A Population-Based Cohort Study of Premorbid Intellectual, Language, and Behavioral Functioning in Patients With Schizophrenia, Schizoaffective Disorder, and Nonpsychotic Bipolar Disorder
Abstract
OBJECTIVE: The premorbid intellectual, language, and behavioral functioning of patients hospitalized for schizophrenia, schizoaffective disorder, or nonpsychotic bipolar disorder was compared with that of healthy comparison subjects. METHOD: The Israeli Draft Board Registry, which contains measures of intellectual, language, and behavioral functioning for the unselected population of 16- to 17-year-olds, was merged with the National Psychiatric Hospitalization Case Registry, which contains diagnoses for all patients with psychiatric hospitalizations in Israel. The database was used to identify adolescents with no evidence of illness at their draft board assessment who were later hospitalized for nonpsychotic bipolar disorder (N=68), schizoaffective disorder (N=31), or schizophrenia (N=536). The premorbid functioning of these subjects was compared to that of nonhospitalized individuals matched for age, gender, and school attended at the time of the draft board assessment. The diagnostic groups of hospitalized subjects were also compared. RESULTS: Relative to the comparison subjects, subjects with schizophrenia showed significant premorbid deficits on all intellectual and behavioral measures and on measures of reading and reading comprehension. Subjects with schizophrenia performed significantly worse on these measures than those with a nonpsychotic bipolar disorder, who did not differ significantly from the comparison subjects on any measure. Subjects with schizoaffective disorder performed significantly worse than the comparison subjects only on the measure of nonverbal abstract reasoning and visual-spatial problem solving and performed significantly worse than subjects with nonpsychotic bipolar disorder on three of the four intellectual measures and on the reading and reading comprehension tests. CONCLUSIONS: The results support a nosologic distinction between nonpsychotic bipolar disease and schizophrenia in hospitalized patients.
Converging evidence indicates that subtle cognitive and behavioral abnormalities sometimes precede the first episode of psychotic illness. Apparently healthy children and adolescents who later develop psychotic illnesses may manifest lower than normal intelligence, withdrawn social behavior, conduct and adjustment abnormalities, and soft neurological deficits (1–19).
The few studies of premorbid functioning of patients who later received a diagnosis of an affective disorder have indicated that, relative to a healthy comparison group, future bipolar disorder patients exhibit impaired premorbid intellectual and social functioning (9, 20–22). However, premorbid abnormalities associated with affective disorders seem to be less severe than those associated with schizophrenia (21). The group differences are less consistent, with premorbid cognitive and behavioral abnormalities reported in some (9, 20–22), but not all (1, 3, 14, 15, 23) studies of nonschizophrenic affective psychosis, possibly owing to differences in inclusion of affective disorder patients with or without psychotic symptoms.
The current study used a population database that linked data on intellectual, behavioral, and language functioning from the Israeli Draft Board Registry with psychiatric follow-up data contained in the Israeli National Psychiatric Hospitalization Case Registry. The purpose of the study was to compare premorbid intellectual, behavioral, and language functioning of future patients later hospitalized with schizophrenia, nonpsychotic bipolar disorder, or schizoaffective disorder. Since test scores were assigned by the draft board when the adolescents were apparently healthy, they are independent of the diagnosis. Hence, a finding of premorbid differences between diagnostic groups would suggest trait versus state differences, thus supporting the nosologic distinctions between these diagnostic groups.
Method
Draft Board Assessment
The study is based on draft board assessments of intellectual, medical, and psychiatric eligibility for military service of the unselected population of Israeli adolescents between age 16 and 17 years. The population includes individuals who will be eligible for military service, as well as those who will be exempted owing to medical, psychiatric, or social reasons.
The draft board assessment consists of 1) a physical examination, review of systems, and psychiatric history, all conducted by a physician; 2) assessment of language ability; 3) a battery of tests measuring intellectual functioning; and 4) a structured interview assessing personality and behavioral traits. The assessments of intellectual abilities, language, and behavior are conducted by college-age individuals who are trained in a 4-month course on administration of draft board tests. The assessment and its validation are described in detail elsewhere (24).
The assessment of language skills includes four subtests: 1) reading comprehension test, which measures the ability to understand ideas presented in unfamiliar passages of increasing length; 2) reading abilities test, which measures the ability to correctly read sentences of increasing difficulty (similar to the reading and spelling subtests of the Wide Range Achievement Test [25]); 3) writing ability test, in which the ability to spell increasingly difficult words is assessed by having the subject transcribe dictated sentences; and 4) the examiner’s overall impression of the fluency and quality of speech (i.e., speaking ability). Each of the four language tests is scored on a 5-point scale, and the sum of the scores provides a measure of language mastery (24).
The intellectual assessment includes four tests (26): 1) the Otis-R, a modified, Otis-type verbal intelligence test adapted from the U.S. Army Alpha Instructions Test, which measures the ability to understand and carry out verbal instructions (score range=0–21); 2) Similarities-R, a revised version of the “similarities” subtest of the Wechsler Adult Intelligence Scale that assesses verbal abstraction and categorization (range=0–30); 3) Arithmetic-R, which measures mathematical reasoning, concentration, and concept manipulation (range=0–25); and 4) Raven’s Progressive Matrices-R, a modified version of Raven’s Progressive Matrices that measures nonverbal abstract reasoning and visual-spatial problem-solving abilities (range=0–30). All scores are based on number of correct answers. The sum of the scores for the four tests forms a validated measure of general intelligence (24).
The behavioral assessment, which has been described in detail elsewhere (24, 27), consists of a structured interview that evaluates five behavioral attributes on a 5-point scale: 1) social functioning, which entails social adeptness and ability to achieve social closeness; 2) individual autonomy, which encompasses personal autonomy, maturity, and internally directed behavior; 3) organization ability, which includes compliance with timetables, self-mastery, and self-care; 4) physical activity, including involvement in extracurricular physical activities; and 5) functioning in structured environments, such as school or work.
National Psychiatric Hospitalization Case Registry
The Israeli National Psychiatric Hospitalization Case Registry is a complete listing of all psychiatric hospitalizations in the country. The record for each hospitalization includes the ICD (ICD-9 or ICD-10, depending on the year) diagnosis assigned and coded at discharge by a board-certified psychiatrist at the facility. All inpatient psychiatric facilities in the country, including day hospitals and psychiatric units in general hospitals, are required by law to report all discharges to the registry.
Study Population
The National Psychiatric Hospitalization Case Registry was linked with the Draft Board Registry by the managers of the psychiatric case registry. The registry files were linked by using an algorithm to preserve medical record confidentiality (28) and in compliance with procedures approved by the local institutional review board. The linking variable was the national identification number (equivalent to the U.S. Social Security number). The merged file included data for all adolescents assessed by the draft board between 1985 and 1995 who were reported to the National Psychiatric Hospitalization Case Registry between 1970 and 1996 and given at least one diagnosis of schizophrenia (N=1,857; 1,350 male and 507 female subjects), schizoaffective disorder (N=297; 202 male and 95 female subjects), or nonpsychotic bipolar disorder (N=331; 205 male and 126 female subjects) during the follow-up period. This allowed for a follow-up period of as many as 11 years since the draft board assessment. Other diagnostic groups, such as bipolar disorder with psychotic symptoms, were not included, since the number of subjects who met the criteria for a distinctive diagnosis (see the next paragraph) was too small to provide meaningful data.
To limit this analysis to apparently healthy individuals with no obvious signs of disease during the draft board assessment, adolescents who were assigned a psychiatric diagnosis by the draft board or who had a psychiatric hospitalization at any time in their lives before the draft board assessment or within 1 year from the date of the draft board assessment were excluded. To ensure that the groups were diagnostically distinct, subjects were included in the analysis only if they had at least 4 years of follow-up after their first hospitalization and had no change in diagnosis between their first hospitalization and any subsequent hospitalizations. By using these criteria, the resulting files used for the analyses included data for 536 persons (390 male and 146 female subjects) with schizophrenia, 31 (23 male and eight female subjects) with schizoaffective disorder, and 68 (38 male and 30 female subjects) with nonpsychotic bipolar disorder.
Each future patient was matched with a single same-sex comparison adolescent. Matched comparison subjects were randomly selected male and female adolescents who attended the same high school at the same time as the future patient and were assessed by the draft board at the same age as the future patient but were not assigned a psychiatric diagnosis by the draft board and did not appear in the psychiatric hospitalization registry during the follow-up period. The future patients and the comparison subjects were matched by high school attended at the time of testing in the attempt to control for the effects of educational and social opportunities. In addition, as students in the same high school come from the same residential area, the matching also controlled in part for the effects of socioeconomic status.
Statistical Analyses
Repeated measures analyses of variance (ANOVA) was performed for sets of four intellectual, four language, and five behavioral measures to compare premorbid performance between each patient and the respective comparison subject. For each measure, a difference score (score for the comparison subjects minus score for the patient) was calculated. Because of differences in the scales used for the intellectual measures, standardized scores were calculated by dividing each difference score by the standard deviation of the differences for that measure. The within-subjects factor was the measure. Gender and diagnostic group (schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder) were the between-subjects factors. The effect of disease was tested by comparing the mean of the differences to zero. Since the behavioral measures were administered to male subjects only, the ANOVA model for the behavioral measures did not include gender. For effects involving the within-subjects factor, the F statistic test was based on the Huynh-Feldt adjustment for the degrees of freedom. For descriptive purposes, ANOVAs were performed for each of the dependent variables. In all post hoc analyses, Tukey’s honestly significant difference was used for testing the effects of diagnosis. For post hoc analysis of the interaction of diagnosis with gender, the Scheffé procedure was used to examine specific interactions by eliminating one of the diagnoses or combining two of the diagnoses. Similar repeated measures ANOVAs were performed for direct comparison of the performance of the three patient groups. Univariate ANOVA was used to compare age at illness onset, and univariate analysis of covariance (ANCOVA) with follow-up time as a covariate was used to compare the number and duration of admissions between the three patient groups.
To evaluate the predictive values of the premorbid test scores, two multivariate logistic regression analyses were performed for each pair of diagnostic groups. One model included gender but did not include the behavioral measures (because behavioral measures were administered only to male subjects), and the other analysis, conducted on data for male subjects only, included the behavioral measures.
For each combination of diagnosis, gender, and measure, effect sizes were calculated as the mean of the differences between the measures for the future patients and the comparison subjects divided by the square root of 1 minus the correlation between the patients’ and comparison subjects’ measures times the standard deviation of the differences (29). To describe differences related to gender and diagnosis, effect sizes were calculated as the difference between means divided by the pooled standard deviation. By using Cohen’s criteria (29), an effect size of ≥0.20 and <0.50 was considered small, ≥0.50 and <0.80 medium, and ≥0.80 large. Similar ranges based on effect sizes of –0.20, –0.50, and –0.80 were also considered small, medium, and large, respectively.
Results
Table 1 presents data on the hospitalization history of the patient groups. We found no significant differences in age at first admission between the three patient groups (F=2.49, df=2, 629, p<0.09). In contrast, significant differences in the number of admissions (F=6.78, df=2, 628, p=0.001) and in overall time in the hospital (F=4.87, df=2, 628, p=0.008) were evident, with schizoaffective disorder patients having significantly (p<0.05, Tukey) more admissions than both the schizophrenia and nonpsychotic bipolar disorder groups and spending more time in hospital than the bipolar disorder group. In addition, female patients had significantly fewer admissions than male patients (F=4.37, df=1, 629, p<0.04). No significant interaction of gender and diagnosis was found for any measure.
For the intellectual measures, repeated measures ANOVA of the differences between future patients and comparison subjects indicated significant main effects for disease status (F=20.08, df=1, 629, p<0.0005), diagnosis (F=4.705, df=2, 629, p=0.009), and gender (F=4.35, df=1, 629, p<0.04), but no significant interactions of gender and diagnosis and no main effect or interaction involving the measures. Descriptive analyses showed that future schizophrenia patients were significantly impaired on all premorbid intellectual measures (all F>42.88, df=1, 534, all p<0.0005, effect sizes=0.32–0.60) (Figure 1). Future schizoaffective disorder patients were significantly impaired only on the Raven’s Progressive Matrices-R (F=8.16, df=1, 29, p=0.008, effect size=0.56), but this group had effect sizes above 0.20 (“small”) for the Otis-R (effect size=0.27) and the Arithmetic-R (effect size=0.21). In contrast, there were no significant impairments in premorbid intellectual functioning in the future nonpsychotic bipolar disorder patients; all effect sizes for that group were less than 0.20 (Figure 1). Post hoc tests showed that the intellectual deficits were significantly (p<0.05, Tukey) more severe in the schizophrenia group than in the nonpsychotic bipolar disorder group. Female future patients had more severe premorbid intellectual impairments than male future patients (effect sizes=0.07–0.14).
Repeated measures ANOVA indicated a significant language measures effect (F=12.58, df=2.88, 1814.74, p<0.0005), as well as an interaction of language measures and diagnosis (F=3.18, df=5.77, 1814.74, p=0.004) and of language measures and gender (F=2.96, df=2.88, 1814.74, p<0.04), but there was no three-way interaction of language measures, diagnosis, and gender, nor were there main effects or interactions of gender and diagnosis. Future schizophrenia patients were significantly impaired on the reading and reading comprehension tests (F=19.23 and F=69.20, df=1, 535, p<0.0005, effect sizes=0.22 and 0.42, respectively), relative to the comparison subjects, but performed better than the comparison subjects on the writing test (F=11.10, df=1, 535, p=0.001, effect size=–0.15) (Figure 1). The other two groups were not significantly impaired, although the effect sizes for writing, speaking, and reading comprehension in the schizoaffective disorder group were –0.46, 0.26, and 0.35, respectively (Figure 1). The interaction of language measures and gender was the result of the significantly larger difference between female future patients and their comparison subjects than between male future patients and their comparison subjects on the reading and reading comprehension tests (F=4.78 and F=4.95, df=1, 629, p<0.03, and p<0.03, effect sizes=0.26 and 0.11, respectively).
Since the behavioral interview was administered only to male subjects, the comparison between the three diagnostic groups on the behavioral measures was based on data for male patients only. For the behavioral measures, a repeated measures ANOVA indicated a significant disease status effect (F=5.50, df=1, 448, p<0.02) and a diagnosis effect (F=3.69, df=2, 448, p<0.03) but no other main effects or interactions. Univariate analyses showed that future schizophrenia patients were significantly impaired on all behavioral measures, relative to the comparison subjects (all F>9.68, df=1, 389, all p<0.002, effect sizes=0.17–0.28) (Figure 1). Future schizoaffective disorder patients also exhibited premorbid deficits on four of the five behavioral measures (effect sizes=0.27–0.39) (Figure 1), but none of the differences on the individual measures reached statistical significance. Future nonpsychotic bipolar disorder patients were not significantly different from their comparison subjects on any measure, but they showed better premorbid social functioning (effect size=–0.20). Post hoc testing showed that the behavioral deficits were significantly (p<0.05, Tukey) more severe in the schizophrenia group than in the nonpsychotic bipolar disorder group.
Figure 2 shows direct comparisons of the three future patient groups’ mean scores on the intellectual, language, and behavioral measures. The direct comparison of premorbid intellectual performance indicated significant main effects of diagnosis (F=33.96, df=2, 629, p<0.0005) and intellectual measures (F=96.17, df=2.94, 1851.23, p<0.0005) and a significant interaction of intellectual measures and gender (F=4.32, df=2.94, 1851.23, p=0.005). No other significant main effects or interactions were found. Univariate analyses showed that both future schizophrenia and schizoaffective disorder patients had significantly (p<0.05, Tukey) lower (worse) Raven’s Progressive Matrices-R, Otis-R, and Arithmetic-R scores than future nonpsychotic bipolar disorder patients. In addition, future schizophrenia patients had significantly (p<0.05, Tukey) lower Similarities-R scores, compared to future nonpsychotic bipolar disorder patients (Figure 2). The effect sizes were small to medium (effect size=0.43–0.66) for the schizophrenia group and medium to large (effect size=0.50–0.85) for the schizoaffective disorder group. Descriptive analyses also showed that the significant interaction of intellectual measures and gender was accounted for by the significantly lower scores on the Arithmetic-R subtest for female than for male patients (p<0.05, Tukey) (effect size=0.41).
We also found significant main effects of diagnosis (F=157.708, df=2.95, 1858.88, p<0.0005) and language measures (F=4.60, df=4, 626, p=0.001), as well as significant interactions of language measures and diagnosis (F=7.11, df=5.91, 1858.88, p<0.0005) and of language measures and gender (F=6.19, df=2.95, 1858.88, p<0.0005). The interaction of language measures and gender was accounted for by the significantly higher scores on the writing test for female than for male patients (p<0.05, Tukey) (effect size=0.29). The interaction of language measures and diagnosis was a result of significantly lower reading and reading comprehension scores for future schizophrenia and schizoaffective patients than for future nonpsychotic bipolar disorder patients (p<0.05, Tukey) (Figure 2). Effect sizes for reading and reading comprehension were medium for both the schizophrenia (effect size=0.53 and 0.62, respectively) and schizoaffective disorder (effect size=0.54 and 0.75, respectively) groups.
We found a significant main effect for diagnosis for the behavioral measures (F=4.65, df=2, 448, p=0.01). Post hoc analysis showed that future schizophrenia patients had significantly (p<0.05, Tukey) lower (worse) overall scores than future nonpsychotic bipolar disorder patients, although no individual measures were significantly different between the patient groups. Nevertheless, effect sizes were small (effect size=0.24–0.44) for the schizophrenia group on all measures, and small (effect size=0.21–0.46) for social functioning, organization ability, and functioning in structured environments in the schizoaffective disorder group, compared to the bipolar disorder group (Figure 2).
Finally, we examined whether the premorbid measures could be used for classification. A logistic regression model distinguishing schizophrenia from nonpsychotic bipolar disorder for both genders was significant (model χ2=54.61, df=9, p<0.0005). The best predictors were gender (i.e., being male) (Wald χ2=12.96, df=1, p<0.0005, odds ratio=2.79, 95% CI=1.59–4.88), low Raven’s Progressive Matrices-R score (Wald χ2=10.71, df=1, p=0.001, odds ratio=1.10, 95% CI=1.04–1.15), low reading comprehension score (Wald χ2=9.11, df=1, p=0.003, odds ratio=1.77, 95% CI=1.22–2.56), and high writing score (Wald χ2=9.29, df=1, p=0.002, odds ratio=1.82, 95% CI=1.24–2.68). Using a classification cutoff point of 0.90, which best divided the groups, we obtained a sensitivity of 52.8% and specificity of 80.9% (model χ2=48.73, df=9, p<0.0005, by Scheffé post hoc test). The behavioral measures did not enhance the discrimination between schizophrenia and nonpsychotic bipolar disorder in male subjects. The other pairs of diagnostic groups could not be distinguished by using the premorbid measures.
Discussion
The results of this study confirm the existence of premorbid differences in intellectual functioning between hospitalized patients assigned a diagnosis of nonpsychotic bipolar disorder and those assigned a diagnosis of schizophrenia. Adolescents later diagnosed with schizophrenia performed worse on all premorbid intellectual measures, relative to the comparison subjects, but future nonpsychotic bipolar disorder patients did not differ from comparison subjects on any premorbid measures of intellectual functioning. As confirmation of this finding, a direct comparison of future schizophrenia and future nonpsychotic bipolar disorder patients also indicated a significant difference between the two patient groups in intellectual functioning.
This study also reveals premorbid differences in reading abilities between schizophrenia and nonpsychotic bipolar disorder patients. Although adolescents who were later diagnosed with schizophrenia performed worse on the reading and reading comprehension tests relative to the comparison subjects, future nonpsychotic bipolar patients did not exhibit premorbid deficits on any language measure. Similarly, a direct comparison of future schizophrenia patients with future nonpsychotic bipolar disorder patients indicated a significant difference between the two groups on the reading and reading comprehension measures.
The results of this study also suggest differences in premorbid behavioral functioning between male patients hospitalized for nonpsychotic bipolar disorder and those hospitalized for schizophrenia. Male subjects later diagnosed with schizophrenia performed worse than the comparison subjects on all premorbid behavioral measures, while those later diagnosed with nonpsychotic bipolar disorder did not differ on any of these premorbid measures from the comparison subjects. The direct comparison of the two groups also indicated that on all measures, male subjects who were later assigned a diagnosis of schizophrenia performed worse than those who were later assigned a diagnosis of nonpsychotic bipolar disorder.
In contrast to findings regarding future schizophrenia patients, in whom subtle premorbid intellectual and social abnormalities have been consistently documented (e.g., references 7, 14, 20, 21, 30), poor premorbid functioning has been less consistently found in persons who later are assigned a diagnosis of nonpsychotic bipolar disorder. Studies directly comparing the premorbid functioning of persons with schizophrenia and those with affective psychosis have shown greater impairments in intellectual and social functioning in the schizophrenia group (22, 31, 32). Studies comparing social and intellectual functioning during childhood in future affective psychosis patients, compared with healthy comparison subjects, have reported mixed results: some found no significant premorbid social or intellectual abnormalities in future affective psychosis patients (3, 14, 23, 30), and others have found such abnormalities (20, 21, 33).
To our knowledge, this study is the first to specifically assess premorbid intellectual, language, and behavioral functioning in future bipolar disorder patients without psychotic symptoms. Our results show no premorbid behavioral, language, or intellectual abnormalities in this group of patients, relative to normal comparison subjects. It is possible that some of the discrepancies between our findings and those of previous studies were a result of the previous studies’ inclusion of affective disorder patients both with and without psychotic symptoms (e.g., reference 21) and lack of sufficient follow-up to establish diagnostic stability. Also, some previous studies did not find evidence of premorbid language deficits in schizophrenia (6, 30), although this discrepancy is probably due to low power (30), use of different measures of language functioning, and inclusion of affective disorder patients in the study group (21).
This study is also one of the first to assess premorbid intellectual, language, and behavioral functioning in schizoaffective disorder. Because of the limited statistical power for this diagnostic group, emphasis in the interpretation of findings was placed on effect size and the pattern of results and not merely on statistical significance. Schizoaffective disorder patients showed premorbid deficits on three of the four intellectual measures, as well as on four of the five behavioral measures. In addition, future schizoaffective disorder patients scored worse than future nonpsychotic bipolar disorder patients on all four premorbid intellectual measures and on the reading and reading comprehension tests, and their scores were very similar to those of the future schizophrenia patients on most premorbid measures. These results are similar to those of studies comparing the neuropsychological performance of patients already diagnosed with schizophrenia and schizoaffective disorder, in which the cognitive performance of schizoaffective disorder patients was indistinguishable from that of schizophrenia patients, and both groups performed worse than their respective comparison subjects (34–37). Yet, a recent study (3) that assessed premorbid IQ in schizoaffective disorder patients and schizophrenia patients did not find significant differences between the schizoaffective disorder patients and normal comparison subjects.
Finally, we demonstrated that premorbid language and intellectual measures could be used to distinguish schizophrenia from nonpsychotic bipolar disorder. It has been suggested that a higher level of intellectual functioning is associated with better mental health (38), better stress coping (39–41), and less antisocial behavior (42). In addition, poor premorbid functioning has been suggested as a predictor of vulnerability to psychosis among patients with major depressive disorder (43). Furthermore, studies comparing neuropsychological functioning among psychotic and nonpsychotic depressed patients have reported better performance among nonpsychotic patients than among psychotic unipolar depressed patients or schizophrenia patients (44–46). The evidence in this study of normal intellectual functioning in future nonpsychotic bipolar disorder patients, compared with future schizophrenia patients, supports the hypothesis that, despite the predisposition for mental illness, normal intellectual functioning might be a protective factor against psychosis. Further support for this hypothesis emerged in the process of subject matching for this study. When normal comparison subjects and future schizophrenia patients were matched with future nonpsychotic bipolar disorder patients by using gender and school attended, thus partly controlling for socioeconomic status, the two patient groups differed only on measures of intellectual functioning and reading ability: the future nonpsychotic bipolar disorder patients exhibited normal performance on all intellectual measures and on the reading ability test, while the future schizophrenia patients performed worse than the comparison subjects (data not shown). However, an alternative explanation is that poor performance in the measured functions may instead reflect a vulnerability marker of brain dysfunction or an early stage of an evolving pathologic process that eventually leads to schizophrenia.
Since this study is based on clinical rather than research diagnoses, concerns regarding the validity and reliability of the diagnoses are pertinent. As has been suggested since the early 1980s, there may be a tendency, particularly in long-term psychiatric hospitals, to assign a diagnosis of schizophrenia to patients who would have received a diagnosis of bipolar affective disorder if a structured interview had been conducted and research diagnostic criteria had been applied (47). Of particular concern is the diagnosis of schizoaffective disorder, which has remained problematic both clinically and in research (48, 49). However, the wide acceptance of DSM and ICD criteria since the 1980s has changed this situation considerably. A study by Pulver et al. (50) that examined the accuracy of diagnoses of affective disorders and schizophrenia in public hospitals found convincing evidence that the tendency for overdiagnosis of schizophrenia has diminished and that agreement between chart and research diagnoses was very good. The training of the psychiatrists who contributed to the registry used in our study and the treatment settings represented in the registry are very similar to the those described by Pulver et al. (50), and there is no reason to assume that the diagnostic skills of the diagnosing physicians would be different. In addition, our criterion of limiting inclusion of patients with repeated hospitalizations to those who had the same diagnosis for all hospitalizations is quite stringent, adding diagnostic reliability. Nevertheless, the usefulness of this criterion could be limited by the possibility that once a diagnosis has been assigned, it might affect the diagnoses assigned on any future admissions. Finally, as part of an ongoing study, we found that the consensus between registry and research diagnoses for schizophrenia and mood disorders exceeded 90%.
Anther limitation of this report is that it examined only three major mental disorders: schizophrenia, schizoaffective disorder, and nonpsychotic bipolar disorder. Diagnostic groups such as depression with or without psychotic features were not included in the analysis, either because of the lack of stable and distinctive diagnoses or because the number of subjects in the relevant age group was too small to provide meaningful results. It is noteworthy that diagnostic stability in the registry was high (>66%) for the three diagnostic groups assessed in this study (51) and was similar to values obtained by using research diagnoses (52). In addition, although schizoaffective disorder patients had significantly more admissions than the two other groups, this difference did not increase the likelihood of changing diagnoses, as these patients were uniformly hospitalized with a diagnosis of schizoaffective disorder throughout the follow-up period. Finally, some effects may have represented prodromal illness. However, age of first admission was not significantly correlated with any dependent variables in any of the diagnostic groups, suggesting that the findings were not related to a prodromal stage of illness.
The main strength of this study is the use of prospective, albeit historical prospective, assessments rather than retrospective assessments. Since the diagnosis of schizophrenia has been associated more consistently than other diagnoses with premorbid abnormalities, retrospective assessments of premorbid functioning might be biased by the index diagnosis. The premorbid differences between hospitalized affective disorder patients and schizophrenia patients are particularly interesting, since hospitalized, rather than ambulatory, affective disorder patients tend to be more severely ill and should therefore be more similar to schizophrenia patients. Because the premorbid assessments that showed differences between the groups were conducted in individuals presumed to be healthy, the results could not have been biased by the distinction between schizophrenia and affective disorder, giving further credence to the distinction. It is interesting to note that the mean age at the first hospitalization in the three patient groups was almost identical, probably because of the exclusion of patients who were hospitalized before the age of testing and because of the limited follow-up period, up to age 27. However, this limitation is not likely to affect the findings of poorer premorbid functioning in the patients with schizophrenia, as patients with childhood schizophrenia have been reported to have particularly poor premorbid functioning (53). A longer follow-up time most likely would have identified more patients with nonpsychotic bipolar disorder, which has been reported to have a later age at onset, compared with schizophrenia or schizoaffective disorder (54, 55).
In conclusion, this study demonstrated that impaired premorbid intellectual, language, and behavioral functioning are specific to future schizophrenia patients relative to future nonpsychotic bipolar disorder patients. The study also indicated that, as groups, future nonpsychotic bipolar disorder patients and future schizophrenia patients can be distinguished on the basis of their intellectual performance. A persistent hypothesis supported by some data (56) is that schizophrenia and affective disorder are variations of a common entity, in contrast to the Kraepelinian distinction between dementia praecox (schizophrenia) and manic depressive psychosis or the more recent classification suggested by Kendler et al. (57) between schizophrenia and other major psychiatric disorders. The data presented here highlight the premorbid similarities and differences between schizophrenia, schizoaffective disorder, and bipolar disorder and support a nosologic distinction between schizophrenia and nonpsychotic bipolar disorder.
 |
Received Aug. 14, 2001; revision received June 17, 2002; accepted June 21, 2002. From the Department of Psychiatry and the Department of Biomathematical Sciences, Mount Sinai School of Medicine, New York; the Department of Psychiatry, Chaim Sheba Medical Center; the School of Social Work, Bar-Ilan University, Ramat Gan, Israel; the Ministry of Health, Division of Mental Health, Jerusalem, Israel; and Israel Defense Forces, Division of Mental Health, Tel-Hashomer, Israel. Address reprint requests to Dr. Davidson, Chaim Sheba Medical Center, Tel-Hashomer 52621 Israel; [email protected] (e-mail).
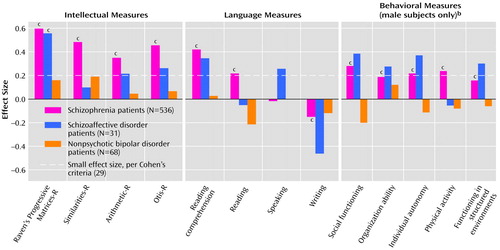
Figure 1. Effect Size for Performance on Measures of Premorbid Intellectual, Language, and Behavioral Functioninga in Patients Hospitalized for Schizophrenia, Schizoaffective Disorder, or Nonpsychotic Bipolar Disorder, Compared With Normal Subjects Matched for Age, Sex, and School Attended
aThe measures are part of the assessment administered by the Israeli draft board. See text for descriptions.
bN=390 for schizophrenia patients; N=23 for schizoaffective disorder patients; N=38 for nonpsychotic bipolar disorder patients.
cSignificantly different from matched comparison subjects (p<0.05, repeated measures analysis of variance).
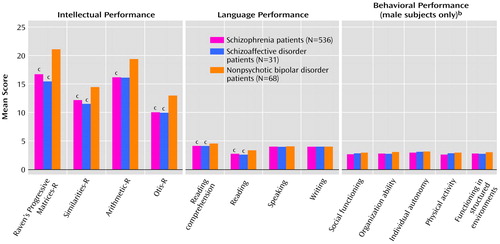
Figure 2. Scores on Measures of Premorbid Intellectual, Language, and Behavioral Functioninga in Patients Hospitalized for Schizophrenia, Schizoaffective Disorder, or Nonpsychotic Bipolar Disorder
aThe measures are part of the assessment administered by the Israeli draft board. See text for descriptions.
bN=390 for schizophrenia patients; N=23 for schizoaffective disorder patients; N=38 for nonpsychotic bipolar disorder patients.
cSignificantly different from patients with nonpsychotic bipolar disorder (p<0.05, Tukey).
1. Cannon M, Caspi A, Moffitt TE, HonaLee H, Taylor A, Murray RM, Poulton R: Evidence for early childhood, pan-developmental impairment specific to schizophreniform disorder: results from a longitudinal birth cohort. Arch Gen Psychiatry 2002; 59:449-456Crossref, Medline, Google Scholar
2. Cannon TD, Bearden CE, Hollister JM, Rosso IM, Sanchez LE, Hadley T: Childhood cognitive functioning in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull 2000; 26:379-393Crossref, Medline, Google Scholar
3. Gilvarry C, Takei N, Russell A, Rushe T, Hemsley D, Murray RM: Premorbid IQ in patients with functional psychosis and their first-degree relatives. Schizophr Res 2000; 41:417-429Crossref, Medline, Google Scholar
4. Nicolson R, Lenane M, Singaracharlu S, Malaspina D, Giedd JN, Hamburger SD, Gochman P, Bedwell J, Thaker GK, Fernandez T, Wudarsky M, Hommer DW, Rapoport JL: Premorbid speech and language impairments in childhood-onset schizophrenia: association with risk factors. Am J Psychiatry 2000; 157:794-800Link, Google Scholar
5. Rosso IM, Bearden CE, Hollister JM, Gasperoni TL, Sanchez LE, Hadley T, Cannon TD: Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull 2000; 26:367-378Crossref, Medline, Google Scholar
6. Cannon M, Jones P, Huttunen MO, Tanskanen A, Huttunen T, Rabe-Hesketh S, Murray RM: School performance in Finnish children and later development of schizophrenia: a population-based longitudinal study. Arch Gen Psychiatry 1999; 56:457-463Crossref, Medline, Google Scholar
7. Cornblatt B, Obuchowski M, Roberts S, Pollack S, Erlenmeyer-Kimling L: Cognitive and behavioral precursors of schizophrenia. Dev Psychopathol 1999; 11:487-508Crossref, Medline, Google Scholar
8. Davidson M, Reichenberg A, Rabinowitz J, Weiser M, Kaplan Z, Mark M: Behavioral and intellectual markers for schizophrenia in apparently healthy male adolescents. Am J Psychiatry 1999; 156:1328-1335Abstract, Google Scholar
9. Isohanni I, Jarvelin MR, Nieminen P, Jones P, Rantakallio P, Jokelainen J, Isohanni M: School performance as a predictor of psychiatric hospitalization in adult life: a 28-year follow-up in the Northern Finland 1966 Birth Cohort. Psychol Med 1998; 28:967-974Crossref, Medline, Google Scholar
10. Kremen WS, Buka SL, Seidman LJ, Goldstein JM, Koren D, Tsuang MT: IQ decline during childhood and adult psychotic symptoms in a community sample: a 19-year longitudinal study. Am J Psychiatry 1998; 155:672-677Link, Google Scholar
11. Malmberg A, Lewis G, David A, Allebeck P: Premorbid adjustment and personality in people with schizophrenia. Br J Psychiatry 1998; 172:308-313Crossref, Medline, Google Scholar
12. McGlashan TH: Early detection and intervention of schizophrenia: rationale and research. Br J Psychiatry Suppl 1998; 172:3-6Crossref, Medline, Google Scholar
13. Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, Patton GC, Jackson HJ: Prediction of psychosis: a step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl 1998; 172:14-20Crossref, Medline, Google Scholar
14. Done DJ, Crow TJ, Johnstone EC, Sacker A: Childhood antecedents of schizophrenia and affective illness: social adjustment at ages 7 and 11. Br Med J 1994; 309:699-703Crossref, Medline, Google Scholar
15. Jones PB, Bebbington P, Foerster A, Lewis SW, Murray RM, Russell A, Sham PC, Toone BK, Wilkins S: Premorbid social underachievement in schizophrenia: results from the Camberwell Collaborative Psychosis Study. Br J Psychiatry 1993; 162:65-71Crossref, Medline, Google Scholar
16. Marcus J, Hans SL, Auerbach JG, Auerbach AG: Children at risk for schizophrenia: the Jerusalem Infant Development Study, II: neurobehavioral deficits at school age. Arch Gen Psychiatry 1993; 50:797-809Crossref, Medline, Google Scholar
17. Walker E, Lewine RJ: Prediction of adult-onset schizophrenia from childhood home movies of the patients. Am J Psychiatry 1990; 147:1052-1056Link, Google Scholar
18. Keefe RS, Mohs RC, Losonczy MF, Davidson M, Silverman JM, Horvath TB, Davis KL: Premorbid sociosexual functioning and long-term outcome in schizophrenia. Am J Psychiatry 1989; 146:206-211Link, Google Scholar
19. Erlenmeyer-Kimling L, Cornblatt B: Biobehavioral risk factors in children of schizophrenic parents. J Autism Dev Disord 1984; 14:357-374Crossref, Medline, Google Scholar
20. David AS, Malmberg A, Brandt L, Allebeck P, Lewis G: IQ and risk for schizophrenia: a population-based cohort study. Psychol Med 1997; 27:1311-1323Crossref, Medline, Google Scholar
21. Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, Murray RM: Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. Am J Psychiatry 1997; 154:1544-1550Abstract, Google Scholar
22. Van Os J, Takei N, Castle DJ, Wessely S, Der G, Murray RM: Premorbid abnormalities in mania, schizomania, acute schizophrenia and chronic schizophrenia. Soc Psychiatry Psychiatr Epidemiol 1995; 30:274-278Crossref, Medline, Google Scholar
23. Lewine RR, Watt NF, Prentky RA, Fryer JH: Childhood social competence in functionally disordered psychiatric patients and in normals. J Abnorm Psychol 1980; 89:132-138Crossref, Medline, Google Scholar
24. Gal R: The selection, classification and placement process, in A Portrait of the Israeli Soldier. Westport, Conn, Greenwood Press, 1986, pp 76-96Google Scholar
25. Jastak S, Wilkinson GS: The Wide Range Achievement Test, Revised. Wilmington, Del, Jastak Associates, 1984Google Scholar
26. Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
27. Rabinowitz J, Reichenberg A, Weiser M, Mark M, Kaplan Z, Davidson M: Cognitive and behavioural functioning in men with schizophrenia both before and shortly after first admission to hospital: cross-sectional analysis. Br J Psychiatry 2000; 177:26-32Crossref, Medline, Google Scholar
28. Rabinowitz J: A method for preserving confidentiality when linking computerized registries (letter). Am J Public Health 1998; 88:836Crossref, Medline, Google Scholar
29. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
30. Jones P, Rodgers B, Murray R, Marmot M: Child development risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet 1994; 344:1398-1402Crossref, Medline, Google Scholar
31. Dalkin T, Murphy P, Glazebrook C, Medley I, Harrison G: Premorbid personality in first-onset psychosis. Br J Psychiatry 1994; 164:202-207Crossref, Medline, Google Scholar
32. Foerster A, Lewis S, Owen M, Murray R: Pre-morbid adjustment and personality in psychosis: effects of sex and diagnosis. Br J Psychiatry 1991; 158:171-176Crossref, Medline, Google Scholar
33. Van Os J, Jones P, Lewis G, Wadsworth M, Murray R: Developmental precursors of affective illness in a general population birth cohort. Arch Gen Psychiatry 1997; 54:625-631Crossref, Medline, Google Scholar
34. Evans JD, Heaton RK, Paulsen JS, McAdams LA, Heaton SC, Jeste DV: Schizoaffective disorder: a form of schizophrenia or affective disorder? J Clin Psychiatry 1999; 60:874-882Crossref, Medline, Google Scholar
35. Zihl J, Gron G, Brunnauer A: Cognitive deficits in schizophrenia and affective disorders: evidence for a final common pathway disorder. Acta Psychiatr Scand 1998; 97:351-357Crossref, Medline, Google Scholar
36. Manschreck TC, Maher BA, Beaudette SM, Redmond DA: Context memory in schizoaffective and schizophrenic disorders. Schizophr Res 1997; 26:153-161Crossref, Medline, Google Scholar
37. Miller LS, Swanson-Green T, Moses JA Jr, Faustman WO: Comparison of cognitive performance in RDC-diagnosed schizoaffective and schizophrenic patients with the Luria-Nebraska Neuropsychological Battery. J Psychiatr Res 1996; 30:277-282Crossref, Medline, Google Scholar
38. Cederblad M, Dahlin L, Hagnell O, Hansson K: Intelligence and temperament as protective factors for mental health: a cross-sectional and prospective epidemiological study. Eur Arch Psychiatry Clin Neurosci 1995; 245:11-19Crossref, Medline, Google Scholar
39. Luthar SS, Zigler E: Vulnerability and competence: a review of research on resilience in childhood. Am J Orthopsychiatry 1991; 61:6-22Crossref, Medline, Google Scholar
40. Luthar SS: Vulnerability and resilience: a study of high-risk adolescents. Child Dev 1991; 62:600-616Crossref, Medline, Google Scholar
41. Masten AS, Garmezy N, Tellegen A, Pellegrini DS, Larkin K, Larsen A: Competence and stress in school children: the moderating effects of individual and family qualities. J Child Psychol Psychiatry 1988; 29:745-764Crossref, Medline, Google Scholar
42. Kandel E, Mednick SA, Kirkegaard-Sorensen L, Hutchings B, Knop J, Rosenberg R, Schulsinger F: IQ as a protective factor for subjects at high risk for antisocial behavior. J Consult Clin Psychol 1988; 56:224-226Crossref, Medline, Google Scholar
43. Sands JR, Harrow M: Vulnerability to psychosis in unipolar major depression: is premorbid functioning involved? Am J Psychiatry 1995; 152:1009-1015Link, Google Scholar
44. Basso MR, Bornstein RA: Neuropsychological deficits in psychotic versus nonpsychotic unipolar depression. Neuropsychology 1999; 13:69-75Crossref, Medline, Google Scholar
45. Bertolino A, Kumra S, Callicott JH, Mattay VS, Lestz RM, Jacobsen L, Barnett IS, Duyn JH, Frank JA, Rapoport JL, Weinberger DR: Common pattern of cortical pathology in childhood-onset and adult-onset schizophrenia as identified by proton magnetic resonance spectroscopic imaging. Am J Psychiatry 1998; 155:1376-1383Link, Google Scholar
46. Jeste DV, Heaton S, Paulsen JS, Ercoli L, Harris MJ, Heaton RK: Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996; 153:490-496Link, Google Scholar
47. Lipton AA, Simon FS: Psychiatric diagnosis in a state hospital: Manhattan State revisited. Hosp Community Psychiatry 1985; 36:368-373Abstract, Google Scholar
48. Forrester A, Owens DG, Johnstone EC: Diagnostic stability in subjects with multiple admissions for psychotic illness. Psychol Med 2001; 31:151-158Crossref, Medline, Google Scholar
49. Maj M, Pirozzi R, Formicola AM, Bartoli L, Bucci P. Reliability and validity of the DSM-IV diagnostic category of schizoaffective disorder: preliminary data. J Affect Disord 2000; 57:95-98Crossref, Medline, Google Scholar
50. Pulver AE, Carpenter WT, Adler L, McGrath J: Accuracy of the diagnoses of affective disorders and schizophrenia in public hospitals. Am J Psychiatry 1988; 145:218-220Link, Google Scholar
51. Rabinowitz J, Slyuzberg M, Ritsner M, Mark M, Popper M, Ginath Y: Changes in diagnosis in a 9-year national longitudinal sample. Compr Psychiatry 1994; 35:361-365Crossref, Medline, Google Scholar
52. Schwartz JE, Fennig S, Tanenberg-Karant M, Carlson G, Craig T, Galambos N, Lavelle J, Bromet EJ: Congruence of diagnoses 2 years after a first-admission diagnosis of psychosis. Arch Gen Psychiatry 2000; 57:593-600Crossref, Medline, Google Scholar
53. Kaplan HI, Sadock BJ (eds): Kaplan and Sadock’s Synopsis of Psychiatry: Behavioral Sciences, Clinical Psychiatry. Baltimore, Williams & Wilkins, 1998Google Scholar
54. Johnson L, Andersson-Lundman G, Aberg-Wistedt A, Mathe AA: Age of onset in affective disorder: its correlation with hereditary and psychosocial factors. J Affect Disord 2000; 59:139-148Crossref, Medline, Google Scholar
55. Dell’Osso L, Akiskal HS, Freer P, Barberi M, Placidi GF, Cassano GB: Psychotic and nonpsychotic bipolar mixed states: comparisons with manic and schizoaffective disorders. Eur Arch Psychiatry Clin Neurosci 1993; 243:75-81Crossref, Medline, Google Scholar
56. Crow TJ: A continuum of psychosis, one human gene, and not much else—the case for homogeneity. Schizophr Res 1995; 17:135-145Crossref, Medline, Google Scholar
57. Kendler KS, Karkowski LM, Walsh D: The structure of psychosis: latent class analysis of probands from the Roscommon Family Study. Arch Gen Psychiatry 1998; 55:492-499Crossref, Medline, Google Scholar



