Effects of Alcohol Dependence Comorbidity and Antipsychotic Medication on Volumes of the Thalamus and Pons in Schizophrenia
Abstract
OBJECTIVE: Postmortem and in vivo brain imaging studies have identified abnormalities in the thalamus and the pons in both schizophrenia and alcoholism. The authors sought to determine whether patients with both schizophrenia and alcohol dependence would manifest exaggerated volume deficits in either structure. METHOD: Volumetric measures of the left and right thalamus and the pons were derived from magnetic resonance imaging scans obtained from 27 patients with schizophrenia, 19 patients with schizophrenia and comorbid alcohol dependence, 25 patients with alcohol dependence without comorbid axis I disorders, and 51 healthy comparison subjects. RESULTS: The alcohol-dependent patients had significant volume deficits in both the thalamus and the pons. Among patients with schizophrenia, there were no differences in thalamus volumes between those with and without comorbid alcohol dependence. However, patients with schizophrenia who were taking atypical antipsychotic medications had bilateral thalamic deficits, whereas those taking typical neuroleptics did not. Patients with schizophrenia and comorbid alcohol dependence had deficits in the pons. CONCLUSIONS: Patients with schizophrenia and comorbid alcohol dependence are at risk for alcohol-related reduction of pontine structures that are not necessarily affected by schizophrenia per se. The effect of alcohol dependence on the thalamus in schizophrenic patients may be mitigated by the type of neuroleptic medication they receive.
The pons and thalamus are both critical nodes in multiple circuits linking the cerebellum to the motor and sensory cortices and to association areas in the frontal and prefrontal cortex that subserve higher-order behavior (1–3). The pons is primarily a white matter structure of the brainstem composed of several systems of large white matter tracts and nuclear relays. The thalamus is a highly differentiated gray matter structure that comprises many subnuclei, each with specialized functional links to multiple cortical sites. Both the pons and the thalamus are adversely affected by excessive alcohol consumption. Central pontine myelinosis has been associated with alcoholism (4), and its defining neuroradiological sign on clinical T2-weighted magnetic resonance imaging (MRI) scans is a hyperintense triangular lesion (5). Damage to the pontine system in uncomplicated alcoholism is seen in vivo with increased T2 relaxation times (6) and in pathological reports of a lower than normal number of cholinergic neurons in the locus ceruleus, a substructure of the pons (7–9). Alcohol also has deleterious effects on the thalamus that are observed as deficits in overall volume (10) and in the volumes of specific nuclei (11) and deficits in the number and size of neurons, particularly in the anterior thalamus (12). Furthermore, the size of the thalamus has been associated with odor identification ability in patients with alcoholism (13).
The pons has also been implicated in the pathophysiology of schizophrenia, although relatively few reports have examined this relationship, and evidence for an overall volume deficit has not emerged (14). However, pathology studies have found lower levels of choline acetyltransferase in the pons of patients with schizophrenia, relative to nonschizophrenic comparison subjects (15), and greater numbers of neurons in the pedunculopontine nucleus (16). In vivo studies have reported lower N-acetylaspartate/creatine ratios in the pons of patients with schizophrenia, relative to comparison subjects (17), but no pontine area deficit, as measured on a single sagittal slice (14). In contrast to the limited amount of research on the pons, more studies have examined the role of thalamic pathology in the development of schizophrenia (e.g., reference 18) and in the cognitive dysmetria associated with the disorder (e.g., reference 19). In vivo evidence for gross volume deficits in the whole thalamus has been mixed, although a meta-analysis identified a small-to-moderate, statistically significant effect size for thalamic volume deficits (20). Although evidence for gross thalamic volume deficits is equivocal, the evidence for an abnormally small size of specific nuclei, particularly the mediodorsal nucleus, in schizophrenia is stronger (21, 22). Further, in vivo spectroscopic studies have reported abnormally low levels of thalamic N-acetylaspartate/creatine in schizophrenia (23, 24).
Neuroleptic medication can affect thalamic volume. One study reported that the thalamus was smaller in neuroleptic-naive patients but enlarged in patients treated with neuroleptics and that the extent of enlargement was dose dependent regardless of whether the patients were treated with typical or atypical antipsychotic medications (25). Similar findings have been reported for other diencephalic structures, such as the basal ganglia (26, 27), with different effects related to the type of antipsychotic medication. In one study, caudate volumes, enlarged in patients treated with typical antipsychotic medications, declined after 24 weeks of treatment with atypical antipsychotic medications (28). In another study, mean basal ganglia volume increased over a 2-year period in patients predominantly treated with typical antipsychotic medications but decreased in patients predominantly treated with atypical antipsychotic medications (29). Structural neuroimaging studies have not reported differential effects of typical and atypical antipsychotic medications on thalamic volume. A recent positron emission (PET) study, however, reported that a typical antipsychotic medication induced significantly higher dopamine D2 receptor binding in the thalamus, compared with atypical antipsychotics (30), suggesting a mechanism by which the volume differences associated with the various antipsychotic medication types could occur.
Alcohol abuse and dependence occur with high prevalence in schizophrenia and pose exceptional risks of brain damage to patients with this disorder, especially in regions that are vulnerable to the toxic effects of alcohol. In regions already compromised by schizophrenia, such as the frontal lobes and the cerebellum, even relatively mild alcohol abuse has a particularly deleterious effect (31, 32). This compounding effect has also been demonstrated for ataxia, a behavioral manifestation of cerebellar damage (33). In this study, we sought to determine whether a compounded deleterious effect of alcoholism on schizophrenia holds true for either the pons or the thalamus—the principal intermediary nodes of the frontocerebellar circuit—and whether these effects may be mitigated by the type of antipsychotic medication patients receive.
Method
Study Participants
All subjects (Table 1) were men and gave written informed consent to participate in MRI research. A detailed description of the selection criteria and the clinical and demographic characteristics of the study subjects have been provided in earlier reports (31, 32). Patients with schizophrenia were recruited from an unlocked voluntary research ward at the Veterans Affairs (VA) Palo Alto Health Care System. The group with schizophrenia included 27 patients who met the DSM-III-R criteria for schizophrenia and did not meet the criteria for current or lifetime alcohol abuse or dependence or any other axis I diagnosis. The group with schizophrenia and comorbid alcohol dependence included 19 patients who met the DSM-III-R criteria for schizophrenia and who also met either the DSM-III-R criteria for alcohol dependence or the Research Diagnostic Criteria (as determined from the Schedule for Affective Disorders and Schizophrenia [36]) for alcoholism. Participants with a diagnosis of nonalcohol substance dependence, but not those with a diagnosis of nonalcohol substance abuse, were excluded. All but three of the patients with either schizophrenia alone or schizophrenia and comorbid alcohol dependence were currently taking antipsychotic medications: 29 were taking typical antipsychotic medications, and 13 were taking atypical antipsychotic medications. (Data on type of medication were missing for one patient.) The alcohol-dependent patients (N=25) were drawn from a group of patients who met the DSM-III-R or DSM-IV criteria for a diagnosis for alcohol dependence but not for schizophrenia or any other axis I disorder. These subjects had earlier undergone brain imaging while they were inpatients at the VA Palo Alto Health Care System (37) and were participating in follow-up studies that included administration of the Structured Clinical Interview for DSM-III-R or the Structured Clinical Interview for DSM-IV, medical screening, clinical review of MRI scans, and an updated assessment of alcohol consumption (38, 39). The median time between their last drink and MRI scanning was 204 days (range=1–1,994 days).
Healthy comparison participants (N=51), recruited from the community by advertisement and word of mouth, were screened to exclude any potential subjects with axis I disorder, substance abuse in the year before entry into the study, or consumption of more than four drinks a day for more than a month. Lifetime alcohol consumption of all subjects was assessed by using a semistructured interview based on an interview developed by Skinner (40, 41) and used previously by the authors (e.g., references 37, 42).
Group Comparisons of Demographic Variables
One-way analysis of variance (ANOVA) was used to test for demographic differences across the four groups. Significant differences were found for education (F=9.03, df=3, 118, p=0.0001), general intelligence estimated with the National Adult Reading Test (34) (F=4.29, df=3, 95, p<0.01), and total lifetime consumption of alcohol (F=59.24, df=3, 103, p=0.0001). No significant differences were found for age (F=2.16, df=3, 118, n.s.) and handedness, as measured by a quantitative test (35) (F=0.78, df=3, 111, n.s.). Follow-up Scheffé tests (alpha ≤0.05) revealed that the alcohol-dependent group consumed more alcohol than the group with schizophrenia and comorbid alcohol dependence, which consumed more than the schizophrenia group or the healthy comparison group. However, the three patient groups had equivalent numbers of years of education and equivalent IQs. The group with comorbid disorders did not differ significantly from the schizophrenia group in Brief Psychiatric Rating Scale (BPRS) scores.
Among the patients with schizophrenia, there were no group differences in age, BPRS scores, IQ, years of education, or handedness by medication type (typical versus atypical) or by the presence or absence of alcohol dependence comorbidity.
Image Acquisition and Analysis
MRI scans were acquired on a 1.5-T General Electric Signa scanner by using a three-dimensional spoiled gradient recalled sequence (TR=24 msec; TE=5 msec; flip angle=40°; 124 slices; field of view=24 cm; 256×196 matrix; reconstructed resolution=0.9×0.9×1.5 mm; acquired resolution=0.9×1.2×1.5 mm). Image data were reformatted to 1-mm isotropic voxels and aligned along the anterior-posterior commissure plane and interhemispheric fissure. All images were coded to allow processing to be performed by researchers who were blind to the subject’s identity, age, and sex.
The ventral (basilar) pons and the thalamus were manually traced by K.L.S. following visible borders on every third 1-mm- thick sagittal slice, with the medial slice at mid-sagittal plane. All measurements were completed twice, and the volume was the mean of the two measurements. The most lateral boundaries of both structures were first estimated by marking the limits on the coronal and axial views. The shape of the pons was smooth and ovoid (Figure 1). The dorsal borders were formed by the medial and lateral lemnisci that appeared as a strip of reduced signal intensity. The most lateral aspects were traced until partial voluming made it difficult to distinguish the ventral pons from the superior cerebellar peduncles, approximately 10 mm from the midline, where the brainstem is transected by the calcarine fissure. Visible borders of the left and right thalamus were first apparent at approximately 15–18 mm from each side of the midline. The rostral border of the thalamus forms part of the floor of the lateral ventricles and is bounded by the fornix and the body of the caudate nucleus (laterally). Caudally, the sulcus hypothalamicus marks the boundary of the thalamus and the subthalamus. The dorsal surface of the thalamus, the pulvinar, forms a part of the floor of the third ventricle and is clearly delimited by the retropulvinal (subarachnoid) cistern. The ventral boundaries of the thalamus are the third ventricles (medially) and the internal capsule (laterally). The left and right thalami were randomly reversed in orientation on the display screen to prevent measurement bias.
Statistical Analysis
Because the size of the pons was associated with intracranial volume and the size of the thalamus with age (see Results), we applied a two-step regression analysis based on the data from the healthy comparison subjects to calculate intracranial-volume- and age-corrected z scores (37). Measures for the pons and thalamus were regressed against an estimate of intracranial volume that was based on a three-dimensional volume of the cerebrum (32). The resulting regression coefficients were used to calculate intracranial-volume-residualized scores on the basis of the regression analysis. These values were then regressed on age, and values from individual subjects were converted to standardized z scores as the final unit of analysis. The expected mean of the comparison subjects was z=0 (SD=1). Use of standardized z scores permitted assessment of the effects of disease, having removed the effects of variation due to normal aging and differences in intracranial volume. Data were analyzed first as absolute volumes and then as age-adjusted and intracranial-volume-adjusted scores. Group differences in pons and thalamus volumes were assessed with four-group, one- or two-way ANOVAs with follow-up Scheffé tests (alpha=0.05). Absolute values for the pons and thalamus were also subjected to analysis of covariance (ANCOVA), with intracranial volume as the covariate in the analysis of pons volumes and age as the covariate in the analysis of thalamus volumes.
Results
Among healthy comparison subjects, the correlation of absolute regional volume with age was significant for the bilateral thalamus (left plus right) (r=–0.53, N=51, p<0.0001) but not the pons (r=0.15, N=51, n.s.). Intracranial volume was significantly correlated with neither the pons volume (r=0.21, N=51, n.s.) nor the bilateral thalamic volume (r=0.02, N=51, n.s.) in the healthy comparison group; however, in all groups combined, pons volume and intracranial volume were correlated significantly (r=0.29, N=122, p=0.001). Further, a one-way ANOVA indicated that the four groups did not differ in intracranial volume (F=0.41, df=3, 118, p=0.75).
Means and standard deviations for z scores and unadjusted volumes of the left and right thalamus for each group are listed in Table 2 and plotted in Figure 2. Thalamic volume z scores showed a group effect (F=7.05, df=3, 118, p=0.0002) but no hemisphere or group-by-hemisphere interaction. Follow-up Scheffé tests indicated that the alcohol-dependent group had a deficit relative to the healthy comparison group (p=0.0008) and the schizophrenia group (p=0.002) but that neither the schizophrenia group nor the group with comorbid disorders had significant deficits. The ANCOVA, with unadjusted volumes and age as the covariates, yielded essentially the same results. The addition of lifetime alcohol use as a covariate in an ANCOVA assessing z scores for the bilateral thalamus eliminated the group effect.
Table 2 shows MRI values for the patients with schizophrenia and the patients with schizophrenia and comorbid alcohol dependence grouped by medication type. The contribution of antipsychotic medication type to thalamic volume in the schizophrenia patients as a group was assessed with a two-by-two group ANOVA for diagnosis (schizophrenia versus schizophrenia and comorbid alcohol dependence) and medication type (typical versus atypical). This analysis revealed an effect for medication type (F=4.21, df=1, 38, p<0.05), no diagnosis effect (F=0.85, df=1, 38, n.s.), and no interaction of medication type and diagnosis (F=0.23, df=1, 38, n.s.). The patients with schizophrenia, regardless of alcohol comorbidity, who were taking atypical medications had greater thalamic volume deficits than the patients who were taking typical medications.
Means and standard deviations for z scores and unadjusted volumes for the pons for each group are listed in Table 2 and plotted in Figure 2. A one-way ANOVA of the z scores for pons volume yielded a group effect (F=3.56, df=3, 118, p=0.02). Follow-up Scheffé tests identified significant differences between the alcohol-dependent and healthy comparison groups (p<0.04). In addition, the mean pons volume for both the alcohol-dependent group and the group with schizophrenia and comorbid alcohol dependence fell below the 99% confidence interval for the healthy comparison group. Unpaired t tests identified significant volume deficits in both the alcohol-dependent group (t=3.05, df=74, p=0.003) and the group with schizophrenia and comorbid alcohol dependence (t=2.12, df=68, p<0.05), relative to the healthy comparison group. The ANCOVA, with unadjusted volumes and intracranial volume as covariates, yielded essentially the same results. Group effects were eliminated when lifetime alcohol use was entered as a covariate in an ANCOVA assessing the z scores for the pons. The contribution of antipsychotic medication type to pontine volume in schizophrenia patients (with and without alcohol comorbidity) was assessed by a two-by-two group ANOVA for diagnosis (schizophrenia versus schizophrenia and comorbid alcohol dependence) and medication type (typical versus atypical). This analysis revealed no significant main effects or interactions.
Discussion
This study confirms the well-documented vulnerability of the thalamus and pons to chronic alcohol use and adds new information about the contribution of alcoholism to the volume of these structures in patients with schizophrenia. Although both the thalamus and the pons have been identified as structures involved in the pathophysiology of schizophrenia, our study found no significant deficits in gross volume in either structure in these particular patients with schizophrenia. Many other recent studies of medicated patients have also reported negative findings (e.g., references 21, 43–45), although a meta-analysis found a small but significant effect for thalamic volume deficits (20). Our negative finding for the pons is consistent with other studies (14).
Patients with schizophrenia who have comorbid alcoholism manifest deficits in the pons but not in the thalamus. It is possible that antipsychotic medication (particularly typical neuroleptics) can mitigate the effects of alcoholism in certain brain areas. We found that patients with schizophrenia who were taking atypical antipsychotic medications, regardless of whether they had comorbid alcoholism, showed greater thalamic volume deficits than those taking typical antipsychotic medications, an effect not found for the pons. Recent longitudinal in vivo imaging studies have shown an increase in basal ganglia volumes in patients taking typical antipsychotic medications and a decrease in patients taking atypical antipsychotic medications (28, 29). However, the findings of studies of antipsychotic medication type and thalamic volume are less clear. One longitudinal study found that antipsychotic medication increased gross thalamus size, but this effect occurred with both typical and atypical antipsychotic medications in a dose-dependent manner (25). Although a meta-analysis found no effect for medication status (medicated versus medication-naive), it did not consider medication type (20). However, a recent PET study found a significantly lower D2 binding index in the thalamus in patients treated with atypical antipsychotic medications than in those treated with typical antipsychotic medications (30). The D2 receptor binding associated with typical antipsychotic medications could normalize the thalamic volume deficits reported for neuroleptic-naive (46) and first-episode schizophrenia patients (47), or even increase the volume beyond the normal range. Unfortunately, the number of subjects in our study was relatively small, and we did not have medication dose and history data with which to more fully deconstruct the interactive effects of alcohol toxicity and neuroleptic enhancement on the thalamus. Further, this study included only men, thus precluding generalization of the findings to women.
In other studies involving this group of patients with schizophrenia and comorbid alcohol dependence, we have found deficits in the prefrontal cortex (31) and gray matter of the cerebellar hemispheres and vermis (32) that are greater than those observed in patients with either disease alone. The exacerbated prefrontal cortex deficits (31) occurred despite far lower lifetime alcohol consumption by the patients with schizophrenia and comorbid alcohol dependence than by those with alcohol dependence alone. In our study of the cerebellum (32), the schizophrenia group did not exhibit significant volume deficits, whereas deficits in the cerebellum were found in both the alcohol-dependent group and the group with schizophrenia and comorbid alcohol dependence, indicating that the volume deficits in the comorbidity group were attributable solely to the presence of alcohol dependence and that the comorbidity group appeared particularly vulnerable, as their lifetime exposure to alcohol was much less than that of the alcohol-dependent group. The effects of alcohol comorbidity in patients with schizophrenia were manifest differently on the structures examined in the current study. The thalamus was not smaller in volume in patients with schizophrenia, whether or not they had comorbid alcoholism, than in healthy comparison subjects. It is possible that the putative hypertrophy associated with typical neuroleptic use protected the thalamus from alcohol-related shrinkage, although this explanation is speculative and requires more rigorous investigation. By contrast, the volume of the pons in the comorbidity group was not afforded such protection and showed a level of volume deficit similar to that found in the alcohol-dependent group, who had consumed five times more alcohol in their lifetimes than the group with comorbid schizophrenia and alcohol dependence. Such pontine dysmorphology, along with cerebellar and prefrontal dysmorphology, puts schizophrenia patients with comorbid alcoholism at particular risk for impairment in cognitive and motor functions involving fronto-ponto-cerebellar circuitry (1, 48–50).
 |
 |
Presented at the International Congress on Schizophrenia Research, Whistler, B.C., Canada, April 28–May 1, 2001. Received Aug. 29, 2002; revision received Dec. 3, 2002; accepted Dec. 5, 2002. From the Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine; the Neuroscience Program, SRI International, Menlo Park, Calif.; and the University of Michigan School of Medicine, Ann Arbor, Mich. Address reprint requests to Dr. Sullivan, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 401 Quarry Rd., Stanford, CA 94305-5723; [email protected] (e-mail). Supported by grants AA-05965, AA-10723, AA-12388, and AA-12999 from the National Institute on Alcohol Abuse and Alcoholism and NIMH grants MH-58007 and MH-30854. The authors thank Barton Lane, M.D., for advice and guidance in delineating anatomical boundaries for morphometry.
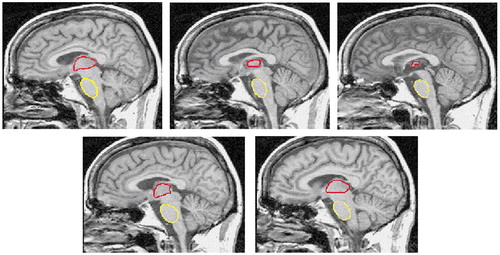
Figure 1. Outlines of Thalamus and Pons on Five Medial Slices From the 12 Sagittal Slices Used for Delineating These Structures in a 46-Year-Old Alcoholic Mana
aOutline of thalamus shown in red. Outline of pons shown in yellow.
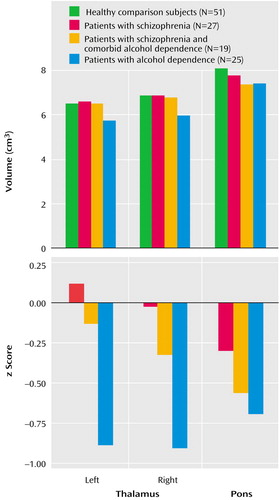
Figure 2. Volume Measures of the Pons and Thalamus in Healthy Comparison Subjects, Patients With Schizophrenia, Patients With Schizophrenia and Comorbid Alcohol Dependence, and Patients With Alcohol Dependence
1. Andreasen NC, Paradiso S, O’Leary DS: “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull 1998; 24:203-218Crossref, Medline, Google Scholar
2. Schmahmann JD, Pandya DN: Anatomic organization of the basilar pontine projections from prefrontal cortices in rhesus monkey. J Neurosci 1997; 17:438-458Crossref, Medline, Google Scholar
3. Haber S, McFarland NR: The place of the thalamus in frontal cortical-basal ganglia circuits. Neuroscientist 2001; 7:315-324Crossref, Medline, Google Scholar
4. Adams RD, Victor M, Mancall EL: Central pontine myelinolysis: a hitherto undescribed disease occurring in alcoholic and malnourished patients. Arch Neurol Psychiatry 1959; 81:154-172Crossref, Medline, Google Scholar
5. Kleinschmidt-DeMasters BK, Anderson CA, Rubinstein D: Asymptomatic pontine lesions found by magnetic resonance imaging: are they central pontine myelinolysis? J Neurol Sci 1997; 149:27-35Crossref, Medline, Google Scholar
6. Sullivan EV, Pfefferbaum A: Magnetic resonance relaxometry reveals central pontine abnormalities in clinically asymptomatic alcoholic men. Alcohol Clin Exp Res 2001; 25:1206-1212Crossref, Medline, Google Scholar
7. Arango V, Underwood MD, Mann JJ: Fewer pigmented neurons in the locus coeruleus of uncomplicated alcoholics. Brain Res 1994; 650:1-8Crossref, Medline, Google Scholar
8. Baker KG, Halliday GM, Harper CG: Effect of chronic alcohol consumption on the human locus coeruleus. Alcohol Clin Exp Res 1994; 18:1491-1496Crossref, Medline, Google Scholar
9. Halliday G, Ellis J, Heard R, Caine D, Harper C: Brainstem serotonergic neurons in chronic alcoholics with and without the memory impairment of Korsakoff’s psychosis. J Neuropathol Exp Neurol 1993; 52:567-579Crossref, Medline, Google Scholar
10. Kril JJ, Halliday GM, Svoboda MD, Cartwright H: The cerebral cortex is damaged in chronic alcoholics. Neuroscience 1997; 79:983-998Crossref, Medline, Google Scholar
11. Harding A, Halliday G, Caine D, Kril J: Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 2000; 123:141-154Crossref, Medline, Google Scholar
12. Belzunegui T, Insausti R, Ibanez J, Gonzalo LM: Effect of chronic alcoholism on neuronal nuclear size and neuronal population in the mammillary body and the anterior thalamic complex of man. Histol Histopathol 1995; 10:633-638Medline, Google Scholar
13. Shear PK, Butters N, Jernigan TL, DiTraglia GM, Irwin M, Schuckit MA, Cermak LS: Olfactory loss in alcoholics: correlations with cortical and subcortical MRI indices. Alcohol 1992; 9:247-255Crossref, Medline, Google Scholar
14. Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC: An MRI study of midbrain morphology in patients with schizophrenia: relationship to psychosis, neuroleptics, and cerebellar neural circuitry. Biol Psychiatry 2001; 49:13-19Crossref, Medline, Google Scholar
15. Karson CN, Casanova MF, Kleinman JE, Griffin WS: Choline acetyltransferase in schizophrenia. Am J Psychiatry 1993; 150:454-459Link, Google Scholar
16. Garcia-Rill E, Biedermann JA, Chambers T, Skinner RD, Mrak RE, Husain M, Karson CN: Mesopontine neurons in schizophrenia. Neuroscience 1995; 66:321-335Crossref, Medline, Google Scholar
17. Eluri R, Paul C, Roemer R, Boyko O: Single-voxel proton magnetic resonance spectroscopy of the pons and cerebellum in patients with schizophrenia: a preliminary study. Psychiatry Res 1998; 84:17-26Crossref, Medline, Google Scholar
18. Jones EG: Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 1997; 23:483-501Crossref, Medline, Google Scholar
19. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum M: Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 1999; 46:908-920Crossref, Medline, Google Scholar
20. Konick LC, Friedman L: Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry 2001; 49:28-38Crossref, Medline, Google Scholar
21. Byne W, Buchsbaum MS, Kemether E, Hazlett EA, Shinwari A, Mitropoulou V, Siever LJ: Magnetic resonance imaging of the thalamic mediodorsal nucleus and pulvinar in schizophrenia and schizotypal personality disorder. Arch Gen Psychiatry 2001; 58:133-140Crossref, Medline, Google Scholar
22. Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L: Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 2002; 159:59-65Link, Google Scholar
23. Omori M, Murata T, Kimura H, Koshimoto Y, Kado H, Ishimori Y, Ito H, Wada Y: Thalamic abnormalities in patients with schizophrenia revealed by proton magnetic resonance spectroscopy. Psychiatry Res 2000; 98:155-162Crossref, Medline, Google Scholar
24. Ende G, Braus DF, Walter S, Henn FA: Lower concentration of thalamic N-acetylaspartate in patients with schizophrenia: a replication study. Am J Psychiatry 2001; 158:1314-1316Link, Google Scholar
25. Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC: Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry 1998; 155:1711-1717Link, Google Scholar
26. Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M: Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. Am J Psychiatry 1994; 151:1430-1436Link, Google Scholar
27. Keshavan MS, Bagwell WW, Haas GL, Sweeney JA, Schooler NR, Pettegrew JW: Changes in caudate volume with neuroleptic treatment (letter). Lancet 1994; 344:1434Crossref, Medline, Google Scholar
28. Scheepers FE, de Wied CC, Hulshoff Pol HE, van de Flier W, van der Linden JA, Kahn RS: The effect of clozapine on caudate nucleus volume in schizophrenic patients previously treated with typical antipsychotics. Neuropsychopharmacology 2001; 24:47-54Crossref, Medline, Google Scholar
29. Corson PW, Nopoulos P, Miller DD, Arndt S, Andreasen NC: Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. Am J Psychiatry 1999; 156:1200-1204Abstract, Google Scholar
30. Xiberas X, Martinot JL, Mallet L, Artiges E, Loc HC, Maziere B, Paillere-Martinot ML: Extrastriatal and striatal D(2) dopamine receptor blockade with haloperidol or new antipsychotic drugs in patients with schizophrenia. Br J Psychiatry 2001; 179:503-508Crossref, Medline, Google Scholar
31. Mathalon DH, Pfefferbaum A, Lim KO, Rosenbloom MJ, Sullivan EV: Compounded brain volume deficits in schizophrenia-alcoholism comorbidity. Arch Gen Psychiatry 2003; 60:245-252Crossref, Medline, Google Scholar
32. Sullivan EV, Deshmukh A, Desmond JE, Mathalon DH, Rosenbloom MJ, Lim KO, Pfefferbaum A: Contribution of alcohol abuse to cerebellar volume deficits in men with schizophrenia. Arch Gen Psychiatry 2000; 57:894-902Crossref, Medline, Google Scholar
33. Sullivan EV, Rosenbloom MJ, Pfefferbaum A: Compounded deficits of balance and gait in schizophrenics comorbid for alcoholism (abstract), in Proceedings of the 2002 Annual Meeting of the American College of Neuropsychology. Nashville, Tenn, ACNP, 2002Google Scholar
34. Nelson HE: The National Adult Reading Test (NART). Windsor, UK, Nelson, 1982Google Scholar
35. Crovitz HF, Zener KA: Group test for assessing hand and eye dominance. Am J Psychol 1962; 75:271-276Crossref, Medline, Google Scholar
36. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 2nd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1975Google Scholar
37. Pfefferbaum A, Lim KO, Zipursky RB, Mathalon DH, Lane B, Ha CN, Rosenbloom MJ, Sullivan EV: Brain gray and white matter volume loss accelerates with aging in chronic alcoholics: a quantitative MRI study. Alcohol Clin Exp Res 1992; 16:1078-1089Crossref, Medline, Google Scholar
38. Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, Lim KO: Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res 1995; 19:1177-1191Crossref, Medline, Google Scholar
39. Pfefferbaum A, Sullivan EV, Rosenbloom MJ, Mathalon DH, Lim KO: A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch Gen Psychiatry 1998; 55:905-912Crossref, Medline, Google Scholar
40. Skinner HA: Development and Validation of a Lifetime Alcohol Consumption Assessment Procedure. Toronto, Canada Addiction Research Foundation, 1982Google Scholar
41. Skinner HA, Sheu WJ: Reliability of alcohol use indices: the lifetime drinking history and the MAST. J Stud Alcohol 1982; 43:1157-1170Crossref, Medline, Google Scholar
42. Pfefferbaum A, Rosenbloom MJ, Crusan K, Jernigan TL: Brain CT changes in alcoholics: the effects of age and alcohol consumption. Alcohol Clin Exp Res 1988; 12:81-87Crossref, Medline, Google Scholar
43. Portas CM, Goldstein JM, Shenton ME, Hokama HH, Wible CG, Fischer I: Volumetric evaluation of the thalamus in schizophrenic male patients using magnetic resonance imaging. Biol Psychiatry 1998; 43:649-659Crossref, Medline, Google Scholar
44. Arciniegas D, Rojas DC, Teale P, Sheeder J, Sandberg E, Reite M: The thalamus and the schizophrenia phenotype: failure to replicate reduced volume. Biol Psychiatry 1999; 45:1329-1335Crossref, Medline, Google Scholar
45. Deicken RF, Eliaz Y, Chosiad L, Feiwell R, Rogers L: Magnetic resonance imaging of the thalamus in male patients with schizophrenia. Schizophr Res 2002; 58:135-144Crossref, Medline, Google Scholar
46. Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr: PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 1996; 153:191-199Link, Google Scholar
47. Ettinger U, Chitnis XA, Kumari V, Fannon DG, Sumich AL, O’Ceallaigh S, Doku VC, Sharma T: Magnetic resonance imaging of the thalamus in first-episode psychosis. Am J Psychiatry 2001; 158:116-118Link, Google Scholar
48. Deshmukh A, Rosenbloom MJ, Pfefferbaum A, Sullivan EV: Clinical signs of cerebellar dysfunction in schizophrenia and alcoholism. Schizophr Res 2002; 57:281-291Crossref, Medline, Google Scholar
49. Schmahmann J: Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends Cogn Sci 1998; 2:362-371Crossref, Medline, Google Scholar
50. Sullivan EV, Harding AJ, Pentney R, Dlugos C, Martin PR, Parks MH, Desmond JE, Chen SH, Pryor MR, De Rosa E, Pfefferbaum A: Disruption of frontocerebellar circuitry and function in alcoholism. Alcohol Clin Exp Res 2003; 27:301-309Crossref, Medline, Google Scholar



