by Eli Lilly and Company in which the effects of olanzapine and haloperidol were compared failed to find a difference between the two agents in the proportion of patients with abnormal elevations in nonfasting plasma glucose levels (26).
A link between diabetes and certain agents is also supported by the finding that both olanzapine and clozapine may induce insulin resistance (27, 28). However, the conference participants agreed that increases in the risk for diabetes could also be explained by their liabilities for causing weight gain.
The conference participants acknowledged that a considerable amount of data support a link between certain second-generation antipsychotics and the risk for type II diabetes. However, because none of the studies provided level-1 evidence, identifying any of the agents as causally associated with an increased risk for diabetes would be premature. Cross-sectional studies that used large databases from the VA and the United Kingdom were informative, although the clinical diagnosis of diabetes was not supported by a change in laboratory test values after the administration of antipsychotic medications.
Individuals with schizophrenia should be evaluated for undiagnosed diabetes by using the criteria recommended by the American Diabetes Association (29). Factors that indicate a high risk for undiagnosed diabetes include a BMI greater than 25, a first-degree relative with diabetes, habitual physical inactivity, being a member of a high-risk ethnic population (African American, Hispanic American, Native American, Asian American, Pacific Islander), having delivered a baby heavier than 9 lb or having had gestational diabetes, hypertension, an HDL cholesterol level ≤35 mg/dl and/or a triglyceride level ≥250 mg/dl, history of abnormal findings on a glucose tolerance test or fasting plasma glucose test, and history of vascular disease. The consensus was that the intensity of an ongoing monitoring strategy should depend both on baseline risk factors (such as weight gain) and clinical course (for example, development of symptoms of diabetes such as weight loss, polyuria, and polydipsia). Psychiatrists should document the patient’s family history of diabetes, weight, race, history of gestational diabetes, blood pressure, and lipid profile.
The American Diabetes Association recommends a fasting plasma glucose test for screening patients for undiagnosed diabetes or impaired glucose tolerance; a random plasma glucose test can be used to diagnose diabetes, especially in the presence of symptoms. Although an oral glucose tolerance test is considered the “gold standard” for epidemiological studies, the inconvenience for the average patient, as well as standardization in practice, is a barrier to the more widespread use of this test. Consequently, the conference participants agreed that an oral glucose tolerance test was probably not a realistic method for screening patients with schizophrenia in mental health care settings. The conference participants recommended that if a fasting plasma glucose level cannot be obtained, the use of the hemoglobin A1c test, performed by using a method certified by the National Glycohemoglobin Standardization Program (30), could be considered for screening for this population. Although the hemoglobin A1c test is not recommended by the American Diabetes Association, others have suggested that this test is useful for screening (31), but if confirmatory tests are needed. Because of the need for standardization and training in self-monitoring of plasma glucose levels, the conference participants did not recommend the use of this method for the diagnosis of diabetes.
Patients who are starting treatment with antipsychotic agents or whose antipsychotic agent is being changed should be evaluated with a fasting plasma glucose test, or, if this is not possible, their hemoglobin A1c level should be measured. In the absence of symptoms of diabetes or significant weight gain (one BMI unit), patients should be monitored for development of symptoms of diabetes 4 months later and then yearly. (Others have recommended more frequent glucose monitoring [23, 32, 33].) An abnormal test value (fasting plasma glucose value ≥126 mg/dl, random plasma glucose value >200 mg/dl, or hemoglobin A1c value >6.1%) suggests the poent and follow-up.
Patients and, when present, caregivers should also be informed about the clinical symptoms of diabetes, including polyuria, polydipsia, and weight change (particularly weight loss). Psychiatrists should inquire about these symptoms on a yearly basis and more often if patients are gaining weight or receiving antipsychotics associssibility of diabetes and should lead to a consultation with an internist or other primary health care provider for further assessment. Fasting glucose levels between 100 mg/dl and 125 mg/dl are indicative of prediabetes and should also prompt closer assessmated with weight gain.
Quality of Evidence
Quality of evidence for an association between specific antipsychotics and risk for diabetes: level 2.
Recommendations
| 1. | Mental health care providers should be aware of risk factors for diabetes for all patients with schizophrenia.
| ||||||||||||||||||||||||||||||||||
| 2. | If a patient presents to a mental health care provider with symptoms of diabetes, a random plasma glucose test should be performed. If the value is elevated (>126 mg/dl if fasting or >200 mg/dl if nonfasting), the patient should be referred to an internist or primary health care provider. If the patient contacts the mental health care provider by telephone and describes symptoms of diabetes, the patient should be urged to seek prompt evaluation by an internist or primary health care provider. | ||||||||||||||||||||||||||||||||||
Hyperlipidemia
Certain antipsychotics may be associated with hyperlipidemias. These lipid abnormalities may increase the risk of coronary heart disease.
Background
Elevated cholesterol and triglyceride levels are associated with coronary heart disease, including ischemic heart disease and myocardial infarction (34, 35). A 10% increase in cholesterol level is associated with a 20%–30% increase in the risk of coronary heart disease; and lowering the cholesterol level by 10% decreases the risk by 20%–30% (36). Elevated triglyceride levels are also associated with an increase in the risk of coronary heart disease. Triglyceride levels greater than 250 mg/dl are associated with a twofold higher risk of cardiovascular disease, compared with lower levels (35, 37). Treatment guidelines recommend aggressive interventions for reducing elevated lipid levels (38). These interventions vary from lifestyle changes (including weight loss, diet change, or exercise) to the use of drug therapy, depending on the patient’s level of risk. Despite their high risk for coronary heart disease, patients with schizophrenia were found in one study to be less likely than other individuals to receive medications for lowering cholesterol (39).
There is also concern that patients with schizophrenia may be particularly vulnerable to the metabolic syndrome, or syndrome X. The metabolic syndrome, which is associated with both diabetes and cardiovascular disease, includes abdominal obesity, elevated fasting plasma glucose levels, elevated triglyceride levels, low HDL cholesterol levels, and hypertension.
Several retrospective reports—reviewed by Meyer (40) and Wirshing et al. (37)—found elevations of lipids in patients who were taking newer antipsychotics. Early case reports focused on clozapine and found elevated levels of triglycerides but not elevated total cholesterol levels (41, 42). Similar comparisons of cohorts have reported increased levels of triglycerides in patients who were taking olanzapine (43). One study found that olanzapine was associated with higher triglyceride and cholesterol levels, compared with risperidone (44). The strongest data in this area come from a study that used the United Kingdom General Practice Research Database (45). The study included 18,309 individuals with a diagnosis of schizophrenia and compared the odds ratios for developing hyperlipidemias after receiving first- and second-generation antipsychotics. Patients who received olanzapine had significantly increased odds of developing hyperlipidemia, compared to patients who received no antipsychotic (odds ratio=4.65, p<0.0001) and compared to patients who received a first-generation antipsychotic (odds ratio=3.36, p=0.0002). In contrast, risperidone was not associated with an increased risk in either comparison.
The conference participants agreed that substantial evidence indicated that clozapine and olanzapine were associated with increased triglyceride levels, although the published studies did not reach the standard of level-1 evidence. Moreover, there is a lack of data from trials in which patients were randomly assigned to receive different antipsychotics and had their lipid profiles monitored at baseline and during chronic treatment. As a result, the participants agreed that there were insufficient data to provide clinicians with accurate information about the relative risk of elevated triglyceride levels with different antipsychotics. However, the participants agreed that individuals with schizophrenia—as a group—were at a higher risk for atherosclerotic heart disease. As a result, regular monitoring of lipid profiles should occur according to guidelines established for patients at increased risk for coronary heart disease. Guidelines have been established by the National Cholesterol Education Program (http://www.nhlbi.nih.gov/about/ncep/) and the U.S. Preventive Services Task Force (http://www.ahcpr.gov/clinic/ajpmsuppl/lipidrr.htm) (46). The U.S. Preventive Services Task Force guidelines may be more straightforward and more easily implemented in psychiatric settings. These guidelines recommend routine lipid screening for men age ≥35 years and for women age ≥45 years. For younger individuals (age 20–35 years for men and age 20–45 years for women), screening and treatment are recommended when there is increased risk for coronary heart disease. In other words, since schizophrenia patients as a group may be considered to be at high risk for coronary heart disease, the recommendations for screening and treatment should be followed for all patients age 20 years and older.
Quality of Evidence
Quality of evidence for an association between specific antipsychotics and risk for hyperlipidemias: level 2.
Recommendations
| 1. | Mental health providers should be aware of the lipid profile of each patient with schizophrenia they treat. Psychiatrists should follow National Cholesterol Education Program guidelines (http://www.nhlbi.nih.gov/about/ncep/) or U.S. Preventive Services Task Force guidelines (http://www.ahcpr.gov/clinic/ajpmsuppl/lipidrr.htm) for screening and treating patients who are at a high risk for cardiovascular disease.
| ||||||||||||||||||||||||||||
| 2. | Mental health providers should ensure that National Cholesterol Education Program or U.S. Preventive Services Task Force guidelines are followed for patients with abnormal cholesterol (total, LDL, HDL) and triglyceride levels. When patients with abnormal levels are identified (for example, a patient with an LDL level >130 mg/dl), the patient should be referred to a primary health care provider, or, in the absence of such a provider, treatment may be implemented by the mental health care provider. | ||||||||||||||||||||||||||||
QT Prolongation
Certain antipsychotics may be associated with ECG abnormalities that may indicate an increased risk of potentially fatal arrhythmias.
Background
Prolongation of the QT interval of the ECG is associated with the development of torsade de pointes, a ventricular arrhythmia that can cause syncope and may progress to ventricular fibrillation and sudden death (47). (Since the QT interval becomes shorter as the heart rate becomes more rapid, the interval duration is usually corrected for heart rate and referred to as the QTc interval.) The average QTc interval in healthy adults is approximately 400 msec, and the risk of torsade de pointes increases as the interval lengthens. A QTc interval of 500 msec or greater is considered to be a substantial risk factor for torsade de pointes.
Several drugs are known to prolong the QT interval. They include a number of antipsychotics and some tricyclic antidepressants. Thioridazine has been associated with an increased risk of ventricular arrhythmias and sudden death associated with a prolonged QT interval (48). There is also concern that drugs that inhibit the metabolism of antipsychotic agents may raise the plasma level of the antipsychotic and increase the QT interval.
A recent focus on the QT interval and antipsychotics emerged during trials of two second-generation antipsychotics, sertindole and ziprasidone. Sertindole in the amount usually administered in a clinical dose was found to increase the QTc interval by 22 msec, and the increase was dose dependent. There was evidence of increased risk of arrhythmias and unexpected deaths with sertindole (47). This medication was never marketed in the United States, although it is currently available in parts of Europe.
In initial trials, ziprasidone was found to increase the QTc interval by 6–10 msec, according to data from ECGs administered at random times (49). The U.S. Food and Drug Administration (FDA) was concerned that the prolongation might be considerably longer at ziprasidone’s maximal plasma concentration or when ziprasidone was administered with a drug that inhibited its metabolism. This concern led to a study that was carried out by Pfizer at the request of FDA (Pfizer Pharmaceuticals, unpublished data presented to the U.S. Food and Drug Administration, 2000). In that study, patients were randomly assigned to receive ziprasidone, risperidone, olanzapine, quetiapine, thioridazine, or haloperidol. ECGs were administered when the patient had the maximal plasma concentration for each agent. In addition, each agent was administered in conjunction with a drug that inhibited its metabolism. The results for the mean increase in the QTc interval (in milliseconds) were as follows: ziprasidone 20.3, risperidone 11.6, olanzapine 6.8, quetiapine 14.5, thioridazine 35.6, and haloperidol 4.7. The intervals were not substantially affected by the inhibitor.
The results of the study led to the approval of ziprasidone with the warning that ziprasidone’s “larger prolongation of QTc length compared to several other antipsychotic drugs raises the possibility that the risk of sudden death may be greater for…[ziprasidone] than for other available drugs for treating schizophrenia” (49).
In addition, thioridazine and mesoridazine (which is metabolized to thioridazine) as well as pimozide received a change in their package inserts indicating that they should not be used as first-line antipsychotics. Further, these three agents are now contraindicated for patients who are receiving drugs that inhibit the cytochrome P450 2D6 isoenzyme, patients with reduced levels of this enzyme, patients with congenital long QT syndrome, and patients with a history of cardiac arrhythmias. In addition, it is recommended that a baseline ECG and measurement of potassium concentration be completed before therapy with these drugs. Other data (50) led to restrictions in the use of droperidol, including the recommendation that patients be monitored with ECG before treatment and for 2–3 hours after treatment (51).
The conference participants agreed that in the absence of increased risk factors for QT interval prolongation or cardiac arrhythmias, ziprasidone can be prescribed without ECG monitoring. However, patients who are to be treated with ziprasidone—and patients who are treated with thioridazine, mesoridazine, or pimozide —should receive a baseline ECG before treatment is initiated if any of the following cardiac risk factors are present: known heart disease, a personal history of syncope, a family history of sudden death at an early age (under age 40 years, especially if both parents had sudden death), or congenital long QT syndrome.
Quality of Evidence
Quality of evidence for an association of specific antipsychotics with prolonged QT interval: level 1.
Recommendations
| 1. | Mental health care providers should not prescribe thioridazine, mesoridazine, or pimozide for patients with known heart disease, a personal history of syncope, a family history of sudden death at an early age (under age 40 years, especially if both parents had sudden death), or congenital long QT syndrome. | ||||
| 2. | If ziprasidone is prescribed for patients with any of the risk factors described in the previous recommendation, the patient should receive a baseline ECG before treatment is initiated. A subsequent ECG is indicated if the patient presents with symptoms associated with a prolonged QT interval (e.g., syncope). | ||||
Elevated Prolactin Levels and Sexual Side Effects
Certain antipsychotics are associated with increases in plasma prolactin level. Elevated prolactin levels can cause galactorrhea and menstrual irregularities in women, and galactorrhea and sexual dysfunction in men. Hyperprolactinemia can also cause osteoporosis if it impairs sex steroid production.
Background
Hyperprolactinemia is a common endocrine disorder that can cause galactorrhea (abnormal lactation) and menstrual disturbances in women, and sexual dysfunction (decreased libido, impotence, and ejaculatory dysfunction) and galactorrhea in men. Antipsychotic medications—particularly first-generation agents and risperidone—can cause hyperprolactinemia by their activity at dopamine D2 receptors that suppress the secretory activity of pituitary lactotrophs (52). Since dopamine inhibits prolactin release, blockade of dopamine receptors in the pituitary elevates plasma prolactin levels. The frequency of elevated prolactin levels associated with antipsychotics tends to be greater in women than in men. Although relatively few data are available about the prevalence of prolactin-related menstrual disturbances and galactorrhea, both are relatively common. Estimates of the prevalence of menstrual disturbances associated with antipsychotic use vary from 15% to 91% in different studies (53). However, these disturbances may have multiple causes. In one study, galactorrhea was present in 19% of a group of women with schizophrenia who were treated with neuroleptics (54). Although earlier studies did not show that neuroleptic-induced hyperprolactinemia increases the risk of breast cancer (55, 56), this finding was contradicted by a recent report (57). In that study, large New Jersey databases were used to identify 52,819 women who were exposed to dopamine antagonists and a similar number who were not exposed. Dopamine antagonists were associated with a 16% increase in the risk of breast cancer (adjusted hazard ratio=1.16, 95% CI=1.07–1.26).
Hyperprolactinemia impairs sexual function by inhibiting gonadotropin-releasing hormone and subsequently luteinizing hormone and follicle-stimulating hormone by means of a short feedback loop between the pituitary and hypothalamus. Since sexual dysfunction is common in men and women with schizophrenia, it is conceivable that an elevated prolactin level is an important contributor to this problem. However, hyperprolactinemia secondary to antipsychotics is most often of minimal to moderate degree (58), and the increased prolactin levels associated with antipsychotics may not be high enough to interfere with normal ovarian or testicular function. However, even a small degree of hyperprolactinemia can impair libido and potency, and hyperprolactinemia associated with use of antipsychotics may sometimes be of considerable magnitude, specifically in some patients who take risperidone. Of course, sexual dysfunction in patients is frequently caused by factors not related to prolactin and also may result from nonpharmacologic causes. Some of these causes (i.e., large prolactinomas) and the resulting high prolactin levels (>200 ng/ml) almost invariably lead to sexual and menstrual dysfunction, while conditions that cause more moderate hyperprolactinemia (e.g., nonfunctioning macroadenomas, microprolactinomas, growth hormone-producing pituitary tumors, other sellar tumors, and idiopathic hyperprolactinemia) may or may not cause sexual dysfunction depending on the extent of the prolactin elevation and the individual’s sensitivity to elevated prolactin levels. One study found that after 2 years of taking antipsychotics, 75% of the women and 34% of the men in a group of patients had elevated prolactin levels (59). In women, there was a significant negative correlation between prolactin levels and sex hormone levels.
Before the introduction of second-generation antipsychotics, prolactin elevation was an inevitable risk of treatment with antipsychotics. Prolactin elevation is less of a concern with some of the second-generation agents. The exception is risperidone, which results in prolactin elevations that are similar to those associated with first-generation antipsychotics. A meta-analysis by Kleinberg and others (60) found that prolactin levels in patients who were taking 2–16 mg/day of risperidone were similar to prolactin levels in patients who were taking 20 mg/day of haloperidol. On the other hand, the prolactin levels of patients who were taking 1–16 mg/day of risperidone were significantly higher than those of patients who were taking 10 mg/day of haloperidol. This report did not find a relationship between prolactin levels and the frequency of adverse effects that could have been related to hyperprolactinemia. Studies of other second-generation antipsychotics have found that these agents may result in transient elevations in prolactin levels but that these levels tended to return to the normal range within a few days (61–65).
The conference participants agreed that clinicians should take a careful history to determine if the patient has any signs and symptoms of an elevated prolactin level before beginning treatment with antipsychotic medications, especially first-generation antipsychotic medications and risperidone. If hyperprolactinemia is suspected, the patient’s prolactin level should be measured, and if an elevated prolactin level is found, the cause should be determined. When hyperprolactinemia occurs during treatment and is associated with menstrual or sexual dysfunction, consideration should be given to changing the patient’s medication to a prolactin-sparing agent in order to avoid an expensive workup to determine the cause of the elevated prolactin level. Psychiatrists should be aware that even minimal to moderate hyperprolactinemia can be the harbinger of a serious underlying problem, such as a pituitary tumor.
Quality of Evidence
Quality of evidence for an association between specific antipsychotics and elevation in prolactin level: level 1.
Recommendations
| 1. | Mental health providers should be aware of the signs and symptoms associated with elevated prolactin levels. The following questions should be asked before beginning treatment with an antipsychotic and yearly thereafter for all patients.
| ||||||||||||||||
| 2. | If clinically indicated, the patient’s plasma prolactin level should be measured, and, if the plasma prolactin level is elevated, a workup to determine the cause should be initiated. | ||||||||||||||||
| 3. | If patients are receiving antipsychotics known to be associated with elevation in prolactin levels, the screening questions listed with the first recommendation should be asked at each visit after starting the antipsychotic or until the dose is stable, and then the questions should be asked yearly. | ||||||||||||||||
| 4. | If a patient responds positively to any of the screening questions and is taking a prolactin-elevating medication, the patient’s prolactin level should be measured and, if possible, other potential causes of an elevated prolactin level should be ruled out. Consideration should also be given to a medication change to a prolactin-sparing medication. If the signs and symptoms disappear and the prolactin level decreases to the normal range after a change to a prolactin-sparing medication, an endocrine workup can be avoided. | ||||||||||||||||
Extrapyramidal Side Effects, Akathisia, and Tardive Dyskinesia
Drugs with dopamine receptor antagonist properties have the potential to produce acute or short-term reversible adverse effects such as dystonia, akathisia, and parkinsonism. In addition, late occurring and potentially irreversible involuntary movement disorders such as tardive dyskinesia can also occur.
Background
Conventional antipsychotics produced a significant risk of extrapyramidal side effects and tardive dyskinesia. Extrapyramidal side effects, particularly akathisia, can be a factor in medication nonadherence (66, 67). In addition, these adverse effects can contribute to psychomotor retardation and lack of energy or spontaneity, as well as diminished affect. Akathisia can interfere with functional ability and cause considerable subjective distress. Movement disorders in general can add to the stigmata associated with schizophrenia and can provide unnecessary obstacles to optimum social and vocational adjustment. Even mild involuntary movements can make patients appear odd or peculiar. Early occurring extrapyramidal side effects are a risk factor for tardive dyskinesia in patients who take conventional antipsychotics (68, 69). Much discussion has focused on the high doses of first-generation antipsychotics with which second-generation antipsychotics were compared in initial trials. However, a seven-arm, double-blind study involving patients with relatively chronic history of schizophrenia who were undergoing treatment for an acute exacerbation found that among patients who received 4 mg/day of haloperidol the frequency of use of antiparkinsonian drugs was 42.3%, a rate that was not significantly different from the rates for patients who were taking 8 mg/day or 16 mg/day of haloperidol (49.3% and 47.1%, respectively) (70).
Meta-analyses indicate that when second-generation antipsychotic medications are used at recommended doses, all of these medications are associated with significantly lower rates of extrapyramidal side effects and lower levels of antiparkinsonian use, compared with (generally high-potency) conventional antipsychotics (reviewed in reference 2). Some newer antipsychotics (e.g., risperidone and olanzapine) have a dose-response relationship for extrapyramidal side effects, while with others (e.g., clozapine, quetiapine) this relationship is not apparent. (Not enough data are available to determine if this relationship exists for aripiprazole.)
On the basis of available data for the second-generation drugs, tardive dyskinesia appears to occur significantly less frequently with clozapine, risperidone, olanzapine, and quetiapine than with first-generation antipsychotics (2). Fewer data are available for ziprasidone and aripiprazole, but early evidence suggests a low risk of tardive dyskinesia with these drugs as well.
Some patients can have preexisting motor abnormalities, ranging from apparent parkinsonian signs to abnormal involuntary movements, before the initiation of any antipsychotic medications. However, the overwhelming majority of cases of extrapyramidal symptoms appear to be due in large part to exposure to antipsychotic medication. (It is also necessary to recognize that other psychotropic drugs, e.g., antidepressants and lithium, are capable of producing some extrapyramidal signs in particularly vulnerable individuals.)
It is therefore important to examine all patients for movement disorders before the initiation of antipsychotic drug treatment (with either first-generation or second-generation antipsychotics). Patients should be monitored for extrapyramidal side effects (including akathisia) at weekly intervals during acute treatment and until their medication dose is stabilized for at least 2 weeks. Patients who are taking first-generation antipsychotic medications should be examined for tardive dyskinesia at least every 6 months, and those who are taking second-generation antipsychotics should be examined for these symptoms every year. Patients at high risk for extrapyramidal symptoms (i.e., elderly patients and those who have experienced dystonic reactions, clinically significant parkinsonism, and/or akathisia) who are taking first-generation antipsychotic medications should be examined every 3 months, and those who are taking second-generation antipsychotic medications should be examined every 6 months.
Quality of Evidence
Quality of evidence for a reduced risk of tardive dyskinesia with second-generation antipsychotics: level 1.
Recommendations
| 1. | Mental health care providers should examine patients before initiation of any antipsychotic medications to determine the possible preexistence of parkinsonian signs or abnormal involuntary movements. | ||||
| 2. | Patients should be evaluated weekly until the medication dose has been stabilized for at least 2 weeks after the introduction of first- or second-generation antipsychotics and for 2 weeks after any significant dose increase. | ||||
| 3. | Mental health care providers should examine patients for tardive dyskinesia every 6 months while the patient is taking a first-generation antipsychotic medication and every 12 months while the patient is taking a second-generation antipsychotic medication, unless the patient is at high risk for extrapyramidal symptoms (i.e., elderly patients and patients who have experienced acute dystonic reactions, other clinically significant extrapyramidal side effects, or akathisias). Patients at high risk should be examined every 3 months while they are receiving a first-generation antipsychotic medication and every 6 months while they are receiving a second-generation antipsychotic medication. | ||||
Cataracts
Certain antipsychotics may be associated with an increased risk of ocular lens opacities.
Background
Cataracts are ocular lens opacities that can lead to loss of vision. Cataracts are usually characterized according to their location as nuclear, cortical, and capsular or subcapsular. The severity of visual impairment caused by a cataract is largely determined by the location of the opacity. Visual impairment is greater when the cataract is situated more posteriorly and less severe for anterior locations. Increasing age and diabetes can predispose patients to the development of cataracts. Certain drugs, including corticosteroids, miotics, gold-based medications, amiodarone, allopurinol, and phenothiazines, have been found to increase the risk of developing cataracts. For example, corticosteroids can cause central posterior subcapsular cataracts. This location tends to have serious visual consequences. In contrast, the cataracts that may be caused by phenothiazines are likely to be anterior subcapsular and therefore less likely to impair vision.
A recent epidemiologic study that used the United Kingdom’s General Practice Research Database did not find an overall increase in the risk for cataracts among patients treated with antipsychotics (71). On the other hand, patients who were treated with chlorpromazine (at doses greater than 300 mg/day) or patients who received prochlorperazine had a significantly higher risk of developing cataracts (cataract type was not specified). These observations support the findings of earlier studies that phenothiazine antipsychotics were associated with an increased risk of cataracts.
Data from animal studies suggest that quetiapine might be associated with cataract development (Physicians’ Desk Reference, 2004, p. 689). Focal triangular cataracts were found in beagle dogs that received quetiapine for 6 or 12 months. The dogs received four times the maximum human dose of quetiapine on a milligram-per-kilogram basis. Cataracts were not found in other species, including monkeys. To date there is insufficient evidence to associate quetiapine with an increased risk of cataract development in humans. A recent report found 34 cases of lens opacities in 620,000 patient exposures to quetiapine in the United States (72). Most of the patients with opacities had known risk factors, including use of medications known to increase the risk of cataracts, ocular trauma, hypertension, or diabetes. Others were reported to have had cataracts at baseline. The remaining patients could have developed idiopathic cataracts.
Nevertheless, the observation of cataracts in dogs that were given quetiapine led to the recommendations included in the product label that patients treated with quetiapine should have a slit-lamp eye examination or a similarly sensitive eye examination shortly after initiation of treatment and at 6-month intervals. The concern about cataracts in association with quetiapine administration has led to uncertainty among clinicians about the appropriate monitoring of vision for patients who receive this and other medications.
Cataracts should be suspected in patients who report a gradual decline in vision or an increase in blurring of vision. Distance vision is most likely to be affected. The diagnosis of a cataract requires a slit-lamp examination. Examination with an ophthalmoscope is inadequate and can detect only fairly large, clinically symptomatic cataracts. Patients should be referred to an ophthalmologist or an optometrist for a slit-lamp examination.
The conference participants agreed that clinicians should follow the recommendations included in the quetiapine package insert until there is more definitive evidence regarding the risk of cataracts. Because patients with schizophrenia often have risk factors for lens opacities, such as diabetes, hypertension, and poor nutrition, clinicians should inquire about visual changes and ensure that guidelines for visual monitoring are followed. A reasonable frequency for visual monitoring is yearly for patients older than age 40 years and every 2 years for younger patients.
Quality of Evidence
The quality of evidence for an association of cataracts with specific antipsychotics could not be graded.
Recommendations
| 1. | Mental health care providers should monitor visual changes in patients with schizophrenia. On a yearly basis psychiatrists should inquire whether patients have experienced a change in vision and should specifically inquire about the quality of distance vision and about blurry vision. | ||||
| 2. | Patients who are older than age 40 years should have a yearly ocular evaluation; younger patients should have an evaluation every 2 years. | ||||
Myocarditis in Patients Who Take Clozapine
Case reports indicate that clozapine is associated with a risk of fatal myocarditis.
Background
Case reports suggest that clozapine is associated with an increased risk of myocarditis (73, 74). These reports were reinforced by post-marketing surveillance. A letter to physicians from Novartis, one of the manufacturers of clozapine, reported 30 cases of myocarditis with 17 fatalities among 205,493 patients treated with clozapine in the United States (75). Seven cases with one fatality among 15,600 patients were reported in Canada, 30 cases with eight fatalities among 24,108 patients were reported in the United Kingdom, and five cases with five fatalities among 8,000 patients were reported in Australia. These reports led to inclusion of a warning on the clozapine label in the United States that clinicians should consider the possibility of myocarditis in clozapine-treated patients who present with certain symptoms.
The symptoms of myocarditis include unexplained fatigue, dyspnea, tachypnea, fever, chest pain, palpitations, and other signs of heart failure. Eighty percent of cases of myocarditis occurred within 6 weeks of the patient’s starting clozapine. The mortality rate from myocarditis approaches 40%. Abnormalities in laboratory test findings associated with myocarditis may include increased white blood cell count, eosinophilia, increased erythrocyte sedimentation rate, and increased cardiac enzyme levels. Measurement of the plasma level of cardiac troponin may be useful in the diagnosis of myocarditis (76). In addition, patients with myocarditis may demonstrate ST abnormalities and T wave inversions on the ECG.
Since myocarditis among patients who take clozapine remains uncommon, the conference participants did not recommend routine monitoring for myocarditis. On the other hand, they did recommend that clinicians who prescribe clozapine be alert for the symptoms of myocarditis in patients who receive this medication.
Quality of Evidence
Quality of evidence for an association of myocarditis with clozapine: level 3.
Recommendations
| 1. | Mental health care providers who treat patients with clozapine should be aware of the signs and symptoms of myocarditis.
| ||||||||||||||||
| 2. | If myocarditis is identified, clozapine should be stopped and the patient should be urgently evaluated by a primary health care provider. | ||||||||||||||||
Discussion
Adhering to the recommendations of this conference (which are summarized in Table 1) will result in a significant change in the role of psychiatrists and other mental health care providers involved in the prescribing of antipsychotic medications for patients with schizophrenia. The conference participants acknowledged that psychiatrists and other mental health care providers may have to assume a role in physical health monitoring that, in the care of individuals without schizophrenia, would be the responsibility of primary care providers. There are a number of reasons for recommending that mental health care providers assume this role. Patients who are treated in public mental health settings may have very limited access to primary care providers. Moreover, primary care providers may not be knowledgeable about the health risks associated with antipsychotic medications and the resulting health monitoring that is indicated for patients with schizophrenia. Finally, patients with schizophrenia may be seen with greater frequency by mental health care providers than by their primary care colleagues. As a result, important changes in blood pressure, weight, or glucose metabolism may be detected at an earlier stage by mental health care providers. The conference participants also recognized that their recommendations may differ substantially from current standards of practice and that mental health care providers should not be subject to legal consequences for implementing current standards rather than the practices suggested in the conference recommendations. Implementing these recommendations will take planning and the support of payers and administrators, as well as the cooperation of providers.
The conference participants were impressed that an intervention as simple as stepping on a scale could yield substantial information about health risks. While in-clinic monitoring of weight should be standard practice, patients with schizophrenia also should be encouraged to self-monitor their weight and to report changes that reach a clinician-defined threshold (the number of pounds associated with an increase of one BMI unit for the individual in question) promptly to the prescribing psychiatrist.
Some of the recommendations in this report may be difficult to implement in certain mental health settings. For example, clinics or private offices may not have the capability to monitor plasma glucose levels or provide ECGs and may not have ready access to weight-management programs. However, the conference participants agreed that monitoring changes in weight and waist size and inquiring about symptoms such as polyuria, polydipsia, galactorrhea, sexual dysfunction, and visual changes have the potential for improving the health of patients with schizophrenia at a relatively low cost. As a result, the participants recommended that every mental health setting that manages individuals with schizophrenia develop the capability of monitoring and charting BMI and patients’ responses to specific inquiries. Monitoring blood pressure was not specifically discussed at the conference. However, the participants noted that blood pressure monitoring is a relatively low-cost procedure that can identify a common, treatable problem and that many patients will not have their blood pressure monitored unless monitoring occurs as part of their care in a mental health clinic.
Several health issues were not addressed at the Mount Sinai conference. The high rate of smoking among patients with schizophrenia contributes to the reduced life span of many patients. Clinicians should inquire about smoking, counsel patients about the health consequences of smoking, and refer them to smoking cessation programs where people with schizophrenia are welcome. The high prevalence of alcoholism and street drug abuse among patients with schizophrenia also was not discussed. Again, clinicians should inquire about these comorbidities as part of psychiatric monitoring.
The recommendations from this conference were intended to apply to the care of adults with schizophrenia. The participants did not address physical health monitoring for elderly patients, children, adolescents, or patients with special health needs, such as those infected with HIV. However, clinicians who treat members of these populations may find that some of the recommendations may be useful in the care of these patients as well.
 |
Received April 22, 2003; revision received Oct. 14, 2003; accepted Oct. 31, 2003. From the Department of Veterans Affairs Greater Los Angeles Healthcare System. Address reprint requests to Dr. Marder, MIRECC/210A, West Los Angeles Healthcare Center, 11301 Wilshire Blvd., Los Angeles, CA 90073; [email protected] (e-mail). Supported by the Department of Psychiatry of Mount Sinai School of Medicine, the University of Texas Health Science Center at San Antonio, the Mental Illness Research, Education and Clinical Centers of the U.S. Department of Veterans Affairs (VA) Veterans’ Integrated Service Networks 3 and 22, the VA Mental Health Quality Enhancement Research Initiative, and the VA National Center for Health Promotion and Disease Prevention. The consensus meeting on which this article is based did not receive financial support from the pharmaceutical industry. However, individual conference participants disclose the following sources of support: Dr. Buchanan has received grant support from Janssen Pharmaceutica and Novartis Pharmaceuticals Corporation and honoraria from Janssen Pharmaceutica, Eli Lilly and Company, Organon, and GlaxoSmithKline. Dr. Casey has been a consultant to Abbott Laboratories, Inc., AstraZeneca Pharmaceuticals, Aventis, Bristol-Myers Squibb Company, Pfizer Inc., Janssen Pharmaceutica, Eli Lilly and Company, and Novartis Pharmaceuticals Corporation and is a member of the speakers bureaus of Abbott Laboratories, Inc., Bristol-Myers Squibb Company, Eli Lilly and Company, Janssen Pharmaceutica, and Pfizer Inc. Bonnie M. Davis is named in Synaptec’s patent for galantamine, which is licensed to Janssen Pharmaceutica. Dr. Kane has received financial support from Abbott Laboratories, AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Company, Pfizer Inc., Janssen Pharmaceutica, Eli Lilly and Company, Novartis Pharmaceuticals Corporation, Aventis, Organon, and Lundbeck. Dr. Lieberman has received grants or research support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Company, Pfizer Inc., Janssen Pharmaceutica, Eli Lilly and Company, Novartis Pharmaceuticals Corporation, Upjohn, and Hoechst AG; is a consultant to AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Company, Pfizer Inc., Janssen Pharmaceutica, Eli Lilly and Company, Novartis Pharmaceuticals Corporation, and Upjohn; and is a member of the speakers bureaus of Janssen Pharmaceutica and Pfizer Inc. Dr. Marder has received financial support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Company, Otsuka, Pfizer Inc., Janssen Pharmaceutica, Eli Lilly and Company, Novartis Pharmaceuticals Corporation, Aventis, and Lundbeck. Dr. Miller has received financial support from AstraZeneca Pharmaceuticals, Bristol-Myers Squibb Company, Pfizer Inc., Janssen Pharmaceutica, and Eli Lilly and Company. Dr. Pi-Sunyer has received financial support from Abbott Laboratories, Inc., Novo Nordisk, Roche, and Sanofi-Synthelabo. Dr. Wirshing has received financial support from Eli Lilly and Company, Janssen Pharmaceutica, Pfizer Inc., AstraZeneca Pharmaceuticals, and Bristol-Myers Squibb Company.
1. Newman SC, Bland RC: Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry 1991; 36:239–245Crossref, Medline, Google Scholar
2. Marder SR, Essock SM, Miller AL, Buchanan RW, Davis JM, Kane JM, Lieberman J, Schooler NR: The Mount Sinai Conference on the Pharmacotherapy of Schizophrenia. Schizophr Bull 2002; 28:5–16Crossref, Medline, Google Scholar
3. Allison DB, Fontaine KR, Heo M, Mentore JL, Cappelleri JC, Chandler LP, Weiden PJ, Cheskin LJ: The distribution of body mass index among individuals with and without schizophrenia. J Clin Psychiatry 1999; 60:215–220Crossref, Medline, Google Scholar
4. Homel P, Casey D, Allison DB: Changes in body mass index for individuals with and without schizophrenia, 1987–1996. Schizophr Res 2002; 55:277–284Crossref, Medline, Google Scholar
5. Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156:1686–1696Abstract, Google Scholar
6. Wirshing DA, Wirshing WC, Kysar L, Berisford MA, Goldstein D, Pashdag J, Mintz J, Marder SR: Novel antipsychotics: comparison of weight gain liabilities. J Clin Psychiatry 1999; 60:358–363Crossref, Medline, Google Scholar
7. Allison DB, Casey DE: Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry 2001; 62(suppl 7):22–31Google Scholar
8. Conley RR, Mahmoud R: A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. Am J Psychiatry 2001; 158:765–774; correction, 158:1759Link, Google Scholar
9. Volavka J, Czobor P, Sheitman B, Lindenmayer J-P, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA: Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002; 159:255–262Link, Google Scholar
10. Azorin J-M, Spiegel R, Remington G, Vanelle J-M, Péré J-J, Giguere M, Bourdeix I: A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. Am J Psychiatry 2001; 158:1305–1313Link, Google Scholar
11. Bustillo JR, Buchanan RW, Irish D, Breier A: Differential effect of clozapine on weight: a controlled study. Am J Psychiatry 1996; 153:817–819Link, Google Scholar
12. Tollefson GD, Beasley CM Jr, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME: Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry 1997; 154:457–465Link, Google Scholar
13. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report: NIH Publication 98–4083. Bethesda, Md, National Institutes of Health, 1998Google Scholar
14. Preventing and Managing the Global Epidemic of Obesity: Report of the World Health Organization Consultation of Obesity. Geneva, WHO, 1997Google Scholar
15. Janssen I, Katzmarzyk PT, Ross R: Body mass index, waist circumference, and health risk. Arch Intern Med 2002; 162:2074–2079Crossref, Medline, Google Scholar
16. Aquila R: Management of weight gain in patients with schizophrenia. J Clin Psychiatry 2002; 63(suppl 4):33–36Google Scholar
17. Ball MP, Coons VB, Buchanan RW: A program for treating olanzapine-related weight gain. Psychiatr Serv 2001; 52:967–969Link, Google Scholar
18. Arvanitis LA, Miller BG (Seroquel Trial 13 Study Group): Multiple fixed doses of “Seroquel” (quetiapine) in patients with acute exacerbation of schizophrenia: a comparison with haloperidol and placebo. Biol Psychiatry 1997; 42:233–246Crossref, Medline, Google Scholar
19. Dixon L, Weiden PJ, Delahanty J, Goldberg R, Postrado L, Lucksted A, Lehman A: Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000; 26:903–912Crossref, Medline, Google Scholar
20. McIntyre RS, McCann SM, Kennedy SH: Antipsychotic metabolic effects: weight gain, diabetes mellitus, and lipid abnormalities. Can J Psychiatry 2001; 46:273–281Crossref, Medline, Google Scholar
21. Sernyak MJ, Leslie DL, Alarcon RD, Losonczy MF, Rosenheck R: Association of diabetes mellitus with use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002; 159:561–566Link, Google Scholar
22. Henderson DC, Cagliero E, Gray C, Nasrallah RS, Hayden DL, Schoenfeld DA, Goff DC: Clozapine, diabetes mellitus, weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry 2000; 157:975–981Link, Google Scholar
23. Lindenmayer J-P, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M, Lieberman JA: Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. Am J Psychiatry 2003; 160:290–296Link, Google Scholar
24. Koro CE, Fedder DO, L’Italien GJ, Weiss SS, Magder LS, Kreyenbuhl J, Rivicki DA, Buchanan RW: Assessment of independent effect of olanzapine and risperidone on risk of diabetes among patients with schizophrenia: population based nested case-control study. BMJ 2002; 325:243–245Crossref, Medline, Google Scholar
25. Wang PS, Glynn RJ, Ganz DA, Schneeweiss S, Levin R, Avorn J: Clozapine use and risk of diabetes mellitus. J Clin Psychopharmacol 2002; 22:236–243Crossref, Medline, Google Scholar
26. Kinon BJ, Basson BR, Gilmore JA, Tollefson GD: Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry 2001; 62:92–100Crossref, Medline, Google Scholar
27. Melkersson KI, Hulting AL, Brismar KE: Different influences of classical antipsychotics and clozapine on glucose-insulin homeostasis in patients with schizophrenia or related psychosis. J Clin Psychiatry 1999; 60:783–791Crossref, Medline, Google Scholar
28. Melkersson KI, Hulting AL, Brismar KE: Elevated levels of insulin, leptin, and blood lipids in olanzapine-treated patients with schizophrenia or related psychosis. J Clin Psychiatry 2000; 61:742–749Crossref, Medline, Google Scholar
29. Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2003; 26:S5-S20. http://care.diabetesjournals.org/content/vol26/suppl_1/Google Scholar
30. American Diabetes Association Position Statement: Tests of Glycemia in Diabetes. Diabetes Care 2003; 26:S106-S108. http://care.diabetesjournals.org/content/vol26/suppl_1/Google Scholar
31. Barr RG, Nathan DM, Meigs JB, Singer DE: Tests of glycemia for the diagnosis of type II diabetes mellitus. Ann Intern Med 2002; 137:263–272Crossref, Medline, Google Scholar
32. Wirshing DA, Boyd JA, Pien J, Wirshing WC: Weight gain and atypical antipsychotics. Essential Psychopharmacology 2000; 3:17–31Google Scholar
33. Ananth J, Venkatesh R, Burgoyne K, Gunatilake S: Atypical antipsychotic drug use and diabetes. Psychother Psychosom 2002; 71:244–254Crossref, Medline, Google Scholar
34. Kannel WB, Castelli WP, Gordon T, McNamara PM: Serum cholesterol, lipoproteins, and the risk of coronary heart disease. Ann Intern Med 1971; 74:1–12Crossref, Medline, Google Scholar
35. Jeppesen J, Hein HO, Suadicani P, Gyntelberg F: Triglyceride concentration and ischemic heart disease: an eight-year follow-up in the Copenhagen Male Study. Circulation 1998; 95:1–4Google Scholar
36. LaRosa JC, Hunninghake D, Bush D, Criqui MH, Getz GS, Gotto AM Jr, Grundy SM, Rakita L, Robertson RM, Weisfeldt ML, et al (Task Forces of Cholesterol Issues, American Heart Association): The cholesterol facts: a summary of the evidence relating dietary fats, serum cholesterol, and coronary heart disease: a joint statement by the American Heart Association and the National Heart, Lung, and Blood Institute. Circulation 1990; 81:1721–1733Crossref, Medline, Google Scholar
37. Wirshing DA, Boyd JA, Meng LR, Ballon JS, Marder SR, Wirshing WC: The effects of novel antipsychotics on glucose and lipid levels. J Clin Psychiatry 2002; 63:856–865Crossref, Medline, Google Scholar
38. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497Crossref, Medline, Google Scholar
39. Redelmeier DA, Tan SH, Booth GL: The treatment of unrelated disorders in patients with chronic medical diseases. N Engl J Med 1998; 338:1516–1520Crossref, Medline, Google Scholar
40. Meyer JM: Novel antipsychotics and severe hyperlipidemia. J Clin Psychopharmacol 2001; 21:369–374Crossref, Medline, Google Scholar
41. Ghaeli P, Dufresne RL: Serum triglyceride levels in patients treated with clozapine. Am J Health Syst Pharm 1996; 53:2079–2081Crossref, Medline, Google Scholar
42. Spivak B, Roitman S, Vered Y, Mester R, Graff E, Talmon Y, Guy N, Gonen N, Weizman A: Diminished suicidal and aggressive behavior, high plasma norepinephrine levels, and serum triglyceride levels in chronic neuroleptic-resistant schizophrenic patients maintained on clozapine. Clin Neuropharmacol 1998; 21:245–250Medline, Google Scholar
43. Osser DO, Najarian DM, Dufresne RL: Olanzapine increases weight and serum triglycerides. J Clin Psychiatry 1999; 60:767–770Crossref, Medline, Google Scholar
44. Meyer JM: A retrospective comparison of weight, lipid, and glucose changes between risperidone- and olanzapine-treated inpatients: metabolic outcomes after 1 year. J Clin Psychiatry 2002; 63:425–433Crossref, Medline, Google Scholar
45. Koro CE, Fedder DO, L’Italien GJ, Weiss S, Magder LS, Kreyenbuhl J, Revicki D, Buchanan RW: An assessment of the independent effects of olanzapine and risperidone exposure on the risk of hyperlipidemia in schizophrenic patients. Arch Gen Psychiatry 2002; 59:1021–1026Crossref, Medline, Google Scholar
46. Pignone MP, Phillips CJ, Atkins D, Teutsch SM, Mulrow CD, Lohr KN: Screening and treating adults for lipid disorders. Am J Prev Med 2001; 20(3 suppl):77–89Google Scholar
47. Glassman AH, Bigger JT Jr: Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry 2001; 158:1774–1782Link, Google Scholar
48. Menkes DB, Knight JC: Cardiotoxicity and prescription of thioridazine in New Zealand. Aust NZ J Psychiatry 2002; 36:492–498Crossref, Medline, Google Scholar
49. FDA Briefing Document for Zeldox Capsules (Ziprasidone). New York, Pfizer, Inc., July 18, 2000Google Scholar
50. Lischke V, Behne M, Doelken P, Schledt U, Probst S, Vettermann J: Droperidol causes a dose-dependent prolongation of the QT interval. Anesth Analg 1994; 79:983–986Crossref, Medline, Google Scholar
51. US Food and Drug Administration: FDA strengthens warnings for droperidol. FDA Talk Paper, Dec. 5, 2001. http://www.fda.gov/bbs/topics/ANSWERS/2001/ANS01123.htmlGoogle Scholar
52. Ben-Jonathan N, Hnasko R: Dopamine as a prolactin (PRL) inhibitor. Endocr Rev 2001; 22:724–763Crossref, Medline, Google Scholar
53. Dickson RA, Glazer WM: Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999; 35(suppl):S75-S86Google Scholar
54. Windgassen K, Wesselmann U, Schulze Monking H: Galactorrhea and hyperprolactinemia in schizophrenic patients on neuroleptics: frequency and etiology. Neuropsychobiology 1996; 33:142–146Crossref, Medline, Google Scholar
55. Schyve PM, Smithline F, Meltzer HY: Neuroleptic-induced prolactin level elevation and breast cancer: an emerging clinical issue. Arch Gen Psychiatry 1978; 35:1291–1301Crossref, Medline, Google Scholar
56. Mortensen PB: Neuroleptic treatment and other factors modifying cancer risk in schizophrenic patients. Acta Psychiatr Scand 1987; 75:585–590Crossref, Medline, Google Scholar
57. Wang PS, Walker AM, Tsuang MT, Orav EJ, Glynn RJ, Levin R, Avorn J: Dopamine antagonists and the development of breast cancer. Arch Gen Psychiatry 2002; 59:1147–1154Crossref, Medline, Google Scholar
58. Kleinberg DL, Noel GL, Frantz AG: Galactorrhea: a study of 235 cases, including 48 with pituitary tumors. N Engl J Med 1977; 296:589–600Crossref, Medline, Google Scholar
59. Smith S, Wheeler MJ, Murray R, O’Keane V: The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J Clin Psychopharmacol 2002; 22:109–114Crossref, Medline, Google Scholar
60. Kleinberg DL, Davis JM, de Coster R, Van Baelen B, Brecher M: Prolactin levels and adverse events in patients treated with risperidone. J Clin Psychopharmacol 1999; 19:57–61Crossref, Medline, Google Scholar
61. Turrone P, Kapur S, Seeman MV, Flint AJ: Elevation of prolactin levels by atypical antipsychotics. Am J Psychiatry 2002; 159:133–135Link, Google Scholar
62. Goodnick PJ: Ziprasidone: profile on safety. Expert Opin Pharmacother 2001; 2:1655–1662Crossref, Medline, Google Scholar
63. Esel E, Basturk M, Saffet Gonul A, Kula M, Tayfun Turan M, Yabanoglu I, Sofuoglu S: Effects of olanzapine and haloperidol on serum prolactin levels in male schizophrenic patients. Psychoneuroendocrinology 2001; 26:641–647Crossref, Medline, Google Scholar
64. Kasper S, Muller-Spahn F: Review of quetiapine and its clinical applications in schizophrenia. Expert Opin Pharmacother 2000; 1:783–801Crossref, Medline, Google Scholar
65. David SR, Taylor CC, Kinon BJ, Breier A: The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clin Ther 2000; 22:1085–1096Crossref, Medline, Google Scholar
66. Van Putten T: Why do schizophrenic patients refuse to take their drugs? Arch Gen Psychiatry 1974; 31:67–72Crossref, Medline, Google Scholar
67. Robinson D, Woerner MG, Alvir JM, Bilder R, Goldman R, Geisler S, Koreen A, Sheitman B, Chakos M, Mayerhoff D, Lieberman JA: Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999; 56:241–247Crossref, Medline, Google Scholar
68. Kane J, Woerner M, Lieberman J: Tardive dyskinesia: prevalence, incidence, and risk factors. J Clin Psychopharmacol 1998; 8:52S-56SGoogle Scholar
69. Saltz BL, Woerner MG, Kane JM, Lieberman JA, Alvir JM, Bergmann KJ, Blank K, Koblenzer J, Kahaner K: Prospective study of tardive dyskinesia incidence in the elderly. JAMA 1991; 266:2402–2406Crossref, Medline, Google Scholar
70. Zimbroff DL, Kane JM, Tamminga CA, Daniel DG, Mack RJ, Wozniak PJ, Sebree TB, Wallin BA, Kashkin KB (Sertindole Study Group): Controlled, dose-response study of sertindole and haloperidol in the treatment of schizophrenia. Am J Psychiatry 1997; 154:782–791Link, Google Scholar
71. Ruigomez A, Garcia Rodriguez LA, Dev VJ, Arellano F, Raniwala J: Are schizophrenia or antipsychotic drugs a risk factor for cataracts? Epidemiology 2000; 11:620–623Crossref, Medline, Google Scholar
72. Laties AM, Dev VJ, Geller W, Rak I, Brecher M, Nasrallah H: Safety update on lenticular opacities: benign experience with 620,000 US patient exposures to quetiapine, in Proceedings of the 39th Annual Meeting of the American College of Neuropsychopharmacology. Nashville, Tenn, ACNP, 2000, p 354Google Scholar
73. Killian JG, Kerr K, Lawrence C, Celermajer DS: Myocarditis and cardiomyopathy associated with clozapine. Lancet 1999; 354:1841–1845Crossref, Medline, Google Scholar
74. La Grenade L, Graham D, Trontell A: Myocarditis and cardiomyopathy associated with clozapine use in the United States. N Engl J Med 2001; 345:224–225Crossref, Medline, Google Scholar
75. Bess AL, Cunningham SR: Important drug warning (letter). East Hanover, NJ, Novartis Pharmaceuticals Corp, Feb. 11, 2002Google Scholar
76. Feldman AM, McNamara D: Medical progress: myocarditis. N Engl J Med 2000; 343:1388–1398Crossref, Medline, Google Scholar



