A Voxel-Based PET Investigation of the Long-Term Effects of “Ecstasy” Consumption on Brain Serotonin Transporters
Abstract
OBJECTIVE: Recent functional imaging studies have reported evidence of alterations in the serotonergic system induced by 3,4-methylenedioxymethamphetamine (MDMA), or “Ecstasy.” However, these studies have often been limited by small sample size, lack of tracer selectivity, unreliable assessment of MDMA doses, insufficiently matched comparison groups, or region-of-interest analysis. METHOD: Positron emission tomography (PET) using the specific serotonin transporter ligand [11C](+)McN5652 was performed in 117 subjects: 30 current MDMA users, 29 former MDMA users, 29 drug-naive comparison subjects, and 29 users of drugs other than MDMA (polydrug comparison subjects). Self-assessment of drug history was checked by analyzing hair samples. Local serotonin transporter availability was computed by a regularized reference tissue approach. Voxel-based comparison of serotonin transporter availability was performed using statistical parametric mapping (SPM 99). RESULTS: Serotonin transporter availability in current MDMA users was significantly reduced in the mesencephalon, thalamus, left caudate, hippocampus, occipital cortex, temporal lobes, and posterior cingulate gyrus compared with all other groups. Reduction was more pronounced in female than in male subjects. There was no significant difference of serotonin transporter availability among former MDMA users and the drug-naive and polydrug comparison subjects. A negative correlation between serotonin transporter availability and mean MDMA dose was found in occipital visual areas and in the left precentral sulcus of current MDMA users. In addition, there was a significant positive correlation between the serotonin transporter availability and the MDMA abstention period in brainstem and in the basal forebrain in all MDMA users. CONCLUSIONS: These findings support the hypothesis of MDMA-induced protracted alterations of the serotonergic system and indicate that the reduced availability of serotonin transporter, as measured by PET, might be reversible. Women appear to be more susceptible than men to MDMA-induced alterations of the serotonergic system.
There is increasing evidence for the neurotoxicity of the popular recreational drug “Ecstasy” (3,4-methylenedioxymethamphetamine [MDMA]) in humans (1–5). Functional imaging studies have reported decreased availability of the presynaptic serotonin transporter in different brain regions of MDMA users (6–10). However, these studies have been limited by one or more of the following shortcomings: small sample size, rather unreliable assessment of MDMA doses consumed, negligence of concurrent abuse of other psychoactive agents than MDMA, negligence of gender as a confounding variable, lack of selectivity of the tracer used, inappropriate quantification of nonspecific tracer binding, or image analysis based on volume of interest methods (11).
Therefore, the aim of this study was to investigate the long-term effect of MDMA consumption on the serotonergic system. Positron emission tomography (PET) with the highly selective serotonin transporter ligand [11C](+)McN5652 was used in a relatively large number of subjects with special regard to the mentioned methodological problems.
Method
PET using the highly selective serotonin transporter ligand [11C](+)McN5652, which provides good test-retest stability (12), was performed in a total of 117 subjects (13, 14). These subjects were assigned to four different categories on the basis of drug use history: current MDMA users (N=30), former MDMA users (N=29), subjects with no history of illicit drug abuse (N=29), and subjects with an abuse history of psychoactive agents other than MDMA (polydrug users, N=29).
Continuous regular MDMA use was required for the current MDMA user group. Inclusion criteria for the group of former MDMA users were based on the average values in the group of 14 former MDMA users described by McCann et al. (6). Thus, a minimum MDMA abstention period of 20 weeks and a cumulative MDMA dose of at least 250 tablets (for female subjects) or 400 tablets (for male subjects) were required for former MDMA users. A history without illicit drug use was required for drug-naive comparison subjects. Use of alcohol or nicotine that did not fulfill DSM-IV criteria for dependence was not an exclusion criterion. MDMA consumption was an exclusion criterion for polydrug comparison subjects.
The strategy was to first recruit suitable MDMA users and then to match the comparison groups by sociodemographic variables (gender, age, educational level). The polydrug comparison group was intended to match in addition the concomitant drug use of the MDMA groups as closely as possible. Demographic and drug history data are given in Table 1.
Calls for participants were published in newspapers, magazines, and leaflets distributed at techno events. Participants were offered some financial compensation for their efforts (127 euros) and feedback about individual test results. Details relevant for inclusion in the study were obtained from the applicants by initial phone contact. Exclusion criteria were acute major illness, age less than 18, pregnancy or breast-feeding, epilepsy, major depression, or schizophrenia as well as alcohol, opiate, or benzodiazepine dependence. Of the initial approximately 1,500 applicants, 184 were invited for further testing.
During the psychiatric interview, all subjects were tested for psychopathology with the Structured Clinical Interview for DSM-IV (SCID). Subjects suffering from any psychotic or mood disorder (exception: dysthymia) according to DSM-IV criteria that was not substance-related were excluded from the study. The prevalence of axis I mental disorders not related to substance use was in good agreement with the prevalence in the normal population. In particular, there was no statistically significant difference among the four groups of subjects with respect to these disorders. Substance-related disorders, except alcohol-, opiate-, or benzodiazepine-related disorders, were permitted.
Drug history data were obtained by a standardized questionnaire. Plausibility of the drug users’ self-assessment was ascertained by analyzing hair samples. Hair samples were taken at the day of the PET scan either from the head (if hair length >6 cm) or from the pubic/leg area in order to detect drug use during the last 6 months before the PET imaging in all subjects despite differences in the length of the head hair or dyeing of the head hair (15). In addition to MDMA, hair samples were analyzed (gas chromatography/mass spectrometry) for amphetamine, methamphetamine, methylenedioxyamphetamine, methylenedioxy-N-ethylamphetamine, and N-methyl-1,3-benzodioxolbutanamine. The subjective reports of MDMA use or abstinence for the past 6 months before the investigation matched with the hair analyses in 95% of the cases.
Participants abstained from use of all psychoactive drugs for at least 3 days before the PET measurement. Abstention in this period was verified by urine screening for the presence of amphetamines, barbiturates, benzodiazepines, cannabinoids, cocaine metabolites, opiates, methadone, and alcohol at the day of PET imaging.
The study was approved by the local ethics committee and radiation protection authorities. All participants gave their written informed consent after the procedures had been fully explained.
PET imaging was performed on a full-ring system ECAT EXACT 921/47 (Siemens/CTI, Knoxville, Tenn.) in two-dimensional mode. Stabilization of the head position was achieved by a thermoplastic mask immobilization system (Tru-Scan Imaging, Annapolis, Md.). After the transmission scan for attenuation correction, [11C](+)McN5652 was slowly injected (mean dose=466 MBq, SD=76). Mean specific activity at injection time was 83 GBq/μmol (SD=56). At the beginning of tracer injection, a dynamic scan protocol was initiated, including 35 frames with a total acquisition time of 90 minutes (14).
Reconstructed images were realigned and stereotactically normalized to an [11C](+)McN5652 template using the SPM 99 software package (Wellcome Department of Cognitive Neurology, Institute of Neurology, University College, London).
Local distribution volume ratios were obtained by kinetic modeling using the multilinear reference tissue method for reversible binding (16), regularized by singular value decomposition (17). Gray matter of the cerebellum, which is (almost) free of serotonin transporter, was chosen as the reference region (14, 18). Under the conventional assumptions of tracer kinetic modeling, the binding potential, which is obtained by subtracting 1 from the distribution volume ratio, is proportional to the local density of available serotonin transporters (19). Distribution volume ratio images were smoothed with an isotropic three-dimensional Gaussian kernel with 12 mm full-width-at-half-maximum in order to eliminate residual interindividual anatomical differences that remained after stereotactical normalization.
Further details about injected tracer dose, scan schedules, image reconstruction, stereotactical normalization, and application of the multilinear reference tissue method are given elsewhere (10, 17).
Between-group comparisons of distribution volume ratio were performed on a voxel-by-voxel basis using SPM 99 (PET/SPECT model “Compare populations: 1 scan/subject (AnCova)”). No proportional scaling was applied. Gender and age were taken into account as nuisance variables (8, 20).
In order to analyze potential gender differences in more detail, separate voxel-based statistical comparisons of current MDMA users and polydrug comparison subjects were performed for female and male subjects (PET/SPECT model “Compare populations: 1 scan/subject (two-sample t test)”).
Potential correlations of distribution volume ratio with MDMA drug history (typical dose and maximum dose taken during a party event, cumulative dose, duration of abstinence at day of PET, duration of consumption, and age at first ingestion) were analyzed in the group of current MDMA users on a voxel-by-voxel basis using the “Multiple regression” model of SPM 99. Gender was taken into account as a second covariate in each correlation analysis.
A potential correlation of the distribution volume ratio and the MDMA abstention period was also analyzed in the group of all MDMA users (N=59) using voxel-based multiple regression. Gender and age were taken into account as further covariates.
An effect was considered statistically significant if the test reached the one-sided significance level α=0.001 (uncorrected for multiple comparisons) commonly used in the statistical parametric mapping evaluation of PET studies. Only effects in clusters of at least 15 voxels corresponding to a volume of 0.4 ml were considered. In the analysis of gender differences, α=0.005 was used to account for the reduced degrees of freedom caused by the subdivision of the groups.
Results
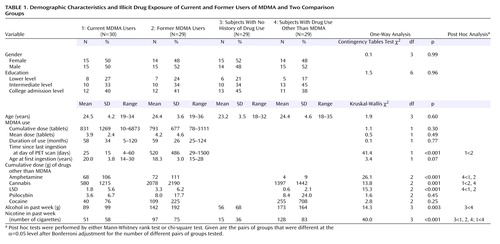
Distribution volume ratio in current MDMA users was significantly reduced in the posterior cingulate gyrus, left caudate, thalamus, occipital cortex (including visual areas), medial temporal lobes (predominantly left) including the hippocampus and parahippocampal regions, and brainstem with mesencephalon and pons (Figure 1). Reduction of distribution volume ratio in occipital visual areas was about 10%, corresponding to a much larger reduction in the binding potential.
When performing the separate between-group comparisons for female and male subjects, female current MDMA users presented reduced serotonin transporter availability in all brain structures that were conspicuous in the comparison of the whole groups except the left caudate (α=0.005, uncorrected) (Figure 2). In addition, there were significant reductions in the region of the left precentral sulcus and in superior and medial aspects of the left temporal lobe, comprising the auditory cortex. In contrast, male current MDMA users presented reduced serotonin transporter availability only in the occipital cortex and medial temporal lobes (Figure 2). There were no statistically significant differences in serotonin transporter availability among former MDMA users and the drug-naive and polydrug comparison subjects.
A significant negative correlation between distribution volume ratio and typical MDMA dose was found in occipital visual areas and in the region of the left precentral sulcus in current MDMA users. There was no significant correlation between distribution volume ratio and any other drug history measure in any brain region in the current MDMA users.
A repeat regression analysis with sex, age, and MDMA abstention period as covariates in the group of all MDMA users (N=59) revealed a significant positive correlation between the distribution volume ratio and the MDMA abstention period in brainstem with mesencephalon and pons and in the basal forebrain (Figure 3).
Discussion
Relative to both comparison groups and to the group of former MDMA users, the distribution volume ratio in current MDMA users was reduced in virtually all gray matter regions (data not shown), in good agreement with previous findings (6). The reduction was statistically significant in the posterior cingulate gyrus, left caudate, thalamus, occipital visual cortex, medial temporal lobes, and brainstem. There were no areas in which serotonin transporter availability in current MDMA users was significantly larger than in any other group. The observed pattern of reduced serotonin transporter availability in current MDMA users is in very good agreement with the pattern of serotonergic innervation as expressed by serotonin transporter density (18).
Reduction of serotonin transporter availability in current MDMA users was more pronounced in female than in male subjects, in agreement with the findings of Reneman et al. (8). This might indicate that women are more susceptible to MDMA-induced alterations of the serotonergic system than men.
The group of current MDMA users was designed to study subjects who consumed and continued to consume MDMA on a regular basis. On the one hand, it was intended to study the current MDMA users without enforcing abstention periods that were significantly longer than the typical abstention periods within their individual MDMA consumption schemes in order to avoid possible acute serotonergic dysfunction during acute abstinence from chronic MDMA use. This kind of effect was described by Jacobsen et al. (21), who found elevated central serotonin transporter availability using [123I]β-CIT SPECT in 15 cocaine-dependent subjects who had been abstinent from drug use a mean of 3.7 days. On the other hand, it was intended to establish a minimum abstention period long enough to rule out acute pharmacological effects. Mas et al. (22) investigated pharmacokinetics of MDMA in recreational MDMA users and reported that after administration of different oral doses of MDMA, plasma levels of MDMA declined following a monoexponential model with mean elimination half-life of about 8 hours. Thus, within 3 days (72 hours) plasma levels of MDMA drop far below 1% of the peak level. On the basis of the results of Jacobsen et al. and Mas et al., a minimum abstention period of 3 days was chosen in order to avoid both acute serotonergic dysfunction due to prolonged MDMA abstinence and acute pharmacological effects as much as possible.
However, fast plasma clearance does not necessarily imply fast clearance of MDMA from brain tissue. MDMA shows an approximately 1000 times lower affinity for the serotonin transporter than the tracer [11C](+)McN5652 (23, 24), so the availability of the serotonin transporter for [11C](+)McN5652 binding should not be affected significantly by the presence of MDMA. This is in accordance with studies demonstrating that administration of SSRIs prevents MDMA-induced neurodegeneration of serotonergic nerve terminals (25) and tends to reduce physiological and mental effects of MDMA (26).
Nevertheless, between-group comparison of the current MDMA users and the polydrug comparison subjects was repeated excluding all subjects who had an MDMA abstention period of less than 2 weeks (N=6). Current MDMA users with a minimum MDMA abstention period of 2 weeks showed significantly reduced distribution volume ratio in all brain structures described earlier except the posterior cingulate gyrus and left caudate. This difference might be explained by the reduced statistical power of the analysis caused by the reduction of the degrees of freedom due to the exclusion of six subjects. Thus, the described reductions of serotonin transporter availability in current MDMA users most likely are not acute pharmacological effects but protracted alterations of the serotonergic system that last (at least) several weeks.
Both current and former MDMA users differed significantly from polydrug comparison subjects with respect to cumulative doses of amphetamine, cannabis, and LSD and with respect to use of nicotine in the past week (Table 1). Staley et al. (27), using [123I]β-CIT SPECT, found higher serotonin transporter availability in the brainstem of healthy smokers than in age-, race-, and gender-matched comparison subjects. Therefore, the comparison of the current MDMA users and the polydrug comparison subjects was repeated using the number of cigarettes smoked in the week prior to PET imaging as an additional nuisance variable. Current MDMA users showed significantly reduced distribution volume ratio in the same brain structures as when confounding effects of nicotine were not taken into account, except in the left caudate. This supports the hypothesis that the observed reductions of distribution volume ratio in the current MDMA users are induced by MDMA consumption rather than being related to the higher nicotine doses used by the polydrug comparison subjects. MDMA users and comparison subjects did not differ with respect to use of cocaine and alcohol, both of which are known to affect serotonin transporter availability (21, 28).
Chang et al. (2), using perfusion SPECT, reported decreased regional cerebral blood flow (rCBF) within 3 weeks after MDMA administration in the visual cortex, the caudate, and the superior parietal and dorsolateral frontal regions. These results suggest protracted (several weeks) vasoconstriction due to MDMA-induced serotonergic dysfunction and therefore are in good agreement with our findings.
In this context it should be noted that distribution volume ratios obtained by the multilinear reference tissue approach are independent of both rCBF and peripheral clearance of the tracer (16). Therefore, the reduction of distribution volume ratio in the current MDMA users most likely is not an artifact caused by decreased tracer delivery due to reduced rCBF.
The observed reduction of serotonin transporter availability in the visual cortex in current MDMA users was further emphasized by a significant negative correlation with the typical MDMA dose, i.e., the dose taken at a typical party event. Yet, the neurophysiological implications remain unclear and occasional reports of impaired vision from MDMA users were too inconsistent to conclude any causal relationship. In contrast, memory deficits are reported throughout a number of studies (29), possibly to some extent reflecting alterations in memory-processing regions in the medial temporal lobe. However, this hypothesis remains to be tested in future studies, e.g., by the analysis of putative correlations of serotonin transporter availability and memory performance.
Interindividual differences in local brain volume were leveled out by nonlinear stereotactical normalization of the PET images to a template. Nevertheless, the observed distribution volume ratio reductions may not be explained solely by reduced local tracer uptake due to reduced availability of the serotonin transporter. Reduced tracer recovery in the reconstructed images due to volume reductions and the limited spatial resolution of the PET system cannot be ruled out. However, there is no evidence that MDMA use might cause significant atrophy effects.
There was no significant difference of serotonin transporter availability in former MDMA users relative to both comparison groups. This might indicate reversibility of serotonin transporter binding as measured by PET, in agreement with previous results of in vivo functional imaging (7, 8). This observation was strengthened by a significant positive correlation between the distribution volume ratio and the MDMA abstention period in brainstem with mesencephalon and pons and in the basal forebrain in the group of all MDMA users.
It should be noted that the exclusion of subjects with DSM-IV axis I mood disorders, which might be mediated by MDMA use, means that the subjects included in the present study were the “best” and are therefore those likely to have suffered less from the process of putative MDMA neurotoxicity. In addition, there may be a further participant-selection effect. Heavier drug users might suffer from withdrawal a minimum of 3 days from any psychoactive drug. Therefore, only subjects with the mental stability to control their withdrawal symptoms were able to participate.
Analysis of hair samples does not reliably detect use of drug in the week prior to hair sampling, since the part of the hair that indicates drug use in this period has not yet appeared above the scalp. Drug use in this period was tested to some extent by the analysis of urine samples taken at the day of PET. Further possible caveats of hair testing, including interindividual and intraindividual differences in the rate of hair growth and in hair color and texture, have been discussed by Ditton (15). However, it should be noted that the 95% agreement of subjective reports and hair analysis of MDMA use or abstinence in the past 6 months before the investigation is rather large, indicating good reliability of the subjective reports.
Concerning the control for multiple comparisons, SPM 99 provides correction for multiple comparisons on the basis of the theory of Gaussian random fields. Correction for multiple comparisons on the level of voxels controls the probability of only one single false positive voxel among the about 50,000 voxels tested throughout the whole brain. This appears extremely conservative, since it does not take into account location, size, and form of clusters of voxels above a given level of the statistics, e.g., T. For example, the probability of being false positive is expected to be smaller for a voxel that belongs to a contiguous cluster of voxels that outlines a given brain structure than for an isolated voxel scattered around somewhere in the brain. To account for this SPM 99 provides corrected statistical tests on the cluster-level or on the set-level (number of clusters). However, this approach appears to be still too conservative in many cases. For example, it does not account for the fact that the probability of being false positive might be smaller for bilateral findings than for unilateral findings. Therefore, it has become common practice in PET/SPECT applications to control for multiple comparisons by using rather high but uncorrected significance levels, i.e., α=0.01 to α=0.001, in combination with a minimum cluster size, e.g., cluster volume >0.4 ml (30). This approach was adopted for the statistical analyses in the present study. Nevertheless, it might be worth noting that voxels with reduced serotonin transporter availability in current MDMA users relative to polydrug comparison subjects that were significant at the corrected α=0.05 voxel level were located in the visual cortex area. The reductions of serotonin transporter availability in the visual cortex, hippocampus, and mesencephalon were statistically significant at the corrected α=0.05 cluster level (p<0.001, p=0.004, and p<0.05, respectively).
In conclusion, our findings yield further evidence for protracted alterations of the brain serotonergic system caused by MDMA consumption. In addition, our results indicate reversibility of serotonin transporter binding as measured by PET. However, it should be noted that the serotonin transporter is a dynamic component of the serotonergic system and is modified by multiple mechanisms. Therefore, altered serotonin transporter availability as measured by PET does not necessarily reflect morphological changes or changes of the number of nerve terminals, whereas reversibility of serotonin transporter binding does not necessarily imply full reversibility of potential neurotoxic effects. Therefore, follow-up studies are required including neuropsychological examinations and functional neuroimaging in order to assess longer-term sequelae of MDMA use.
 |
Received April 17, 2003; revision received Aug. 11, 2003; accepted Oct. 31, 2003. From the Department of Nuclear Medicine and the Department of Psychiatry and Psychotherapy, University Hospital Hamburg. Address reprint requests to Dr. Buchert, Department of Nuclear Medicine, University Hospital Hamburg-Eppendorf, Martinistr. 52, D-20246 Hamburg, Germany; [email protected] (e-mail). The study was supported by the Federal Institute for Drugs and Medical Devices (FZ: Z12.01-68503-206), Germany.
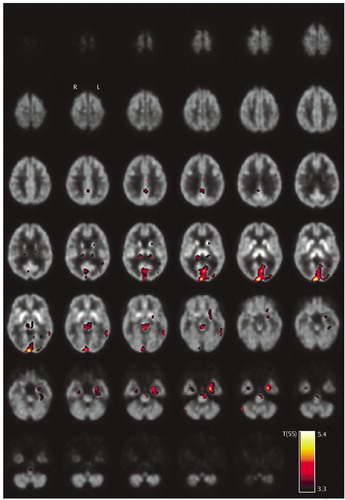
Figure 1. Areas of Significantly Reduced Serotonin Transporter Availability in Current MDMA Users (N=30) Relative to Users of Other Drugs (N=29)a
aThe statistical parametric map is thresholded at α=0.001 (uncorrected) and is overlaid to transversal slices of the [11C](+)McN5652 template. T(55) represents the t statistic at 55 degrees of freedom.
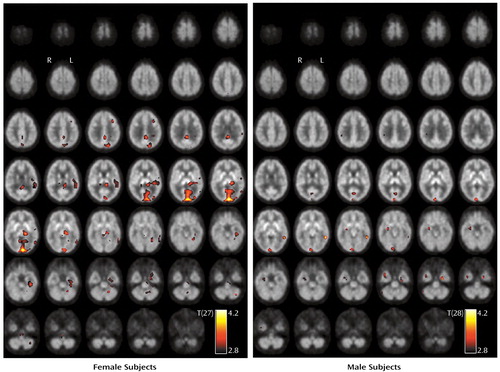
Figure 2. Areas of Significantly Reduced Serotonin Transporter Availability in Current MDMA Users (N=30) Relative to Users of Other Drugs (N=29), by Gendera
aThe statistical parametric maps are thresholded at α=0.005 (uncorrected) and overlaid to transversal slices of the [11C](+)McN5652 template. T(27) and T(28) represent the t statistic at 27 and 28 degrees of freedom, respectively.
Figure 3. Areas of Significant Positive Correlation Between the PET Tracer Distribution Volume Ratio and Duration of MDMA Abstinence Among Current and Former Users (N=59)a
aThe statistical parametric map is thresholded at α=0.001 (uncorrected) and overlaid to transversal slices of the [11C](+)McN5652 template. T(55) represents the t statistic at 55 degrees of freedom.
1. Ricaurte GA, Finnegan KT, Irwin I, Langston JW: Aminergic metabolites in cerebrospinal fluid of humans previously exposed to MDMA: preliminary observations. Ann NY Acad Sci 1990; 600:699–710Crossref, Medline, Google Scholar
2. Chang L, Grob CS, Ernst T, Itti L, Mishkin FS, Jose-Melchor R, Poland RE: Effect of ecstasy [3,4-methylenedioxymethamphetamine (MDMA)] on cerebral blood flow: a co-registered SPECT and MRI study. Psychiatry Res 2000; 98:15–28Crossref, Medline, Google Scholar
3. Kish SJ, Furukawa Y, Ang L, Vorce SP, Kalasinsky KS: Striatal serotonin is depleted in brain of a human MDMA (Ecstasy) user. Neurology 2000; 55:294–296Crossref, Medline, Google Scholar
4. Reneman L, Booij J, Schmand B, van den Brink W, Gunning B: Memory disturbances in “Ecstasy” users are correlated with an altered brain serotonin neurotransmission. Psychopharmacology (Berl) 2000; 148:322–324Crossref, Medline, Google Scholar
5. Buchert R, Obrocki J, Thomasius R, Väterlein O, Petersen K, Jenicke L, Bohuslavizki KH, Clausen M: Long-term effects of ecstasy abuse on the human brain studied by FDG PET. Nucl Med Commun 2001; 22:889–897Crossref, Medline, Google Scholar
6. McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA: Positron emission tomographic evidence of toxic effect of MDMA (“ecstasy”) on brain serotonin neurons in human beings. Lancet 1998; 352:1433–1437Crossref, Medline, Google Scholar
7. Semple DM, Ebmeier KP, Glabus MF, O’Carroll RE, Johnstone EC: Reduced in vivo binding to the serotonin transporter in the cerebral cortex of MDMA (“ecstasy”) users. Br J Psychiatry 1999; 175:63–69Crossref, Medline, Google Scholar
8. Reneman L, Booij J, de Bruin K, Reitsma JB, de Wolff FA, Gunning WB, den Heeten GJ, van den Brink W: Effects of dose, sex, and long-term abstention from use on toxic effects of MDMA (ecstasy) on brain serotonin neurons. Lancet 2001; 358:1864–1869Crossref, Medline, Google Scholar
9. Reneman L, Lavalaye J, Schmand B, de Wolff FA, van den Brink W, den Heeten GJ, Booij J: Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or “ecstasy”): preliminary findings. Arch Gen Psychiatry 2001; 58:901–906Crossref, Medline, Google Scholar
10. Buchert R, Thomasius R, Nebeling B, Petersen K, Obrocki J, Jenicke L, Wilke F, Wartberg L, Zapletalova P, Clausen M: Long-term effects of “Ecstasy” use on serotonin transporters of the brain investigated by PET. J Nucl Med 2003; 44:375–384Medline, Google Scholar
11. Kish SJ: How strong is the evidence that brain serotonin neurons are damaged in human users of ecstasy? Pharmacol Biochem Behav 2002; 71:845–855Crossref, Medline, Google Scholar
12. Szabo Z, McCann UD, Wilson AA, Scheffel U, Owonikoko T, Mathews WB, Ravert HT, Hilton J, Dannals RF, Ricaurte GA: Comparison of (+)-(11)C-McN5652 and (11)C-DASB as serotonin transporter radioligands under various experimental conditions. J Nucl Med 2002; 43:678–692Medline, Google Scholar
13. Buck A, Gucker PM, Schonbachler RD, Arigoni M, Kneifel S, Vollenweider FX, Ametamey SM, Burger C: Evaluation of serotonergic transporters using PET and [11C](+)McN-5652: assessment of methods. J Cereb Blood Flow Metab 2000; 20:253–262Crossref, Medline, Google Scholar
14. Parsey RV, Kegeles LS, Hwang DR, Simpson N, Abi-Dargham A, Mawlawi O, Slifstein M, Van Heertum RL, Mann JJ, Laruelle M: In vivo quantification of brain serotonin transporters in humans using [11C]McN5652. J Nucl Med 2000; 41:1465–1477Medline, Google Scholar
15. Ditton J: Hair testing: just how accurate is it? Surveillance & Society 2002; 1:86–101Crossref, Google Scholar
16. Ichise M, Ballinger JR, Golan H, Vines D, Luong A, Tsai S, Kung HF: Noninvasive quantification of dopamine D2 receptors with iodine-123-IBF SPECT. J Nucl Med 1996; 37:513–520Medline, Google Scholar
17. Buchert R, Wilke F, van den Hoff J, Mester J: Improved statistical power of the multilinear reference tissue approach to the quantification of neuroreceptor ligand binding by regularization. J Cereb Blood Flow Metab 2003; 23:612–620Crossref, Medline, Google Scholar
18. Laruelle M, Vanisberg M-A, Maloteaux J-M: Regional and subcellular localization in human brain of [3H]paroxetine binding, a marker of serotonin uptake sites. Biol Psychiatry 1988; 24:299–309Crossref, Medline, Google Scholar
19. Laruelle M, Wallace E, Seibyl JP, Baldwin RM, Zea-Ponce Y, Zoghbi SS, Neumeyer JL, Charney DS, Hoffer PB, Innis RB: Graphical, kinetic, and equilibrium analyses of in vivo [123I] beta-CIT binding to dopamine transporters in healthy human subjects. J Cereb Blood Flow Metab 1994; 14:982–994Crossref, Medline, Google Scholar
20. Hesse S, Barthel H, Murai T, Muller U, Muller D, Seese A, Kluge R, Sabri O: Is correction for age necessary in neuroimaging studies of the central serotonin transporter? Eur J Nucl Med Mol Imaging 2003; 30:427–430Crossref, Medline, Google Scholar
21. Jacobsen LK, Staley JK, Malison RT, Zoghbi SS, Seibyl JP, Kosten TR, Innis RB: Elevated central serotonin transporter binding availability in acutely abstinent cocaine-dependent patients. Am J Psychiatry 2000; 157:1134–1140Link, Google Scholar
22. Mas M, Farre M, de la Torre R, Roset PN, Ortuno J, Segura J, Cami J: Cardiovascular and neuroendocrine effects and pharmacokinetics of 3, 4-methylenedioxymethamphetamine in humans. J Pharmacol Exp Ther 1999; 290:136–145Medline, Google Scholar
23. Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB: Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol 1988; 149:159–163Crossref, Medline, Google Scholar
24. Shank RP, Vaught JL, Pelley KA, Setler PE, McComsey DF, Maryanoff BE: McN-5652: a highly potent inhibitor of serotonin uptake. J Pharmacol Exp Ther 1988; 247:1032–1038Medline, Google Scholar
25. Hewitt KE, Green AR: Chlormethiazole, dizocilpine and haloperidol prevent the degeneration of serotonergic nerve terminals induced by administration of MDMA (“Ecstasy”) to rats. Neuropharmacology 1994; 33:1589–1595Crossref, Medline, Google Scholar
26. Liechti ME, Baumann C, Gamma A, Vollenweider FX: Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology 2000; 22:513–521Crossref, Medline, Google Scholar
27. Staley JK, Krishnan-Sarin S, Zoghbi S, Tamagnan G, Fujita M, Seibyl JP, Maciejewski PK, O’Malley S, Innis RB: Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse 2001; 41:275–284Crossref, Medline, Google Scholar
28. Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, Gorey JG, Doty L, Geyer C, Lee KS, Coppola R, Weinberger DR, Linnoila M: Reduced central serotonin transporters in alcoholism. Am J Psychiatry 1998; 155:1544–1549Link, Google Scholar
29. Thomasius R, Petersen K, Buchert R, Andresen B, Zapletalova P, Wartberg L, Nebeling B, Schmoldt A: Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology (Berl) 2003; 167:85–96Crossref, Medline, Google Scholar
30. Stamatakis EA, Glabus MF, Wyper DJ, Barnes A, Wilson JT: Validation of statistical parametric mapping (SPM) in assessing cerebral lesions: a simulation study. Neuroimage 1999; 10:397–407Crossref, Medline, Google Scholar



