Effects of Alcoholism and Gender on Brain Metabolism
Abstract
OBJECTIVE: Proton magnetic resonance spectroscopy was used to evaluate gender influences on alcohol-associated changes in brain metabolism. METHOD: Concentrations of N-acetylaspartate, choline-containing compounds, myo-inositol, and creatine plus phosphocreatine in frontal lobe gray matter and white matter were estimated in eight women and 17 men who were recently detoxified from long-term alcoholism. Twelve women and 13 men with no history of alcoholism were used as a comparison group. RESULTS: In male and female alcoholics, frontal lobe white matter concentrations of N-acetylaspartate were significantly lower (–8.8%) than those seen in nonalcoholic comparison subjects. In the frontal lobe gray matter region, a significant alcoholism status-by-gender interaction and follow-up analyses revealed that female alcoholics had significantly lower N-acetylaspartate concentrations (–10.73%) relative to female comparison subjects, while male alcoholics and male comparison subjects had similar levels of this metabolite (<1% difference). CONCLUSIONS: Lower concentrations of white matter N-acetylaspartate, which may indicate neuronal loss or dysfunction, is equally severe in men and women with comparable alcohol abuse histories. However, female alcoholics exhibited significantly less N-acetylaspartate in frontal gray matter relative to female nonalcoholic comparison subjects, which could mean that female alcoholics are more susceptible to gray matter injury than their male counterparts. However, this finding could also be explained by higher-than-expected levels of N-acetylaspartate in the healthy female comparison group.
It is unclear whether there are sex differences in susceptibility to alcohol-induced brain damage. This is highlighted by two recent quantitative magnetic resonance imaging (MRI) studies with conflicting results. In one study, male alcoholics had more brain volume loss in both cortical gray and white matter compared with both alcoholic women and individuals with no history of alcoholism (1). Alternatively, results of another MRI study revealed that female alcoholics were more sensitive than their male counterparts to brain white matter volume loss (2).
In order to evaluate gender differences in alcohol-associated alterations in brain biochemistry, we used proton magnetic resonance spectroscopy (MRS) to measure N-acetylaspartate. N-Acetylaspartate is a marker of neuronal loss or injury as well as mitochondrial dysfunction (3) that can be measured by using MRS. Other quantified metabolites included choline-containing compounds, creatine plus phosphocreatine, and myo-inositol.
Women are thought to achieve higher blood alcohol concentrations than men given the same intake of alcohol (4). Thus, female alcoholics with comparable drinking histories to their male counterparts may experience greater toxicity. Given this potential for differential alcohol toxicity, we hypothesized that female alcoholic individuals in short-term abstinence would have lower concentrations of N-acetylaspartate in frontal lobe white matter and frontal lobe gray matter regions of interest relative to male alcoholics with similar drinking histories.
Method
Twenty-five recently detoxified alcoholics (eight women and 17 men) and 25 nonalcoholic comparison subjects (12 women and 13 men) were recruited from the VA San Diego Healthcare System Alcohol and Drug Treatment Program, Scripps McDonald Center, and surrounding communities. Participants provided written informed consent. The mean ages of the female and male recently detoxified alcoholics were 43.3 years (SD=7.9) and 36.2 years (SD=5.8), respectively, while the female and male comparison subjects were a mean of 35.8 (SD=8.3) and 38.8 (SD=10.0) years old. The recently detoxified alcoholic women were abstinent an average of 39.7 days (SD=20.1) and met our criteria for alcoholism for an average of 10.8 years (SD=4.1) (lifetime intake of ethanol: mean=660.3 kg, SD=235.4). The recently detoxified alcoholic men were abstinent a mean of 26.2 days (SD=11.1) and had a mean of 10.6 years (SD=4.8) of alcoholism (lifetime intake of ethanol: mean=668.2 kg, SD=256.4). The recently detoxified alcoholic women had been abstinent significantly longer than their male counterparts (t=2.16, df=23, p=0.04). A year of alcoholism was defined as 1 year of drinking when an individual consumed, on average, at least 42 drinks per week for an entire calendar year. One drink was considered to be equivalent to one 12-ounce can of beer, 4 ounces of wine, or 1.5 ounces of spirits, each containing approximately 12.5 grams of pure ethanol.
Participants were excluded if they had a history of neurological disease or systemic illness, head injury with loss of consciousness greater than 15 minutes, or history of substance dependence other than alcohol. We verified alcohol abstinence and absence of other drug use with urine toxicology. White matter metabolite data from male participants in this study represent a subgroup used in a previously published report (5).
As seen in Figure 1, two regions of interest were acquired and placed as previously reported (6). Spectra were collected by using a clinical General Electric (GE, Fremont, Calif.) 1.5 Tesla scanner (Signa LX) at the VA San Diego Healthcare System. We used a standard GE short-echo point resolved spectroscopic sequence (echo time=35 msec, repetition time=3000 msec) to acquire data. Sixty-four acquisitions were averaged for each 20-mm3 region of interest.
Spectral analysis was completed as previously reported (5) by using LCModel software, which allows automatic quantitation of metabolite concentrations (7). Free-induction decays were zero-filled to double the points, filtered with a finite discrete convolution to account for field inhomogeneities and eddy currents, and phase corrected. Figure 2 displays an example spectrum. Absolute concentrations of N-acetylaspartate, myo-inositol, choline-containing compounds, and creatine plus phosphocreatine were obtained by scaling the spectrum to the unsuppressed water peak and are reported in “institutional units” (IU). CSF content in each region of interest was estimated for partial volume corrections by fitting a double exponential function to the first point of the free induction decay at eight echo times (30, 35, 40, 62, 100, 200, 500, and 1500 msec) (8). The metabolites were normalized to 100% brain tissue for each region with the following formula: C=Co × (1/[1–FCSF]), where C=concentration, Co=metabolite concentration from LCModel output, and FCSF=estimated CSF content. This correction ensures that observed changes represent alterations in metabolite concentration rather than between-group differences in the proportion of tissue in each region of interest (9).
Results
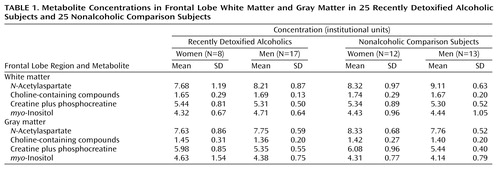
Analysis of covariance (ANCOVA) with two between-subjects factors (alcohol use status and gender) and age as a covariate was used to evaluate the role of gender on alcohol-associated changes in brain metabolism. ANCOVA was used to adjust for group age differences. Means and standard deviations of the metabolites are presented in Table 1.
The ANCOVA was significant (F=3.42, df=4, 45, p<0.02; eta2=0.23) for concentrations of N-acetylaspartate in frontal white matter, with significant main effects seen for alcoholism (F=8.1, df=1, 45, p<0.007) and gender (F=5.9, df=1, 45, p<0.02). The results indicated that regardless of gender, alcoholics had significantly lower N-acetylaspartate. In addition, female subjects had significantly lower N-acetylaspartate concentrations in white matter regardless of alcoholism status. The interaction term (p=0.64) and age covariate (p=0.97) were not significant predictors of N-acetylaspartate. No significant group main effects or interactions were found for choline, creatine plus phosphocreatine, or myo-inositol.
The ANCOVA was significant (F=3.81, df=4, 45, p<0.01; eta2=0.25) for concentrations of N-acetylaspartate in frontal gray matter. The alcoholism status-by-gender interaction was significant (F=6.52, df=1, 45, p<0.02). Simple effects analyses (Tukey honestly significant difference) revealed that recently detoxified alcoholic women had significantly lower N-acetylaspartate concentrations relative to healthy comparison women (mean difference=0.91, 95% confidence interval [CI]=0.13 to 1.69; p<0.05). However, recently detoxified alcoholic men and healthy comparison men had comparable levels of frontal gray matter N-acetylaspartate (mean difference=–0.06, 95% CI=–0.51 to 0.40; p>0.05). Significant main effects were also seen for alcoholism status (F=5.49, df=1, 45, p<0.03) and age (F=6.07, df=1, 45, p<0.02) but not gender.
The ANCOVA was significant for concentrations of frontal gray matter creatine plus phosphocreatine (F=3.01, df=4, 45, p<0.03; eta2=0.21). A significant main effect of gender was found (F=8.66, df=1, 45, p<0.006); regardless of alcoholism status, female subjects had significantly higher levels of creatine plus phosphocreatine than did male subjects. No significant main effects were seen for alcoholism, the alcohol status-by-gender interaction, or age for this metabolite. Further, no significant group main effects or interactions were found for choline or myo-inositol.
Discussion
Recently detoxified male and female alcoholics had significant reductions in concentrations of N-acetylaspartate in frontal white matter relative to healthy comparison participants. These results corroborate our previous finding of reduced N-acetylaspartate in a sample consisting almost entirely of men (5) and do not suggest any unique vulnerability to alcoholism-associated white matter injury in women. However, a significant interaction between alcoholism and gender revealed that female alcoholics had significantly lower frontal gray matter N-acetylaspartate than did healthy female comparison subjects, whereas male alcoholics and male comparison subjects had similar levels of this metabolite. Since the significant gender-by-alcohol status interaction may be driven by female comparison subjects having higher N-acetylaspartate than male comparison subjects, it is possible our effect reflects sampling problems in a study with a small number of subjects. Alternatively, if it is true that female comparison subjects tend to have higher N-acetylaspartate in frontal gray matter than men, as has been reported previously (10), then the interaction might suggest differential vulnerability of frontal gray matter to alcoholism effects in women.
Concentrations of gray matter creatine plus phosphocreatine were higher in female participants than in male participants, regardless of alcohol use status. This finding is consistent with a previous spectroscopic imaging report that evaluated gender differences in metabolism in a similar region of interest (10). The result may reflect increased energy needs in the anterior cingulate gyrus of women (11) and highlights the importance of quantitative spectroscopy, which does not rely on the stability of creatine plus phosphocreatine when interpreting metabolic differences between groups.
 |
Presented in part at the 30th annual meeting of the International Neuropsychological Society, Toronto, Feb. 13–16, 2002. Received Dec. 26, 2001; revisions received July 16 and Dec. 7, 2002; accepted Dec. 13, 2002. From the Veterans Affairs San Diego Healthcare System; University of California, San Diego (UCSD), San Diego; and the San Diego State University/UCSD Joint Doctoral Program in Clinical Psychology, San Diego. Address reprint requests to Dr. Grant, Psychiatry Service (116A), VA San Diego Healthcare System, San Diego, CA 92161; [email protected] (e-mail). Supported by grants SA-325 from the Medical Research Service of the Department of Veterans Affairs and DA-12065 from the National Institute on Drug Abuse to Dr. Grant. The authors thank Dr. Fred Berger for his assistance in participant recruitment and Mr. Dexter Walpole for his assistance in data collection.
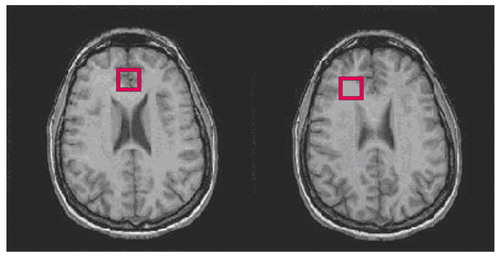
Figure 1. Frontal Gray Matter and Frontal White Matter Regions of Interest Examined in 25 Recently Detoxified Alcoholic Subjects and 25 Nonalcoholic Comparison Subjectsa
aThe image on the left shows the frontal gray matter region of interest, located in the midline just anterior to the genu of the corpus callosum. The image on the right shows the frontal white matter region of interest, located to the right of the anterior horn of the lateral ventricle.
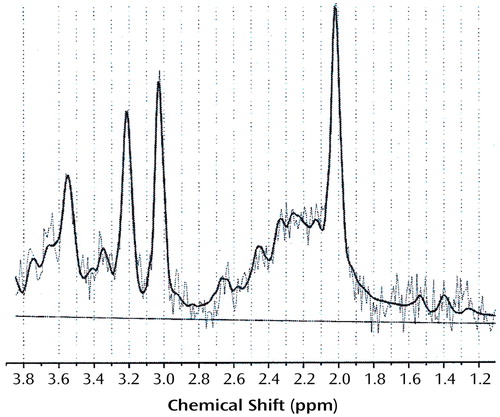
Figure 2. Representative Proton MRS Spectrum From the Frontal Gray Matter Region of Interest of a 54-Year-Old Recently Detoxified Alcoholic Womana
aThe straight line is the fitted baseline, and the bold line represents the fit for the underlying raw data determined with LCModel software. Chemical shifts for the metabolites examined in this study were as follows: N-acetylaspartate=2.02 ppm; choline-containing compounds=3.19 ppm; creatine plus phosphocreatine=3.02 ppm, myo-inositol=3.58 ppm.
1. Crosbie J, Schachar R: Deficient inhibition as a marker for familial ADHD. Am J Psychiatry 2001; 158:1884-1890Link, Google Scholar
2. Hommer DW, Momenan R, Kaiser E, Rawlings RR: Evidence for a gender-related effect of alcoholism on brain volumes. Am J Psychiatry 2001; 158:198-204Link, Google Scholar
3. Demougeot C, Garnier P, Mossiat C, Bertrand N, Giroud M, Beley A, Marie C: N-Acetylaspartate, a marker of both cellular dysfunction and neuronal loss: its relevance to studies of acute brain injury. J Neurochem 2001; 77:408-415Crossref, Medline, Google Scholar
4. Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS: High blood alcohol levels in women: the role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N Engl J Med 1990; 322:95-99Crossref, Medline, Google Scholar
5. Schweinsburg BC, Taylor MJ, Alhassoon O, Videen JS, Brown GG, Patterson TL, Berger F, Grant I: Chemical pathology in brain white matter of recently detoxified alcoholics: a 1H magnetic resonance spectroscopy investigation of alcohol-associated frontal lobe injury. Alcohol Clin Exp Res 2001; 25:924-934Crossref, Medline, Google Scholar
6. Schweinsburg BC, Taylor MJ, Videen JS, Alhassoon OM, Patterson TL, Grant I: Elevated myo-inositol in gray matter of recently detoxified but not long-term abstinent alcoholics: a preliminary MR spectroscopy study. Alcohol Clin Exp Res 2000; 24:699-705Crossref, Medline, Google Scholar
7. Provencher SW: Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993; 30:672-679Crossref, Medline, Google Scholar
8. Ernst T, Kreis R, Ross BD: Absolute quantitation of water and metabolites in the human brain, 1: compartments and water. J Magn Reson B 1993; 102:1-8Crossref, Google Scholar
9. McLean MA, Woermann FG, Barker GJ, Duncan JS: Quantitative analysis of short echo time (1)H-MRSI of cerebral gray and white matter. Magn Reson Med 2000; 44:401-411Crossref, Medline, Google Scholar
10. Grachev I, Apkarian A: Chemical heterogeneity of the living human brain: a proton MR spectroscopy study on the effects of sex, age, and brain region. Neuroimage 2000; 11:554-556Crossref, Medline, Google Scholar
11. Gur RC, Mozley LH, Mozley PD, Resnick SM, Karp JS, Alavi A, Arnold SE, Gur RE: Sex differences in regional cerebral glucose metabolism during a resting state. Science 1995; 267:528-531Crossref, Medline, Google Scholar



