Reduced Superior Temporal Gyrus Volume in Young Offspring of Patients With Schizophrenia
Abstract
OBJECTIVE: The superior temporal gyrus, a heteromodal auditory and language association cortex, has been found to be smaller in patients with schizophrenia than in normal subjects. However, genetic and/or neurodevelopmental underpinnings of superior temporal gyrus alterations are unknown. Nonpsychotic children with greater genetic risk for schizophrenia exhibit language deficits. The authors studied the superior temporal gyrus in nonpsychotic children at risk for schizophrenia. METHOD: Magnetic resonance imaging was used to measure the right and left superior temporal gyrus of 29 young nonpsychotic subjects who had a parent with schizophrenia and 27 age- and sex-matched comparison subjects who had no family psychiatric history. RESULTS: After controlling for age and intracranial volume, the authors found that the volumes of the right and left superior temporal gyrus of the subjects at risk for schizophrenia were significantly smaller than those of the comparison subjects. Comparison subjects, but not at-risk subjects, showed an inverse correlation between age and left superior temporal gyrus volume. CONCLUSIONS: These findings provide new evidence that superior temporal gyrus abnormalities may result from genetically mediated developmental deviance reflecting greater susceptibility to schizophrenia. Further studies and follow-up will lead to greater understanding of the role of the superior temporal gyrus in the premorbid vulnerability to schizophrenia.
Neuroanatomical changes in schizophrenia are well established (1), but their genetic and neurodevelopmental origins are not well understood. Genetically at-risk children present an excellent research opportunity to study premorbid vulnerability markers and developmental brain changes, especially during the crucial period preceding disease onset. Genetically at-risk children and children who later develop schizophrenia have significant neurological, psychological, and social developmental differences from normal subjects (2). Specifically, attention deficits, minor neuromotor deficits, and neuroanatomical differences such as a smaller amygdala and smaller hippocampi have been observed in young at-risk relatives of patients with schizophrenia. In addition, at-risk relatives exhibit deficits in language development such as poor speech comprehension and verbal memory (3–6). Disturbances in thought and language are core features of schizophrenia (7–9), and a faulty development of the language system has been proposed as a causal mechanism for schizophrenia (8).
The superior temporal gyrus, typically larger on the left in right-handed individuals, includes Heschl’s gyrus and the planum temporale and is believed to be a major anatomical substrate for speech, language, and communication. Penfield and Perot (10) observed psychosis-like symptoms with electrical stimulation of the superior temporal gyrus. Neurological injuries to the superior temporal gyrus such as epilepsy, stroke, or tumors cause disturbances of thought, hallucinations, and delusions similar to those seen in schizophrenia (11). The superior temporal gyrus has wide connections to temporolimbic areas, including the hippocampus, amygdala, entorhinal cortex, thalamus, and neocortical association areas in the prefrontal and parietal cortices (12). Although not well understood, the wide and complex connections seem to integrate these brain regions, which are essential for information processing and thought.
The superior temporal gyrus, with a clear role in thought and language processes, is appropriately a major area of interest in schizophrenia research. The widely replicated superior temporal gyrus volume reduction in schizophrenia in relation to symptoms appears to be relatively disease specific, seems to be present at illness onset, and may progress during the course of the illness (13–21). However, it is unclear whether a smaller superior temporal gyrus reflects a premorbid susceptibility trait or a result of persistent psychosis. Studies of nonpsychotic at-risk offspring of parents with schizophrenia allow us to investigate this question. In this preliminary study, we used magnetic resonance imagining (MRI) to examine the superior temporal gyrus in a group of at-risk offspring. Our hypothesis was that the superior temporal gyrus would be smaller in at-risk subjects than in comparison subjects.
Method
Twenty-nine at-risk offspring (15 males and 14 females, mean age=14.9 years, SD=3.4) who had one parent suffering from schizophrenia or schizoaffective disorder and 27 healthy comparison subjects (14 males and 13 females, mean age=16.9 years, SD=5.7) with no family psychiatric history were recruited from the same community. We did not match parental socioeconomic status because the at-risk group, with a parent suffering from schizophrenia, would be expected to have lower socioeconomic status. Matching on this variable would very likely remove illness-related variance. The University of Pittsburgh Institutional Review Board approved the study. All subjects and their parents or guardians provided written consent and assent, when appropriate, after full description of the study. Subjects 14 and younger were evaluated with the Schedule for Affective Disorders and Schizophrenia for School-Age Children (22). Parents and subjects 15 or older were evaluated with the Structured Clinical Interview for DSM-IV (23). Any subject from either group with a lifetime history of psychosis, neurological disorders, chronic medical or psychiatric illnesses, current substance use disorder, psychotropic treatment, or any contraindication for MRI study was excluded.
For MRI acquisition and processing, 124 T1-weighted 1.5-mm coronal slices, without interslice gap, were obtained with a 1.5-T GE scanner (GE Medical Systems, Milwaukee); three-dimensional spoiled gradient recall acquisition coronal, matrix=256×256×192, field of view=24 cm, TR=25 msec, and TE=5 msec. Scans were reviewed to exclude structural abnormalities. Intracranial volume was measured with NIH IMAGE (24). The superior temporal gyrus (gray plus white) was manually traced by one of the authors blind to the clinical data (R.R.) (intrarater reliability=0.98 for the left superior temporal gyrus and intrarater reliability=0.99 for the right superior temporal gyrus) using BRAINS2 (25) on a resampled three-dimensional Talairach image. The methodology of superior temporal gyrus measurement has been described previously (16, 18).
Differences in age, IQ, and intracranial volume were examined by two-tailed unpaired t tests. Multivariate analysis based on the general linear model was conducted with group status (at-risk subjects versus healthy comparison subjects), intracranial volume, and age as predictor variables and superior temporal gyrus measures as dependent variables. We examined age-related differences using partial correlations between age and superior temporal gyrus volumes controlling for intracranial volume. Parental socioeconomic status was not used as a covariate because the grouping was based on parental illness. All tests were two-tailed, and the significance level was set at p≤0.05.
Results
The two groups were not significantly different in age (t=–1.57, df=54, p=0.12), IQ (t=–1.28, df=54, p=0.21), handedness (25 right-handed, one left-handed, and three ambidextrous at-risk subjects and 23 right-handed, one left-handed, one ambidextrous, and two unknown-handed healthy comparison subjects), and gender distribution (χ2=0.03, df=1, p=0.85). The parental socioeconomic status was different between the groups (t=4.75, df=49, p<0.001), which would be expected because one parent of each at-risk subject was suffering from schizophrenia.
The intracranial volume of at-risk subjects (mean=1408.65 cc, SD=174.17) was not significantly different from that of healthy comparison subjects (1473.94 cc, SD=155.73) (t=1.48, df=54, p=0.15). Analyses of variance based on the general linear model showed significant differences in volume between the groups in the right superior temporal gyrus (for at-risk subjects, mean=22.8 cc, SD=2.98; for healthy comparison subjects, mean=25.3 cc, SD=3.5) (F=8.48, df=1, 56, p=0.005) and the left superior temporal gyrus (for at-risk subjects, mean=22.7 cc, SD=3.3; for healthy comparison subjects, mean=25.0 cc, SD=3.6) (F=6.49, df=1, 56, p=0.02) (Figure 1). Similar analysis with IQ as an additional covariate did not change the results (left superior temporal gyrus: F=5.87, df=1, 55, p=0.02; right superior temporal gyrus: F=5.96, df=1, 55, p<0.02).
Left superior temporal gyrus volume showed a significant inverse correlation with age in healthy comparison subjects (partial r=–0.40, df=24, p=0.04) but not in at-risk subjects (partial r=–0.21, df=26, p=0.28).
Discussion
To our knowledge, this is the first report of reduced superior temporal gyrus volume in young offspring genetically at risk for schizophrenia. Superior temporal gyrus volume reductions in these nonpsychotic at-risk subjects suggest that abnormal development rather than degeneration of the superior temporal gyrus is associated with schizophrenia and that such deviant development may be mediated by familial/genetic factors. The absence of the age-related reduction in the superior temporal gyrus in at-risk subjects may be related to a failure of either the normative pruning process that occurs during adolescence or the synaptic/axonal proliferation that occurs earlier in development (26).
Schizophrenia susceptibility genes may influence the integrity of brain regions responsible for thought and language processes and may account for the premorbid language abnormalities in at-risk individuals. Variations in genetic threshold, brain maturational processes such as neuronal pruning and myelination, environmental stressors, and compensatory mechanisms may explain occurrence of or protection from schizophrenia spectrum pathology in genetically vulnerable individuals.
Other structures in the hierarchy of information processing that are interconnected to the superior temporal gyrus, such as the hippocampus, thalamus, or frontal lobe, may also be developmentally impaired in at-risk subjects. For example, the hippocampus has been found to be smaller in patients with schizophrenia as well as at-risk subjects, and hippocampal sclerosis is known to reduce blood flow in the superior temporal gyrus, in association with an impairment of language function (27–31).
The relatively small number of subjects in our study points to a need for an independent replication. The small size of the study group may be the cause for the lack of expected left-greater-than-right asymmetry. This preliminary cross-sectional group comparison will be followed up with longitudinal studies to assess the predictive value and age-related changes in the superior temporal gyrus. Future studies ideally should include subjects carefully matched for age and sex and should incorporate periodic longitudinal clinical and imaging evaluations. Use of other approaches like functional imaging, diffusion tensor imaging, and spectroscopy will help us to understand the physiology of the superior temporal gyrus and other brain structures involved in schizophrenia and help clarify the altered development of functional neuroanatomy and physiology in at-risk individuals.
Received Aug. 6, 2003; revision received Oct. 28, 2003; accepted Oct. 31, 2003. From the Department of Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, and the University of Pittsburgh Medical Center. Address reprint requests to Dr. Keshavan, Clinical Core, Center for Neurosciences in Mental Disorders, STEP Clinic, Rm. 441, Western Psychiatric Institute and Clinic, 3811 O’Hara St., Pittsburgh, PA 15213; [email protected] (e-mail). Supported in part by NIMH grants MH-64023, MH-45203, MH-01180 (Dr. Keshavan), and K24 MH-02037 (Dr. Rosenberg), by a National Alliance for Research on Schizophrenia and Depression Established Investigator Award (Dr. Keshavan), and by the State of Michigan, Joe F. Young Sr. (Dr. Rosenberg). The authors thank Dr. Sri Muddasani for help in the magnetic resonance imaging measurements and Ms. Debra Montrose for help in clinical assessments.
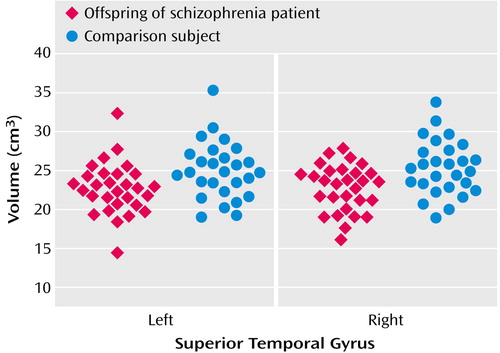
Figure 1. Box Plot of Left and Right Superior Temporal Gyrus Volumes of 29 Young, Nonpsychotic Offspring of Patients With Schizophrenia and 27 Age- and Sex-Matched Comparison Subjects With No Family Psychiatric History
1. McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, Shenton ME: MRI anatomy of schizophrenia. Biol Psychiatry 1999; 45:1099–1119Crossref, Medline, Google Scholar
2. Niemi LT, Suvisaari JM, Tuulio-Henriksson A, Lonnqvist JK: Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res 2003; 60:239–258Crossref, Medline, Google Scholar
3. Amminger GP, Pape S, Rock D, Roberts SA, Ott SL, Squires-Wheeler E, Kestenbaum C, Erlenmeyer-Kimling L: Relationship between childhood behavioral disturbance and later schizophrenia in the New York High-Risk Project. Am J Psychiatry 1999; 156:525–530Abstract, Google Scholar
4. Seidman LJ, Faraone SV, Goldstein JM, Kremen WS, Horton NJ, Makris N, Toomey R, Kennedy D, Caviness VS, Tsuang MT: Left hippocampal volume as a vulnerability indicator for schizophrenia: a magnetic resonance imaging morphometric study of nonpsychotic first-degree relatives. Arch Gen Psychiatry 2002; 59:839–849Crossref, Medline, Google Scholar
5. Cosway R, Byrne M, Clafferty R, Hodges A, Grant E, Abukmeil SS, Lawrie SM, Miller P, Johnstone EC: Neuropsychological change in young people at high risk for schizophrenia: results from the first two neuropsychological assessments of the Edinburgh High Risk Study. Psychol Med 2000; 30:1111–1121Crossref, Medline, Google Scholar
6. O’Driscoll GA, Florencio PS, Gagnon D, Wolff AV, Benkelfat C, Mikula L, Lal S, Evans AC: Amygdala-hippocampal volume and verbal memory in first-degree relatives of schizophrenic patients. Psychiatry Res 2001; 107:75–85Crossref, Medline, Google Scholar
7. Andreasen NC, Grove WM: Thought, language, and communication in schizophrenia: diagnosis and prognosis. Schizophr Bull 1986; 12:348–359Crossref, Medline, Google Scholar
8. Crow TJ: Schizophrenia as failure of hemispheric dominance for language. Trends Neurosci 1997; 20:339–343Crossref, Medline, Google Scholar
9. Hallett S, Quinn D, Hewitt J: Defective interhemispheric integration and anomalous language lateralization in children at risk for schizophrenia. J Nerv Ment Dis 1986; 174:418–427Crossref, Medline, Google Scholar
10. Penfield W, Perot P: The brain’s record of auditory and visual experience: a final summary and discussion. Brain 1963; 86:595–696Crossref, Medline, Google Scholar
11. Galasko D, Kwo-On-Yuen PF, Thal L: Intracranial mass lesions associated with late-onset psychosis and depression. Psychiatr Clin North Am 1988; 11:151–166Crossref, Medline, Google Scholar
12. Pandya DN: Anatomy of the auditory cortex. Rev Neurol 1995; 151:486–494Medline, Google Scholar
13. Hirayasu Y, McCarley RW, Salisbury DF, Tanaka S, Kwon JS, Frumin M, Snyderman D, Yurgelun-Todd D, Kikinis R, Jolesz FA, Shenton ME: Planum temporale and Heschl gyrus volume reduction in schizophrenia: a magnetic resonance imaging study of first-episode patients. Arch Gen Psychiatry 2000; 57:692–699Crossref, Medline, Google Scholar
14. Hirayasu Y, Shenton ME, Salisbury DF, McCarley RW: Hippocampal and superior temporal gyrus volume in first-episode schizophrenia. Arch Gen Psychiatry 2000; 57:618–619Crossref, Medline, Google Scholar
15. Hirayasu Y, Shenton ME, Salisbury DF, Dickey CC, Fischer IA, Mazzoni P, Kisler T, Arakaki H, Kwon JS, Anderson JE, Yurgelun-Todd D, Tohen M, McCarley R: Lower left temporal lobe MRI volumes in patients with first-episode schizophrenia compared with psychotic patients with first-episode affective disorder and normal subjects. Am J Psychiatry 1998; 155:1384–1391Link, Google Scholar
16. Keshavan MS, Haas GL, Kahn CE, Aguilar E, Dick EL, Schooler NR, Sweeney JA, Pettegrew JW: Superior temporal gyrus and the course of early schizophrenia: progressive, static, or reversible? J Psychiatr Res 1998; 32:161–167Crossref, Medline, Google Scholar
17. Kim JJ, Crespo-Facorro B, Andreasen NC, O’Leary DS, Magnotta V, Nopoulos P: Morphology of the lateral superior temporal gyrus in neuroleptic naive patients with schizophrenia: relationship to symptoms. Schizophr Res 2003; 60:173–181Medline, Google Scholar
18. Rajarethinam RP, DeQuardo JR, Nalepa R, Tandon R: Superior temporal gyrus in schizophrenia: a volumetric magnetic resonance imaging study. Schizophr Res 2000; 41:303–312Crossref, Medline, Google Scholar
19. Pearlson GD: Superior temporal gyrus and planum temporale in schizophrenia: a selective review. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:1203–1229Crossref, Medline, Google Scholar
20. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE: Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry 1990; 147:1457–1462Link, Google Scholar
21. Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M, et al: Abnormalities of the left temporal lobe and thought disorder in schizophrenia: a quantitative magnetic resonance imaging study. N Engl J Med 1992; 327:604–612Crossref, Medline, Google Scholar
22. Chambers WJ, Puig-Antich J, Hirsch M, Paez P, Ambrosini PJ, Tabrazi MA, Davies M: The assessment of affective disorders in children and adolescents by semistructured interview: test-retest reliability of the Schedule for Affective Disorders and Schizophrenia for School-Age Children, Present Episode Version. Arch Gen Psychiatry 1985; 42:696–702Crossref, Medline, Google Scholar
23. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
24. Rosband W: NIH Image Manual. Bethesda, Md, National Institutes of Health, 1993Google Scholar
25. Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW II: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992; 4:125–133Crossref, Medline, Google Scholar
26. Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H: Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett 1982; 33:247–252Crossref, Medline, Google Scholar
27. Arnold S, Schlaug G, Niemann H, Ebner A, Luders H, Witte OW, Seitz RJ: Topography of interictal glucose hypometabolism in unilateral mesiotemporal epilepsy. Neurology 1996; 46:1422–1430Crossref, Medline, Google Scholar
28. Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M: Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res 2002; 58:173–183Crossref, Medline, Google Scholar
29. van Erp TGM, Saleh PA, Rosso IM, Huttunen M, Lönnqvist J, Pirkola T, Salonen O, Valanne L, Poutanen V-P, Standertskjöld-Nordenstam C-G, Cannon TD: Contributions of genetic risk and fetal hypoxia to hippocampal volume in patients with schizophrenia or schizoaffective disorder, their unaffected siblings, and healthy unrelated volunteers. Am J Psychiatry 2002; 159:1514–1520Link, Google Scholar
30. Lawrie SM, Whalley H, Kestelman JN, Abukmeil SS, Byrne M, Hodges A, Rimmington JE, Best JJ, Owens DG, Johnstone EC: Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet 1999; 353:30–33Crossref, Medline, Google Scholar
31. Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, Desmond P, McGuire PK: Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet 2003; 361:281–288Crossref, Medline, Google Scholar



