Morphometric Assessment of the Heteromodal Association Cortex in Schizophrenia
Abstract
OBJECTIVE: The heteromodal association cortex has been hypothesized to be selectively involved in the pathophysiology of schizophrenia. To test this hypothesis, the authors measured prefrontal, inferior parietal, and superior temporal gyrus volumes and examined the pattern of connections among these regions. METHOD: Forty-four patients with schizophrenia or schizoaffective disorder and 34 healthy comparison subjects were included in the study. A spoiled gradient recall acquisition in the steady-state three-dimensional magnetic resonance imaging sequence was used for morphometric assessment of the heteromodal association cortex. RESULTS: Patients with schizophrenia had significantly smaller inferior prefrontal region volumes and significant reversal of the normal asymmetry of the inferior parietal cortex. No significant group differences were found in superior temporal gyrus volume. The groups differed significantly in the correlation between inferior prefrontal region volumes and angular gyrus volumes. CONCLUSIONS: The results suggest that patients with schizophrenia may be characterized by selective abnormalities of the heteromodal regions involved in the neuroanatomy of language.
The heteromodal association cortex comprises primarily the prefrontal, superior temporal, and inferior parietal cortices (1). These regions integrate emotional, motor, and sensory inputs in the evaluation and production of complex behavioral responses (1). The heteromodal association cortex has been hypothesized to be selectively involved in the neuroanatomy of schizophrenia (2).
Postmortem and functional and structural imaging studies have supported this hypothesis, with structural magnetic resonance imaging (MRI) studies suggesting that several of the heteromodal association cortex components are characterized by gross structural changes. In particular, there is general consensus that total prefrontal gray matter volume is smaller in patients with schizophrenia, relative to healthy comparison subjects (3). However, there is little agreement about whether this smaller volume represents a global deficit or is restricted to the prefrontal subcomponents. Studies that have examined specific prefrontal regions (4, 5) or have parcellated the prefrontal cortex into subcomponents (6–12) have variously observed smaller volumes of the dorsolateral, superior/medial, inferior, or orbital prefrontal regions in patients with schizophrenia. The lack of consensus among studies is due, in large part, to variability in the number and definitions of the prefrontal regions.
There is also general agreement that superior temporal gyrus volume is smaller in patients with schizophrenia (3); however, some studies have had negative findings (8, 13, 14). Moreover, several studies have shown that reduced superior temporal gyrus volume is associated with hallucinations/delusions or positive formal thought disorder (3). There are also reports of normal asymmetry reversal of the planum temporale (3).
In contrast to the large number of studies of the prefrontal cortex and the superior temporal gyrus, relatively few structural MRI studies have examined the inferior parietal cortex. The inferior parietal cortex comprises the supramarginal and angular gyri. Studies of this region have demonstrated smaller gray matter volume of the total inferior parietal region or one of the component gyri, have shown reversal of the normal asymmetry of this region, or have failed to find any structural abnormalities (4, 8, 15, 16).
The very few studies that have concurrently examined more than one heteromodal association cortex region (4, 5, 16) have examined whether the region’s potential selective involvement in the neuroanatomy of schizophrenia results in unique relationships among these structures. Previous studies of the relationships among heteromodal association cortex regions have produced divergent results. In a factor analytic study, measures of the dorsolateral prefrontal cortex and superior temporal gyrus loaded onto the heteromodal association cortex factor in both patients with schizophrenia and healthy comparison subjects (17). The inferior parietal cortex did not contribute to this factor in either group. In a second study, frontal lobe and superior temporal gyrus volumes were significantly more correlated with each other in healthy comparison subjects than in subjects with schizophrenia (5). In contrast, Niznikiewicz and colleagues (16) observed that volumes of the right and left inferior parietal cortices were significantly correlated with volumes of the right and left inferior and orbital, left middle, and right superior prefrontal regions in patients with schizophrenia but not in healthy comparison subjects. In patients with schizophrenia, volumes of both the right and the left inferior parietal regions were significantly correlated with the left anterior superior temporal gyrus volume (16).
We previously reported the development of a reliable procedure for parcellating the prefrontal cortex into four subcomponents and found that patients with schizophrenia had selective smaller volumes of right and left inferior prefrontal region gray matter (6). The procedure is based on the method proposed by Rademacher and colleagues (18) and utilizes surface sulcal landmarks, information on the functional organization of the brain, and three-dimensional MRI assessment methods. The current study was designed to replicate our previous observation of smaller inferior prefrontal region volumes, extend the evaluation of heteromodal regions to the superior temporal gyrus and inferior parietal cortex, and examine the pattern of correlations among the heteromodal regions. We hypothesized that patients with schizophrenia would be characterized by smaller heteromodal cortical volumes, relative to healthy comparison subjects, and that the pattern of volumetric correlations among the heteromodal regions would differ between the two groups.
Method
Subjects
Forty-four outpatients with DSM-III-R/DSM-IV schizophrenia or schizoaffective disorder were selected for study entry. Patients’ diagnoses were based on the Structured Clinical Interview for DSM-III-R/DSM-IV (SCID) (19, 20), interviews with family informants, and past medical records. Thirty-four healthy comparison subjects were recruited from a group of community volunteers. Comparison subjects were excluded if they had a history of a DSM-III-R/DSM-IV axis I or axis II disorder on the basis of a SCID interview. Patients and comparison subjects were excluded if they had a history of neurological disorder, mental retardation, head injury with loss of consciousness for greater than 30 minutes, or a diagnosis of substance abuse or dependence within the last 12 months. Forty-one patients and 33 comparison subjects were right-handed. Fifteen patients were treated with conventional antipsychotics, seven patients were treated with new-generation antipsychotics other than clozapine, and 16 patients were treated with clozapine. Medication data were missing for six patients. All subjects provided written informed consent before study entry.
MRI Protocol
MRI studies were performed on a 1.5-T Signa GE Scanner (General Electric, Milwaukee). The whole brain was evaluated in the coronal plane by using a spoiled gradient recall acquisition in the steady-state three-dimensional imaging sequence, with the following imaging parameters: TR=35 msec, TE=5 msec, flip angle=45°, number of excitations=1, field of view=24 cm, matrix size=256 ×256, and 1.5-mm slice thickness.
Image Processing and Measurement
The MEASURE system was used to process and analyze images (6, 21). Individual coronal MRI images are stripped of all nonbrain tissue and combined to form a three-dimensional representation of the brain. The three-dimensional representation and the three MRI image orthogonal views (coronal, axial, and sagittal) are displayed on the computer screen. The surface sulcal landmarks for each region are identified and painted on the three-dimensional representation of the brain (see figure 1 in reference 6). The demarcated regions are then painted with different colors, with each paint color defining the set of voxels used to calculate cortical region volume. The paint colors are depicted on the orthogonal views. The painted orthogonal views and three-dimensional brain representation are used in an iterative manner to identify the voxels of each brain region. A stereological method is used to count the gray matter and white matter voxels (21, 22). In this method, a three-dimensional grid of predetermined size is superimposed on the MRI images; the individual grid points were represented by small rotated “L” shapes (see figure 3 in reference 6). Optimal grid size is based on the Cavalieri principle and was calculated by using the formula developed by Gunderson et al. (22). Gray matter volume is calculated by counting the number of L’s that contain a gray matter voxel along the diagonal defined by the angle of the L; white matter volume is calculated by counting the number of remaining L’s within the region of interest (22).
Anatomical Boundaries
Anatomical boundaries for each region are described in Appendix 1 and illustrated in Figure 1, Figure 2, and Figure 3. The boundaries are based on surface sulcal landmarks (23). Prefrontal total gray matter volume is the sum of the four prefrontal region gray matter volumes. Prefrontal total white matter comprises the white matter on each coronal slice identified by the surface paint. If gyri represented on a coronal slice are not part of the prefrontal cortex, then the white matter, defined by the cortical strip and a straight line that connects the apex of each sulcus used to delineate this region from a prefrontal region, is not included in the prefrontal total white matter volume measurement (6). Superior temporal gyrus white matter volume includes all white matter between the cortical strip and the medial border of the region. The inferior parietal total gray matter volume is the sum of the supramarginal and angular gyri volumes. Inferior parietal white matter includes all white matter within the cortical strip and the borders of each gyrus.
Total cranial volume includes the cerebrum, cerebellum, sulcal and ventricular CSF, and brainstem. The inferior boundary of the brainstem is defined by a straight line from the posterior margin of the foramen magnum (most inferior and anterior point in the occipital bone) and the anterior margin of the foramen magnum (most posterior and inferior of the basiocciput). Total brain volume includes all brain structures that define the total cranial volume but excludes the sulcal and ventricular CSF.
Interrater reliability for the prefrontal region, superior temporal gyrus, and inferior parietal region was based on the independent measurement of each region in five brains. The intraclass correlation coefficients were 0.95 for the superior prefrontal region, 0.93 for the middle prefrontal region, 0.91 for the inferior prefrontal region, 0.93 for the orbital prefrontal region, 0.89 for the superior temporal gyrus, 0.93 for the supramarginal gyrus, 0.86 for the angular gyrus, 0.99 for the total brain volume, and 0.99 for the total cranial volume.
Statistical Analyses
To examine effects of diagnosis, gender, and hemisphere, we fitted a mixed analysis of covariance model for repeated measures within each brain region by using the PROC MIXED procedure in SAS Version 8.2 (SAS, Inc., Cary, N.C.), with volumes for the two hemispheres as the repeated measure within subjects. All models were adjusted for age. We used backward hierarchical selection to remove nonsignificant (p>0.05) interaction terms, starting with the following initial model for volume:
volume = age + diagnosis + gender + hemisphere + diagnosis-by-gender + diagnosis-by-hemisphere + gender-by-hemisphere + gender-by-diagnosis-by-hemisphere.
In this procedure, selection began by testing the three-way interaction, dropping that term if it was nonsignificant, then dropping in order the least significant two-way interactions, until only significant interactions and main effects remained. All main effects were retained in the final model. Least squares means and their standard errors were estimated for brain volumes within levels of one factor, averaging over the other terms in the model; where significant interactions were present, least squares means were estimated for all combinations of levels of the factors involved in the interaction. The p values were not adjusted for multiple comparisons. Neither total brain volume nor total cranial volume was included in these models, since there were no significant effects for these measures other than gender differences.
To examine patterns of association among heteromodal regions within each hemisphere, Pearson’s partial correlations were computed between the different gray matter volumes. Each pairwise correlation was adjusted for all other volumes considered, to examine the independent association of the two volumes. These correlations were calculated separately for the comparison subjects and for the patients with schizophrenia. To compare the magnitude of correlations between the two groups, Fisher’s z transformation, z(r)=0.5×log[(1+r)/(1–r)], was applied to each of the compared correlations. The significance of the difference between correlations was then tested by using the asymptotically normal statistic T=(zpatients – zcomparison subjects)/[(1/npatients – 3)+(1/ncomparison subjects – 3)]2(24).
Results
Demographic and clinical characteristics and global brain measurements are presented in Table 1. The comparison group had a greater percentage of female subjects (χ2=4.24, df=1, p=0.04) and was significantly younger (t=–2.88, df=76, p=0.005) than the schizophrenia group. The main effect for diagnosis was not significant for total brain volume (F=0.20, df=1, 74, p=0.65) or total cranial volume (F=0.11, df=1, 74, p=0.74). Male subjects had a larger mean total brain volume (F=38.2, df=1, 74, p=0.0001) and total cranial volume (F=37.7, df=1, 74, p=0.0001). The main effect for age and the gender-by-diagnosis interactions were not significant.
Between-Group Comparisons of Brain Region Volumes
Group mean volumes are presented in Table 2. If there was a statistically significant diagnosis-by-gender or diagnosis-by-hemisphere interaction (p<0.05), then least square means by diagnosis are reported separately within each level of the factor involved in the interaction.
Prefrontal region
There were no significant group differences for either the prefrontal total gray or white matter, the middle prefrontal cortex, and the orbital prefrontal cortex. There was a statistically significant diagnosis-by-hemisphere interaction for superior prefrontal cortex gray matter volumes (F=4.22, df=1, 74, p<0.05). However, group differences were not significant for either hemisphere. There was also a statistically significant diagnosis-by-gender interaction for inferior prefrontal cortex gray matter volume (F=4.67, df=1, 73, p<0.04). The male comparison subjects had significantly larger inferior prefrontal cortex volumes than the male patients with schizophrenia. There were no significant differences between the female comparison subjects and the female patients with schizophrenia.
Superior temporal gyrus
There were no significant group differences, diagnosis-by-hemisphere, or diagnosis-by-gender interactions for either superior temporal gyrus gray or white matter volume.
Inferior parietal cortex
There were statistically significant diagnosis-by-hemisphere interactions for inferior parietal cortex total gray matter (F=9.28, df=1, 74, p=0.003), inferior parietal cortex total white matter (F=5.19, df=1, 74, p=0.03), and supramarginal gyrus (F=14.0, df=1, 74, p=0.0004) volumes. Left inferior parietal cortex gray matter volume was significantly larger in the comparison subjects than in the patients with schizophrenia. No significant group difference was found for right inferior parietal cortex gray matter volume. No significant group differences were found for either left or right inferior parietal cortex white matter volume. The right supramarginal gyrus was significantly larger in patients with schizophrenia. Comparison subjects had larger left supramarginal gyrus volumes, but the difference between groups only approached significance. There were no significant group differences and no significant diagnosis-by-hemisphere or diagnosis-by-gender interactions for the angular gyrus.
Brain Region Volumes by Age and Gender
In the combined group of patients and comparison subjects, significant age effects were found for prefrontal total gray matter (F=5.05, df=1, 74, p=0.03) and superior prefrontal cortex (F=9.71, df=1, 74, p=0.003) (Table 3). All other age effects were nonsignificant (p>0.10). In general, brain region volumes were significantly larger in male subjects than in female subjects, although this difference was not statistically significant for the inferior prefrontal cortex, inferior parietal cortex total gray matter, inferior parietal cortex total white matter, or supramarginal regions.
Brain Region Volumes by Hemisphere
In the combined group of patients and comparison subjects, brain region volume differences between hemispheres were similar in male subjects and female subjects for all regions examined (minimum p value >0.085 for gender-by-hemisphere interactions).
Prefrontal region
A significant diagnosis-by-hemisphere interaction was found for the superior prefrontal cortex (see earlier discussion). The comparison subjects had larger right versus left superior prefrontal cortex volumes (right: mean=24.1, SD=0.5; left: mean=22.8, SD=0.6) (t=–3.67, df=73, p=0.0005), but the patients with schizophrenia showed no hemispheric differences (t=–0.26, df=74, p=0.80). In the combined group of patients and comparison subjects, a significant main effect of hemisphere was found for orbital prefrontal cortex volume (right: mean=11.3, SD=0.3; left: mean=11.0, SD=0.2) (t=–2.57, df=74, p<0.02). There were no main hemisphere effects for the other two prefrontal regions (p>0.12).
Superior temporal gyrus
In the combined group of patients and comparison subjects, no main effect of hemisphere was found for superior temporal gyrus gray matter volume. A significant main effect of hemisphere was found for superior temporal gyrus white matter volumes, with right white matter volumes greater than left white matter volumes (right: mean=5.6, SD=0.4; left: mean=5.5, SD=0.2) (t=–2.04, df=74, p=0.04).
Inferior parietal cortex
Significant diagnosis-by-hemisphere interactions were found for inferior parietal cortex total gray matter, inferior parietal cortex total white matter, and supramarginal gyrus volumes (see earlier discussion). The patients with schizophrenia had larger right than left inferior parietal cortex total gray matter volumes (right: mean=10.5, SD=0.4; left: mean=9.4, SD=0.3) (t=–3.10, df=74, p=0.003) and larger right than left inferior parietal cortex total white matter volumes (right: mean=6.6, SD=0.2; left: mean=6.1, SD=0.2) (t=–2.05, df=74, p=0.04). The hemispheric effect was nonsignificant in the comparison subjects for inferior parietal cortex total gray matter volume (right: mean=10.0, SD=0.4; left: mean=10.5, SD=0.4) (t=1.33, df=74, p=0.18) and for inferior parietal cortex total white matter volume (right: mean=6.1, SD=0.3; left: mean=6.4, SD=0.2) (t=1.23, df=74, p=0.22). The laterality index for the combined volumes of the inferior parietal cortex total gray and total white matter for each study subject is presented in Figure 4.
The comparison subjects had larger left than right supramarginal volumes (right: mean=5.1, SD=0.3; left: mean=6.0, SD=0.3) (t=3.14, df=74, p=0.002), whereas the patients with schizophrenia had larger right than left supramarginal volumes (right: mean=5.9, SD=0.3; left: mean=5.3, SD=0.2) (t=–2.10, df=74, p=0.04).
A significant main effect of hemisphere was found for angular gyrus volume, with the right volume greater than the left volume in the combined group of patients and comparison subjects (right: mean=4.8, SD=0.2; left: mean=4.3, SD=0.1) (t=–3.21, df=74, p=0.002).
Relationships Among Heteromodal Regions
The correlations among the heteromodal region volumes in the comparison subjects and the patients with schizophrenia are presented in Table 4 and Table 5.
Left hemisphere
The volumes of the superior prefrontal cortex and orbital prefrontal cortex were significantly correlated in both the healthy comparison subjects (partial r=0.39, df=76, p<0.05) and the patients with schizophrenia (partial r=0.36, df=76, p<0.05) (Table 4). In the comparison subjects, a significant relationship was found between orbital prefrontal cortex and superior temporal gyrus volumes (partial r=0.50, df=76, p<0.01). The correlation was not significantly different from that observed in the patients with schizophrenia.
In the patients with schizophrenia, significant correlations were found between the inferior prefrontal cortex and angular gyrus volumes (partial r=0.34, df=76, p<0.05) and between the supramarginal and angular gyri volumes (partial r=0.34, df=76, p<0.05). The correlation between the inferior prefrontal cortex and angular gyrus volumes in the patients with schizophrenia was significantly different from that observed in the comparison subjects (partial r=–0.14) (z=–2.08, p<0.04).
Right hemisphere
The superior prefrontal cortex and orbital prefrontal cortex volumes (partial r=0.44, df=76, p<0.05) and the superior temporal gyrus and orbital prefrontal cortex volumes (partial r=0.38, df=76, p<0.05) were significantly correlated in the comparison subjects (Table 5). Neither correlation was significantly different from that observed in the patients with schizophrenia.
In the patients with schizophrenia, the superior prefrontal cortex and middle prefrontal cortex volumes (partial r=0.42, df=76, p<0.01), the inferior prefrontal cortex and middle prefrontal cortex volumes (partial r=0.33, df=76, p<0.05), and the supramarginal and angular gyri volumes (partial r=0.34, df=76, p<0.05) were significantly correlated. None of these correlations was significantly different from that observed in the comparison subjects.
The relationship between inferior prefrontal cortex and angular gyrus volumes was not significant in the comparison subjects (partial r=–0.35, df=76, p<0.10) or in the patients with schizophrenia (partial r=0.26, df=76, p>0.10). In order to examine the hemispheric specificity of the differential relationship between left inferior prefrontal cortex and angular gyrus volumes, we compared the correlations between the volumes of these two structures. The two correlations were significantly different (z=–2.64, p=0.008).
Discussion
In the current study, patients with schizophrenia were observed to have selective structural abnormalities of the prefrontal and inferior parietal heteromodal cortices, relative to healthy comparison subjects. Male patients exhibited significantly smaller volumes bilaterally in the inferior prefrontal cortex. In our previous study, we also found that volume of this region differentiated the two groups but we did not observe a gender effect because of the small number of female subjects (6). No other prefrontal region volume differentiated the two groups.
Several previous studies have examined the inferior prefrontal region. Despite marked methodological differences, these studies have shown a consistent demonstration of altered structure in patients with schizophrenia, relative to healthy comparison subjects (7–11). Two studies found smaller volumes of this region in male patients with schizophrenia. Wible and colleagues (7) found smaller volumes in both the left and right inferior frontal gyrus. The magnitude of the group differences was similar to those in the current study: volumes were 10.5% smaller for the left inferior frontal gyrus and 8.3% smaller for the right inferior frontal gyrus in the patients with schizophrenia. In the study by Sanfilipo and colleagues (11), the inferior lateral region, which would partly correspond to the inferior prefrontal region examined in our study, was bilaterally smaller in male patients with schizophrenia. Neither of these two previous studies included female subjects. In contrast, Crespo-Facorro and colleagues (9) failed to find left or right inferior prefrontal cortex volumetric differences between first-episode patients with schizophrenia and healthy comparison subjects. Goldstein and colleagues (8) failed to find a group difference in inferior frontal gyrus volume, but they did not present the data separately by hemisphere. Three studies used voxel-based analytic procedures based on normalized, reformatted images. Two found lower signal intensity in the left inferior prefrontal gyrus in patients with schizophrenia (25, 26), and one found lower signal intensity in the right inferior prefrontal gyrus in patients with schizophrenia (26). In a predominantly male group of subjects with schizophrenia, Sigmundsson and colleagues (27) found an area of lower signal intensity in the left perisylvian region, which included part of the inferior prefrontal region (i.e., Brodmann’s area 44).
In this study the patients with schizophrenia had a significant reversal of the normal asymmetry of the inferior parietal cortex. The healthy comparison subjects were characterized by larger left inferior parietal total gray and white matter volumes, with the difference due largely to a marked laterality effect for the supramarginal gyrus. The reversal of asymmetry was observed in both male and female patients with schizophrenia. The pattern in the comparison subjects is consistent with previous postmortem and MRI observations of increased size of this region in right-handed subjects in nonpsychiatric populations (28, 29). In an MRI study of 148 healthy subjects (55% of whom were female), Raz and colleagues (29) observed a highly significant leftward asymmetry of the inferior parietal cortex and the absence of any gender differences. In contrast, in a group of 30 subjects, Frederikse and colleagues (30) observed the leftward asymmetry only in male healthy comparison subjects.
Two previous studies have observed reversal of the normal leftward asymmetry of the inferior parietal cortex. Frederikse and colleagues (15) used an image analytic procedure similar to the one used in the current study. In accordance with the current study findings, they observed that both male and female patients with schizophrenia exhibited a rightward asymmetry of the inferior parietal cortex. However, only the male comparison subjects in their study exhibited the normal leftward asymmetry. The female comparison subjects had rightward asymmetry of this region. The observation of rightward asymmetry of the inferior parietal cortex in the female comparison subjects is in contrast to the current results and to those of Raz and colleagues (29). In the other study, Niznikiewicz and colleagues (16) also found a reversal of the normal asymmetry of the inferior parietal cortex in patients with schizophrenia. Their study was limited to male subjects. In the patients with schizophrenia, the rightward asymmetry was the result of hemispheric differences in volume for both the supramarginal and angular gyri, whereas the leftward asymmetry of the healthy comparison subjects was almost completely due to the marked leftward asymmetry of the angular gyrus. In the current study, a similar pattern was seen in the patients with schizophrenia, but the leftward asymmetry of the comparison subjects was due to marked leftward asymmetry of the supramarginal gyrus. Other studies of the inferior parietal cortex have not directly assessed the issue of abnormal inferior parietal cortex asymmetry. Schlaepfer and colleagues (4) reported smaller volumes of this region in patients with schizophrenia, relative to healthy comparison subjects, with group differences most prominent in female subjects (4), but hemispheric differences were not described. Goldstein and colleagues (8) failed to find group differences in inferior parietal cortex total gray matter or angular gyrus volumes, but they did find smaller volumes bilaterally in the posterior portion of the supramarginal gyrus in the patients with schizophrenia.
We found no significant group differences in superior temporal gyrus gray or white matter volumes. These results are in contrast with our previous study, which used the same anatomical landmarks for this region (31), and with the majority of other studies examining superior temporal gyrus volumes (3). However, several studies have failed to find a significant group difference for this region (8, 13, 14). The lack of replication has been attributed to the use of combined gray and white matter superior temporal gyrus measures rather than separate evaluation of superior temporal gyrus gray matter volume (3). In addition to our study, the study by Goldstein and colleagues (8) also used a separate measure for superior temporal gyrus gray matter volume and failed to find a group difference. The patient characteristics that might contribute to the variability in study results are unclear.
There were few significant correlations of volume among the different heteromodal regions in either the healthy comparison subjects or the patients with schizophrenia. The only significant between-group differences in the correlations were for the correlation between left inferior prefrontal region volume and angular gyrus volume and the correlation between right inferior prefrontal region volume and angular gyrus volume.
Patients with schizophrenia are characterized by a broad range of language and hemispheric lateralization abnormalities (3, 32, 33). Crow (34) has hypothesized that these abnormalities may represent the core features of schizophrenia. The current study suggests that these abnormalities may be reflected in structural alterations of the heteromodal cortical areas comprising the language neural circuit (1). Male patients with schizophrenia had smaller volumes of the left inferior prefrontal region, which consists primarily of Broca’s area. Male and female patients had abnormal asymmetry of the inferior parietal cortex. In right-handed nonpsychiatric subjects, whose language abilities are located in the left hemisphere, the left inferior parietal cortex is larger than the right inferior parietal cortex. Although the precise location of Wernicke’s area is not known, it is thought to include the inferior aspects of the supramarginal and angular gyri (1). Furthermore, the only correlations for which there were significant group differences were correlations between the inferior prefrontal region and the angular gyrus. In combination, these results suggest that patients with schizophrenia are characterized by abnormal lateralization of the structure and function of the cerebral hemispheres, which leads to abnormalities in language behavior and is reflected in the differential failure of the normal development of the heteromodal language areas.
The current study has several potential limitations. First, the MEASURE image analysis procedure is dependent on the use of reliable rule-based anatomical definitions that conform to local/individual anatomy rather than arbitrary anatomical cut planes. If local neuroanatomy is more variable due to developmental distortions of the usual sulcal gyral patterns in patients with schizophrenia than in healthy comparison subjects (35), then this variability could lead to differential measurement in the two groups. The high region-of-interest interrater reliability suggests that this potential limitation is not a major concern. Second, the use of the paint method to demarcate the brain regions may be subject to possible inaccuracies, especially in cases of atrophy, where the gray/white and gray/CSF boundaries account for a proportionately greater amount of total gray tissue. Third, the prefrontal cortical regions, other than the inferior prefrontal region, are relatively large and may obscure smaller subregion differences. Fourth, we did not assess the planum temporale, which is part of Wernicke’s area. The failure to measure this region precludes our ability to fully evaluate the heteromodal cortical regions involved in the language neural circuit. Finally, language measures were not available to allow examination of whether the observed structural abnormalities were associated with impairments in language ability.
In summary, we have found evidence for the selective disruption of heteromodal association cortical areas involved in the neuroanatomy of language in patients with schizophrenia. Future studies are needed to delineate the pathophysiological process(es) underlying the structural changes of these brain regions.
 |
 |
 |
 |
 |
Received Feb. 21, 2003; revision received July 3, 2003; accepted July 10, 2003. From the Maryland Psychiatric Research Center; the Department of Psychiatry, Hospital G.U. Gregorio Marañón, Madrid, Spain; the Department of Radiology, University of Maryland School of Medicine, Baltimore; the Division of Psychiatric Neuroimaging, Department of Psychiatry, Johns Hopkins University School of Medicine, Baltimore; and the Department of Psychiatry, Yale University, New Haven, Conn. Address reprint requests to Dr. Buchanan, Maryland Psychiatric Research Center, P.O. Box 21247, Catonsville, MD 21228; [email protected] (e-mail). Supported in part by NIMH grants MH-48225 and MH-40279.
 |
APPENDIX 1.
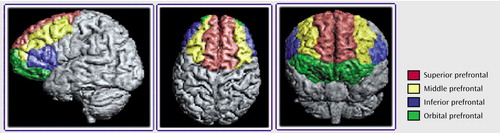
Figure 1. Boundaries of Regions of Interest in the Prefrontal Cortex Traced for a Morphometric Study of the Heteromodal Association Cortex in Schizophrenia
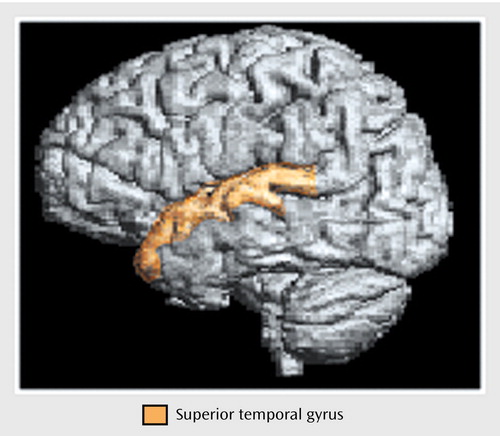
Figure 2. Boundaries of the Superior Temporal Gyrus Traced for a Morphometric Study of the Heteromodal Association Cortex in Schizophrenia
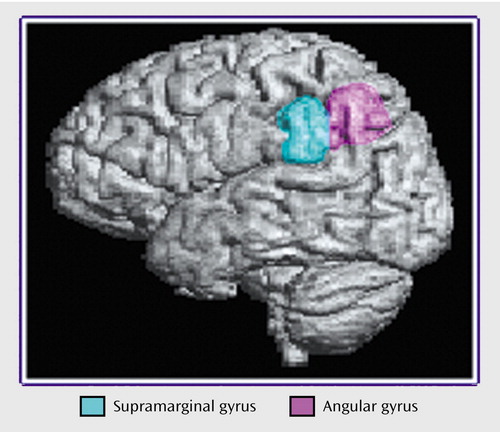
Figure 3. Boundaries of Regions of Interest in the Inferior Parietal Cortex Traced for a Morphometric Study of the Heteromodal Association Cortex in Schizophrenia
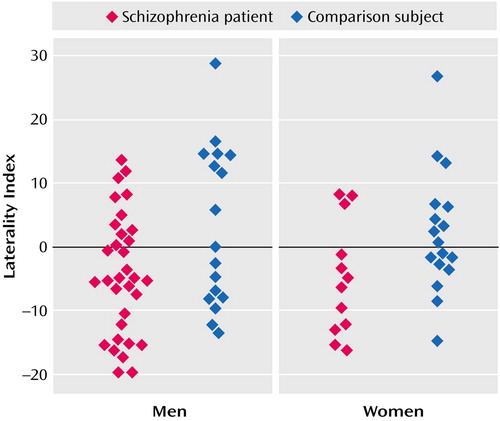
Figure 4. Laterality Index for Inferior Parietal Cortex Volume in Patients With Schizophrenia and Healthy Comparions Subjects, by Gendera
aLaterality index = [(left inferior parietal cortex total volume – right inferior parietal cortex total volume) / (left inferior parietal cortex total volume + right inferior parietal cortex total volume)] × 100. Positive values indicate leftward asymmetry and negative values indicate rightward asymmetry.
1. Mesulam M: Principles of Behavioral and Cognitive Neurology, 2nd ed. New York, Oxford University Press, 2000Google Scholar
2. Pearlson GD, Petty RG, Ross CA, Tien AY: Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology 1996; 14:1–7Crossref, Medline, Google Scholar
3. Shenton ME, Dickey CC, Frumin M, McCarley RW: A review of MRI findings in schizophrenia. Schizophr Res 2001; 49:1–52Crossref, Medline, Google Scholar
4. Schlaepfer TE, Harris GJ, Tien AY, Peng LW, Lee S, Federman EB, Chase GA, Barta PE, Pearlson GD: Decreased regional cortical gray matter volume in schizophrenia. Am J Psychiatry 1994; 151:842–848Link, Google Scholar
5. Woodruff PWR, Wright IC, Shuriquie N, Russouw H, Rushe T, Howard RJ, Graves M, Bullmore ET, Murray RM: Structural brain abnormalities in male schizophrenics reflect fronto-temporal dissociation. Psychol Med 1997; 27:1257–1266Crossref, Medline, Google Scholar
6. Buchanan RW, Vladar K, Barta PE, Pearlson GD: Structural evaluation of the prefrontal cortex in schizophrenia. Am J Psychiatry 1998; 155:1049–1055Link, Google Scholar
7. Wible CG, Shenton ME, Fischer IA, Allard JE, Kikinis R, Jolesz FA, Iosifescu DV, McCarley RW: Parcellation of the human prefrontal cortex using MRI. Psychiatry Res 1997; 76:29–40Crossref, Medline, Google Scholar
8. Goldstein JM, Goodman JM, Seidman LJ, Kennedy DN, Makris N, Lee H, Tourville J, Caviness VS, Faraone SV, Tsuang MT: Cortical abnormalities in schizophrenia identified by structural magnetic resonance imaging. Arch Gen Psychiatry 1999; 56:537–547Crossref, Medline, Google Scholar
9. Crespo-Facorro B, Kim J, Andreasen NC, O’Leary DS, Magnotta V: Regional frontal abnormalities in schizophrenia: a quantitative gray matter volume and cortical surface size study. Biol Psychiatry 2000; 48:110–119Crossref, Medline, Google Scholar
10. Gur RE, Cowell PE, Latshaw A, Turetsky BI, Grossman RI, Arnold SE, Bilker WB, Gur RC: Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry 2000; 57:761–768Crossref, Medline, Google Scholar
11. Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Feiner D, Rotrosen J, Wolkin A: Volumetric measure of the frontal and temporal lobe regions in schizophrenia. Arch Gen Psychiatry 2000; 57:471–480Crossref, Medline, Google Scholar
12. Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JMJ, Ashtari M, Wu H, Lieberman JA: Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res 1999; 90:1–15Crossref, Medline, Google Scholar
13. DeLisi LE, Hoff AL, Neale C, Kushner M: Asymmetries in the superior temporal lobe in male and female first-episode schizophrenic patients: measures of the planum temporale and superior temporal gyrus by MRI. Schizophr Res 1994; 12:19–28Crossref, Medline, Google Scholar
14. Kulynych JJ, Vladar K, Jones DW, Weinberger DR: Superior temporal gyrus volume in schizophrenia: a study using MRI morphometry assisted by surface rendering. Am J Psychiatry 1996; 153:50–56Link, Google Scholar
15. Frederikse M, Lu A, Aylward E, Barta P, Sharma T, Pearlson G: Sex differences in inferior parietal lobule volume in schizophrenia. Am J Psychiatry 2000; 157:422–427Link, Google Scholar
16. Niznikiewicz M, Donnino R, McCarley RW, Nestor PG, Iosifescu DV, O’Donnell B, Levitt J, Shenton ME: Abnormal angular gyrus asymmetry in schizophrenia. Am J Psychiatry 2000; 157:428–437Link, Google Scholar
17. Tien AY, Eaton WW, Schlaepfer TE, McGilchrist IK, Menon R, Powers R, Aylward E, Barta P, Strauss ME, Pearlson GD: Exploratory factor analysis of MRI brain structure measures in schizophrenia. Schizophr Res 1996; 19:93–101Crossref, Medline, Google Scholar
18. Rademacher J, Galaburda AM, Kennedy DN, Filipek PA, Caviness VS Jr: Human cerebral cortex: localization, parcellation, and morphometry with magnetic resonance imaging. J Cogn Neurosci 1992; 4:352–374Crossref, Medline, Google Scholar
19. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
20. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1997Google Scholar
21. Barta PE, Dhingra L, Royall R, Schwartz E: Improving stereological estimates for the volume of structures identified in three-dimensional arrays of spatial data. J Neurosci Methods 1997; 75:111–118Crossref, Medline, Google Scholar
22. Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ: Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS 1988; 96:379–394Crossref, Medline, Google Scholar
23. Ono M, Kubik S, Abernathey CD: Atlas of the Cerebral Sulci. New York, Thieme Medical Publishers, Inc., 1990Google Scholar
24. Kleinbaum D, Kupper L, Miller K: Applied Regression Analysis and Other Multivariable Methods, 2nd ed. Boston, PWS-Kent, 1988, pp 91–93Google Scholar
25. Ananth H, Popescu I, Critchley HD, Good CD, Frackowiak RSJ, Dolan RJ: Cortical and subcortical gray matter abnormalities in schizophrenia determined through structural magnetic resonance imaging with optimized volumetric voxel-based morphometry. Am J Psychiatry 2002; 159:1497–1505Link, Google Scholar
26. Suzuki M, Nohara S, Hagino H, Kurokawa K, Yotsutsuji T, Kawasaki Y, Takahashi T, Matsui M, Watanabe N, Seto H, Kurachi M: Regional changes in brain gray and white matter in patients with schizophrenia demonstrated with voxel-based analysis of MRI. Schizophr Res 2002; 55:41–54Crossref, Medline, Google Scholar
27. Sigmundsson T, Suckling J, Maier M, Williams SCR, Bullmore ET, Greenwood KE, Fukuda R, Ron MA, Toone BK: Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry 2001; 158:234–243Link, Google Scholar
28. Geschwind N, Galaburda AM: Cerebral lateralization: biological mechanisms, associations, and pathology, I: a hypothesis and a program for research. Arch Neurol 1985; 42:428–459Crossref, Medline, Google Scholar
29. Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD: Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 1997; 7:268–282Crossref, Medline, Google Scholar
30. Frederikse ME, Lu A, Aylward E, Barta P, Pearlson G: Sex differences in the inferior parietal lobule. Cereb Cortex 1999; 9:896–901Crossref, Medline, Google Scholar
31. Bryant NL, Buchanan RW, Vladar K, Breier A, Rothman M: Gender differences in temporal lobe structures of patients with schizophrenia: a volumetric MRI study. Am J Psychiatry 1999; 156:603–609Abstract, Google Scholar
32. Goldberg TE, Aloia MS, Gourovitch M, Missar D, Pickar D, Weinberger DR: Cognitive substrates of thought disorder, I: the semantic system. Am J Psychiatry 1998; 155:1671–1676Link, Google Scholar
33. Sommer IEC, Ramsey NF, Kahn RS: Language lateralization in schizophrenia, an fMRI study. Schizophr Res 2001; 52:57–67Crossref, Medline, Google Scholar
34. Crow TJ: Is schizophrenia the price that Homo sapiens pays for language? Schizophr Res 1997; 28:127–141Crossref, Medline, Google Scholar
35. Kikinis R, Shenton ME, Gerig G, Hokama H, Haimson J, O’Donnell BF, Wible CG, McCarley RW, Jolesz FA: Temporal lobe sulco-gyral pattern anomalies in schizophrenia: an in vivo MR three-dimensional surface rendering study. Neurosci Lett 1994; 182:7–12Crossref, Medline, Google Scholar



