Relation of Medial Temporal Lobe Volumes to Age and Memory Function in Nondemented Adults With Down’s Syndrome: Implications for the Prodromal Phase of Alzheimer’s Disease
Abstract
OBJECTIVE: In Down’s syndrome (trisomy 21), a dementia syndrome occurs that is phenotypically similar to Alzheimer’s disease; the initial phase is characterized by memory loss. The authors used an in vivo structural technique in the predementia stage of Alzheimer’s disease in adults with Down’s syndrome to investigate whether atrophy of medial temporal lobe structures occurs in these subjects and whether volumes of these structures correlate specifically with performance on memory tests. METHOD: The subjects were 34 nondemented Down’s syndrome adults (mean age=41.6 years, 17 women and 17 men) and 33 healthy comparison subjects (mean age=41.3, 15 women and 18 men). By using T1-weighted magnetic resonance imaging slices taken perpendicular to the Sylvian fissure, volumes of the hippocampus, amygdala, anterior and posterior parahippocampal gyrus, and temporal pole CSF were measured in both hemispheres. These data were normalized to the total intracranial volume. RESULTS: For Down’s syndrome, smaller volumes of the right and left amygdala, hippocampus, and posterior parahippocampal gyrus were significantly associated with greater age; this association was not seen in the anterior parahippocampal gyrus. The amygdala and hippocampus volumes were positively correlated with memory measures. For the comparison group, there was no relationship between volume and age in any region. CONCLUSIONS: In the predementia phase of Down’s syndrome, significant volume changes in medial temporal lobe structures occur with age and are related to memory. These structures are affected early in Alzheimer’s disease in Down’s syndrome, and their evaluation may help identify people in the preclinical stages of Alzheimer’s disease.
Down’s syndrome is a genetic disorder in which increased production of Aβ peptide due to an extra copy of the amyloid precursor protein gene on chromosome 21 results in a dementia syndrome in later life that is phenotypically similar to Alzheimer’s disease (1). Adults with Down’s syndrome older than 40 years show cognitive decline in distinct phases. Initially, there is no alteration in nonmemory functions. Rather, at this stage there is an isolated, slowly progressive memory decline (2, 3), analogous to the memory loss that is the earliest neuropsychological deficit in typical Alzheimer’s disease (4). This phase is followed by a linear decline in nonmemory cognitive functions coincident with dementia onset (2). Thus, older adults with Down’s syndrome represent a unique population in which to study the prodromal stages of Alzheimer’s disease, allowing the investigation of the neuroanatomic correlates of memory decline at the earliest stages.
By age 40, all adults with Down’s syndrome demonstrate some degree of neuropathologic defects postmortem that meet criteria for Alzheimer’s disease (5, 6); it is even later when significant neurofibrillary tangles accumulate in the neocortex (6, 7). In Down’s syndrome, neurofibrillary tangles also first appear in medial temporal lobe structures, beginning with the entorhinal cortex and CA1/subiculum of the hippocampus (6, 8–10). Whereas we (11) reported that before the development of dementia in Down’s syndrome adults, generalized progressive brain atrophy is not present, the findings from studies of hippocampal size in nondemented adults with Down’s syndrome differ on whether the size of the hippocampus decreases with increasing age (12–15).
Given the memory loss that occurs in nondemented older adults with Down’s syndrome, we hypothesized that brain macrostructural changes occur in the medial temporal lobe in the predementia phase of Alzheimer’s disease, and we sought to establish an in vivo approach to evaluate the degree of Alzheimer’s disease pathology in the brains of adults with Down’s syndrome and to assess the cognitive correlates of this pathology. Our larger strategy is to isolate clinically accessible variables that will indicate the onset of Alzheimer’s disease in the general population before the development of clinical dementia.
In the current study, we used magnetic resonance imaging (MRI) to determine the volumes of the hippocampus, amygdala, and anterior and posterior parahippocampal gyrus in 34 nondemented adults with Down’s syndrome who ranged in age from the third to the seventh decades, and we compared those assessments to measurements in age- and gender-matched healthy comparison subjects without Down’s syndrome. We predicted that the volumes of all the regions studied would be smaller with increasing age in Down’s syndrome and that the volumetric differences across the range of ages would be significantly greater than in the comparison subjects. Since the intact functioning of medial temporal lobe structures is important in aspects of memory processing (16), we correlated the volumes of our regions of interest with memory measures within the Down’s syndrome group. We predicted that smaller volumes would correlate with poorer performance on memory tests but not on tests of general intellectual function or other cognitive measures, such as language, not known to be specifically mediated by the medial temporal lobe memory system.
Method
Subjects
The subjects with Down’s syndrome were participants in a longitudinal study of Down’s syndrome and dementia conducted by the Laboratory of Neurosciences, National Institute on Aging. They volunteered, were recruited by physicians, or were referred by their families. Thirty-four subjects with trisomy 21, as ascertained through karyotyping, and 33 healthy comparison subjects were given MRI scans. The mean ages of the Down’s syndrome and comparison groups were similar: 41.6 years (SD=9.1, range=25.3–61.0) and 41.3 years (SD=10.7, range=26.2–64.5) (two-tailed t test: t=0.13, df=65, p=0.90). Gender distributions were also similar: 17 women and 17 men in the Down’s syndrome group and 15 women and 18 men in the comparison group (Pearson chi-square test: χ2=0.14, df=1, p=0.71).
All of the Down’s syndrome and comparison subjects underwent medical, neurological, and psychiatric evaluations according to published criteria (17); the assessment included a structured examination to exclude extrapyramidal diseases (18). All subjects had Hachinski scale (19) ischemia scores less than 5. No subject had a history of significant head trauma, toxin exposure, diabetes, or drug or alcohol abuse. Psychiatric disorders were diagnosed in four subjects with Down’s syndrome: two had obsessive-compulsive disorder, and two had psychotic disorder not otherwise specified. Twelve Down’s syndrome subjects had hypothyroidism treated with levothyroxine, and all 12 had levels of thyroid-stimulating hormone in the normal range. All subjects had normal results on urinalyses and blood tests that included measurements of electrolytes, glucose, minerals, lipids, folate, vitamin B12, antinuclear antibody, and rheumatoid factor; tests of liver, renal, and thyroid function; and tests for HIV and syphilis. Several of the subjects with Down’s syndrome had functional heart murmurs, and subjects who had not previously been evaluated for valvular heart disease were evaluated with echocardiograms as part of our study. A clinical screening evaluation of the MRIs, performed independently of the volumetric analyses, showed no evidence of stroke, tumor, or mass effect.
Subjects with dementia were excluded from this study. The diagnosis of dementia in Down’s syndrome was made by using modified criteria from DSM-III, which specified an acquired, progressive loss of intellectual function, such as loss of daily living and vocational skills, memory impairment, reduced speech and comprehension, and personality change. The diagnosis was based on interviews with caregivers, clinical examination, and bedside mental status tests using standardized criteria (20). Interrater reliability for our method of diagnosis of dementia in Down’s syndrome has been previously established (11).
A group-by-gender analysis of variance (ANOVA) with subject age as the dependent variable was nonsignificant for main effects and for group-by-gender interaction. The mean total intracranial volume was significantly lower in the Down’s syndrome group (mean=1254 cm3, SD=145) than in the comparison group (mean=1506 cm3, SD=170) (two-tailed t test: t=–6.52, df=65, p<0.001). In a regression analysis with the Down’s syndrome group, subject age did not predict subject total intracranial volume (F=0.89, df=32, p=0.35).
After complete description of the study to each subject or to the holder of a durable power of attorney or legal guardian, written informed consent was obtained. Assent to participate in the study also was obtained from the subjects with Down’s syndrome. The research was approved by the National Institute on Aging institutional review board.
MRI Protocol and Analysis Methods
MRI of the brain was performed on a 0.5-T scanner (Picker Instruments, Cleveland) and on a 1.5-T scanner (General Electric Signa II, Milwaukee). The 0.5-T scan was used to quantify total intracranial volume as measured from 6-mm-thick contiguous coronal slices (TR/TE=2000/20 msec, flip angle=45°, field of view=25 cm, matrix=256×160) obtained perpendicular to the inferior orbitomeatal line. The 1.5-T scanner was used to quantify volumes of medial temporal lobe structures on 5-mm-thick contiguous oblique T1-weighted slices (TR/TE=530/20 msec, flip angle=90°, field of view=16 cm, matrix 256×256) perpendicular to the Sylvian fissure, as ascertained through sagittal scout slices. Several of the subjects with Down’s syndrome could not complete the scan while awake and underwent the scan with intravenous sedation while under the care of an anesthesiologist.
A region-of-interest analysis was applied to determine the total intracranial volume and the volumes of the hippocampus, amygdala, anterior and posterior parahippocampal gyrus, and CSF in the medial temporal lobes. The volume, in cubic centimeters, of each structure was calculated by summing the areas (in square centimeters) of the regions of interest across slices and multiplying by slice thickness. Differences in head size were corrected for by dividing each regional volume by the total intracranial volume and multiplying by 100 (21).
We analyzed the data on a Sun Microsystems (Mountain View, Calif.) workstation with a high-resolution monitor, using a mouse-driven cursor and proprietary tracing software, the details of which have been described previously (22, 23). The scans from the healthy comparison subjects and the subjects with Down’s syndrome were mixed and analyzed by an investigator blind to subject group, age, gender, and cognitive status. All regions of interest were analyzed by one investigator (J.S.K.).
The left and right amygdalae and hippocampal formations were traced according to the method of Watson et al. (24), except that tracing caudally was begun in every case on the slice containing the Sylvian aqueduct and that tracing was not concluded rostrally on the slice with closure of the lateral sulcus, but continued until the anterior amygdalar nucleus was no longer seen. The original criteria, developed by using images from healthy subjects, would have necessitated stopping the tracing on slices that clearly had substantial amygdala remaining. More important, the closure of the lateral sulcus did not appear to be in a consistent spatial relationship to the amygdala in the Down’s syndrome brains. The parahippocampal gyrus was divided along its long axis into anterior and posterior regions of interest. The anterior parahippocampal gyrus region was developed to isolate the entorhinal cortex from the other medial temporal lobe regions of interest (25). For this study we developed a region of interest for the temporal pole lateral ventricle CSF volume. CSF tracing was begun on the slice with the Sylvian aqueduct and proceeded rostrally until temporal pole CSF was no longer seen (Figure 1).
Intrarater reliabilities for determination of volumes for the medial temporal lobe regions of interest were calculated by using the intercorrelation coefficient; values ranged from 0.91 for the right amygdala to 0.99 for the right posterior parahippocampal gyrus (p<0.005 for all the regions of interest) (25). Interrater reliability for total intracranial volume determination also was significant, according to the intercorrelation coefficient (F=47) (23).
Cognitive Assessment
A battery of neuropsychological tests was administered to each subject with Down’s syndrome by trained psychometricians (26). The tests included measures of general intellectual, memory, visuospatial, and language function. The instruments included the Peabody Picture Vocabulary Test—Revised (27), the Down Syndrome Mental Status Examination (orientation, memory, language, praxis, and visuospatial ability) (26), the Hidden Object Memory Test with immediate, 10-second, and 2-minute delayed recall (26), Extended Block Design (26), and measures of confrontation naming, conceptual naming, and object identification (26).
Statistical Analyses
All statistical analyses were conducted by using SPSS for Windows Version 7.0 (Chicago). The analyses were based on regional volumes normalized to total intracranial volume. To test for the effects of age while controlling for gender effects, we performed linear regressions with the regional volumes as dependent variables and gender entered first into the model followed by subject age. Statistical significance for these regression analyses was taken at p<0.05.
Because we hypothesized that differences in medial temporal volumes would be correlated with differences in memory measures, the regional volumes for the Down’s syndrome group were correlated with a series of neuropsychological variables by means of Pearson bivariate correlations. A follow-up set of analyses was performed to determine whether the major correlational effects would hold after we controlled for possible cohort differences in general intellectual function. In these latter analyses, partial correlations were obtained for the same regional volumes and neuropsychological measures while we controlled for subject differences in general cognitive function as represented by scores on the Peabody Picture Vocabulary Test—Revised. Statistical significance was taken at p≤0.001 for correlations of the neuropsychological measures with the MRI brain volumes. This significance threshold was selected to reduce the chance of obtaining spurious findings due to the relatively large number of statistical tests, but not to be so conservative as to miss true effects.
Results
In the Down’s syndrome group, smaller volumes of the right and left amygdala, hippocampus, and posterior parahippocampal gyrus were significantly associated with greater age. This relationship was not present in the right and left anterior parahippocampal gyrus (Table 1). In the comparison group, no relationship was seen between volume of any of these structures and age.
For right and left temporal pole CSF, both the comparison and Down’s syndrome groups showed significant age-related volume differences. Figure 2 shows representative scatterplots of age versus region of interest volumes in the two groups.
In the Down’s syndrome group, no correlation between any regional volume and the score on the Peabody Picture Vocabulary Test—Revised was significant. Table 2 shows partial correlations between regional brain volumes and memory measures for the subjects with Down’s syndrome. For both Pearson bivariate correlations (data not shown) and partial correlations controlling for general intellectual function, the volumes of the right amygdala and left hippocampus were most consistently positively correlated with memory measures of the Down Syndrome Mental Status Examination and the Hidden Object Memory Test. Temporal pole CSF, especially on the left side, was negatively correlated with these memory measures. When partial correlations between regional volumes and scores on tests evaluating other cognitive functions (data not shown) were assessed, no correlations were significant at the p<0.001 level.
Discussion
Because the initial phase of cognitive decline (before dementia) in adults with Down’s syndrome is characterized by a slowly progressive memory loss, we hypothesized that atrophic changes would occur in the medial temporal lobe, the brain region initially affected with Alzheimer’s disease pathology. In our study group of nondemented Down’s syndrome adults and comparison subjects (who were closely matched on age and gender), the Down’s syndrome group showed age-related differences in the bilateral volumes of the amygdala, hippocampus, and posterior parahippocampal gyrus, with older subjects having smaller volumes. On the basis of postmortem studies indicating that adults with Down’s syndrome have prominent neuropathology in medial temporal lobe structures in the early stages of Alzheimer’s disease (6, 8–10), the age-related volumetric differences we found in the Down’s syndrome group are consistent with an Alzheimer’s-related atrophy and show that structural changes in specific brain regions are occurring in the prodrome of Alzheimer’s disease.
Examination of autopsied brain tissue from adults with Alzheimer’s disease without trisomy 21 revealed that the earliest Alzheimer’s disease neuropathology occurs in medial temporal lobe structures of the entorhinal cortex, hippocampus, and amygdala (28, 29); in vivo volumetric MRI of demented individuals with Alzheimer’s disease also indicates that brain atrophy in Alzheimer’s disease occurs first in these same regions (25, 30–34). Further, Huesgen and colleagues (35) showed a large inverse correlation between number of neurofibrillary tangles and MRI-determined hippocampal area in postmortem brain tissue from 13 individuals without trisomy 21 with proven Alzheimer’s disease. Of greater relevance to our work are emerging studies of older nondemented individuals with mild cognitive impairment. Such individuals have memory complaints and objective memory impairment only, and they are thought to be at high risk for Alzheimer’s disease. Hippocampal atrophy determined at this stage predicted subsequent development of Alzheimer’s disease (36). Because it remains uncertain whether all subjects with mild cognitive impairment develop Alzheimer’s disease and long follow-up is required, large cohorts of subjects are necessary for studies. On the other hand, Down’s syndrome subjects universally develop the neuropathology of Alzheimer’s disease, with increasing amyloid deposition associated with greater age.
Although some studies (31, 37–39) have shown that adults from the general population show age-related volume decreases in the hippocampus, other studies (including the present one) have not confirmed this (40–42). Murphy et al. (38) studied healthy subjects, including an elderly group of adults between 60 and 85 years of age, and in addition to finding age-related reductions in hippocampal size, they found that the amygdala and parahippocampal gyrus were significantly smaller than in the young subjects. Because the studies that did show age-related declines in volumes of medial temporal lobe structure included subjects much older than our relatively young comparison group, age-related atrophy in the general population, if it occurs, might not be seen until ages greater than those of our comparison subjects. Thus, because our comparison group did not show smaller volumes of these regions with greater age, it is unlikely that the smaller medial temporal lobe volumes in our older nondemented Down’s syndrome adults are due to age-related atrophy.
We developed the temporal pole CSF region of interest as a nonspecific but sensitive marker for medial temporal lobe atrophy. Its nonspecificity is due to the fact that increases in this region can be secondary to atrophy of any of the surrounding brain structures, such as the hippocampus, the amygdala, and the anterior and posterior parahippocampal gyrus. Its sensitivity is due to the fact that, because in young healthy adults the volume of the temporal pole CSF is smaller than the volume of surrounding structures, relatively minor atrophy of the medial temporal lobe structures can result in proportionally greater volume increases in the CSF. Further, increases in the CSF volume can be due to the minor but cumulative atrophy of all surrounding structures. Consistent with the view that the temporal pole CSF is a sensitive marker of age-related differences, we found that right and left temporal pole CSF were the only regions of interest in the comparison group that showed age-related differences (larger volume with greater age). Although other brain regions, including regions in the medial temporal lobe, previously have been evaluated in healthy subjects across the adult lifespan, age-related volume differences in temporal pole CSF among healthy subjects has not been addressed, to our knowledge.
We also hypothesized that in Down’s syndrome smaller volumes (atrophy) of the medial temporal lobe structures would be associated with poorer performance on cognitive tasks that depend on the intact functioning of this area, i.e., memory performance. This hypothesis was confirmed for the correlation of memory measures with amygdala and hippocampus volumes. The temporal pole CSF volume also consistently correlated with memory performance: the larger the CSF volume, the poorer the performance on memory measures.
In order to exclude the possibility that the relation between memory measures and regional volumes was nonspecific, we evaluated measures of general intellectual function as well as other nonmemory measures. The Peabody Picture Vocabulary Test—Revised, a measure that correlates with general cognitive function (43, p. 542), did not correlate with any regional volume. To further test this point, we performed partial correlations of region of interest volumes with all the neuropsychological measures while controlling for general cognitive function as measured by the Peabody Picture Vocabulary Test. We found that the memory measures remained correlated with amygdala, hippocampal, and temporal pole CSF volumes. Of the other cognitive subtests, none correlated with the medial temporal lobe volumes at the p≤0.001 level.
The specificity of the correlations between memory measures and regional volumes suggests that function of these medial temporal lobe structures is compromised when volume is reduced. The specificity of correlation between amygdala and hippocampus volumes and the memory measures, even when we controlled for general cognitive function, also argues against cohort effect due to subject differences in general intellectual capabilities.
It is possible that age-related cohort differences in Down’s syndrome groups may affect our findings. However, we did not observe differences in total intracranial volume as a function of age. Total intracranial volume might be expected to reflect subject differences in nutritional status and general health during the developmental period. Also, the specificity of correlations between the region of interest volumes and memory measures suggests that the volume differences that occur with age in the Down’s syndrome group cannot be explained solely by these general cohort effects.
One region of interest, the anterior parahippocampal gyrus, isolates the entorhinal cortex from adjacent brain regions (see our earlier work [25] for analysis guidelines). The entorhinal cortex is affected by pathology early in Alzheimer’s disease, but in neither group did it show an age-related decline. This region contains not only entorhinal cortex but also underlying white matter tracts that, if differentially unaffected by Alzheimer’s disease neuropathology, might diminish possible Alzheimer’s-related atrophy seen in the gray matter entorhinal cortex. Studies on normal aging (44, 45) have shown that gray matter but not white matter volume decreases with age.
Although recent studies of structural evidence of early Alzheimer’s disease have used cross-sectional volumetric assessment of medial temporal structures, another potential technique may be subtraction of registered serial MRI scans. Fox et al. (46) showed that rates of change of global cerebral volume measured with this latter technique can identify subjects in the preclinical stages of Alzheimer’s disease. Although such studies are limited to longitudinal analysis of the same subjects, future studies that use MRI morphometry to evaluate longitudinal rates of decline in medial temporal lobe or whole brain structures may provide even greater sensitivity in detecting the preclinical effects of Alzheimer’s disease in Down’s syndrome.
Several features distinguish our study from other MRI studies of medial temporal lobe structures in nondemented adults with Down’s syndrome (12–15). First, our study had relatively large study groups and broad age ranges. With subjects both younger than 30 years and older than 50 years, it was possible to differentiate the effects of early Alzheimer’s disease from mental retardation. Second, in our study all Down’s syndrome subjects and comparison subjects underwent rigorous medical screening, so the effects of confounding medical problems could be avoided. Standardized criteria for exclusion of dementia in mental retardation were used. Third, several medial temporal lobe structures were examined, including the parahippocampal gyrus (a structure that shows early atrophy in adults with Alzheimer’s disease without Down’s syndrome). Finally, both memory and other cognitive measures were evaluated, which allowed us to determine which cognitive functions were disrupted by medial temporal lobe atrophy.
In Down’s syndrome, where mental retardation precedes dementia, the question arises to what degree the changes in medial temporal lobe volumes can be reliably related to Alzheimer’s disease. Prior studies (1, 47) in young, nondemented adults with Down’s syndrome showed that brain size, although smaller than in age-matched healthy comparison subjects, was proportional to the smaller stature that occurs in Down’s syndrome. Further, longitudinal studies showed that progressive generalized brain atrophy and decline in regional cerebral glucose metabolism at rest did not occur (1). Therefore, mental retardation in Down’s syndrome is a static process related to inherent cerebral dysfunction and not to acquired cerebral atrophy or metabolic decline. These results suggest that brain imaging is able to distinguish the progressive brain changes due to dementia from the mental retardation in Down’s syndrome.
Although atrophy of medial temporal lobe structures is accompanied by progressive memory loss, it precedes the appearance of nonmemory cognitive impairment and dementia. MRI quantification of medial temporal lobe volumes may aid in predicting development of dementia during the predementia phase of Alzheimer’s disease, thus aiding early diagnosis. Further studies are needed to assess whether volumes or rates of decline in volumes of medial temporal lobe structures are related to risk and age at onset of dementia. Our findings support further study of nondemented adults with Down’s syndrome as a clinical model of the predementia phase of Alzheimer’s disease in subjects with a 100% risk for Alzheimer’s disease pathology.
 |
 |
Presented in part at the 52nd annual meeting of the Society for Biological Psychiatry, San Diego, May 15–17, 1997, and at the 27th annual meeting of the Society for Neurosciences, New Orleans, Oct. 25–30, 1997. Received Sept. 8, 2000; revision received July 31, 2001; accepted Aug. 11, 2001. From the Laboratory of Neurosciences, National Institute on Aging, Bethesda, Md. Address reprint requests to Dr. Schapiro, Division of Neurology, Children’s Hospital Medical Center, 3333 Burnet Ave., Cincinnati, OH 45229-3039. Supported by the Intramural Program, National Institute on Aging. The authors thank Ms. Diane Teichberg and Ms. Lisa Chang for assisting in the preparation of the magnetic resonance imaging scans for analysis and Ms. Joanna Szczepanik for performing the neuropsychological testing.
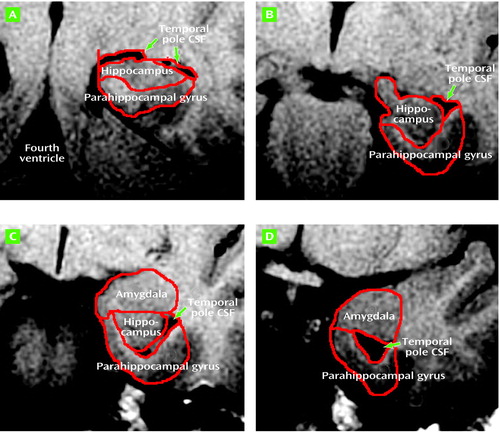
Figure 1. Medial Temporal Lobe Structures Analyzed in MRI Scans in a Studya of Adults With Down’s Syndrome
aRepresentative oblique MRI slices, obtained perpendicular to the Sylvian aqueduct, illustrate the medial temporal region of the left temporal lobe. The slices are presented from caudal (A) to rostral (D).
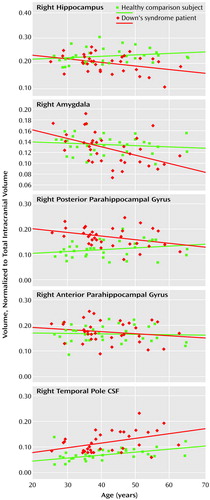
Figure 2. Individual Normalized Medial Temporal Lobe Volumes as a Function of Age in 34 Adults With Down’s Syndrome and 33 Healthy Comparison Subjectsa
aSuperimposed regression lines (controlled for the effects of gender) are shown for each group.
1. Schapiro MB, Haxby JV, Grady CL: Nature of mental retardation and dementia in Down syndrome: study with PET, CT, and neuropsychology. Neurobiol Aging 1992; 13:723-734Crossref, Medline, Google Scholar
2. Haxby JV, Schapiro MB: Longitudinal study of neuropsychological function in older adults with Down syndrome, in Down Syndrome and Alzheimer’s Disease. Edited by Nadel L, Epstein CJ. New York, Wiley-Liss, 1992, pp 35-50Google Scholar
3. Alexander GE, Saunders AM, Szczepanik J, Strassburger T, Pietrini P, Dani A, Furey ML, Mentis MJ, Roses AD, Rapoport SI, Schapiro MB: Relation of age and apolipoprotein E to cognitive function in Down syndrome adults. Neuroreport 1997; 8:1835-1840Crossref, Medline, Google Scholar
4. Haxby JV, Grady CL, Duara R, Schlageter N, Berg G, Rapoport SI: Neocortical metabolic abnormalities precede nonmemory cognitive defects in early Alzheimer’s type dementia. Arch Neurol 1986; 43:882-885Crossref, Medline, Google Scholar
5. Mann DMA, Yates PO, Marciniuk B: Alzheimer’s presenile dementia, senile dementia of Alzheimer type and Down syndrome in middle age form an age-related continuum of pathological changes. Neuropathol Appl Neurobiol 1984; 10:185-207Crossref, Medline, Google Scholar
6. Hof PR, Bouras C, Perl DP, Sparks L, Mehta N, Morrison JH: Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down’s syndrome: quantitative regional analysis and comparison with Alzheimer’s disease. Arch Neurol 1995; 52:379-391Crossref, Medline, Google Scholar
7. Burger PC, Vogel FS: The development of the pathologic changes of Alzheimer’s disease and senile dementia in patients with Down’s syndrome. Am J Pathol 1973; 73:457-476Medline, Google Scholar
8. Mann DMA, Esiri MM: The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down’s syndrome. J Neurol Sci 1989; 89:169-179Crossref, Medline, Google Scholar
9. Hyman BT, West HL, Rebeck GW, Lai F, Mann DMA: Neuropathological changes in Down’s syndrome hippocampal formation: effect of age and apolipoprotein E genotype. Arch Neurol 1995; 52:373-378Crossref, Medline, Google Scholar
10. Ball MJ, Schapiro MB, Rapoport SI: Neuropathological relationships between Down syndrome and senile dementia Alzheimer type, in Neurobiology of Down Syndrome. Edited by Epstein C. New York, Raven Press, 1986, pp 45-58Google Scholar
11. Schapiro MB, Luxenberg JS, Kaye JA, Haxby JV, Friedland RP, Rapoport SI: Serial quantitative CT analysis of brain morphometrics in adult Down’s syndrome at different ages. Neurology 1989; 39:1349-1353Crossref, Medline, Google Scholar
12. Raz N, Torres IJ, Briggs SD, Spencer WD, Thornton AE, Loken WJ, Gunning FM, McQuain JD, Driesen NR, Acker JD: Selective neuroanatomic abnormalities in Down’s syndrome and their cognitive correlates: evidence from MRI morphometry. Neurology 1995; 45:356-366Crossref, Medline, Google Scholar
13. Kesslak JP, Nagata SF, Lott I, Nalcioglu O: Magnetic resonance imaging analysis of age-related changes in the brains of individuals with Down’s syndrome. Neurology 1994; 44:1039-1045Crossref, Medline, Google Scholar
14. Aylward EH, Li Q, Honeycutt NA, Warren AC, Pulsifer MB, Barta PE, Chan MD, Smith PD, Jerram M, Pearlson GD: MRI volumes of the hippocampus and amygdala in adults with Down’s syndrome with and without dementia. Am J Psychiatry 1999; 156:564-568Abstract, Google Scholar
15. Pearlson GD, Breiter SN, Aylward EH, Warren AC, Grygorcewicz M, Frangou S, Barta PE, Pulsifer MB: MRI brain changes in subjects with Down syndrome with and without dementia. Dev Med Child Neurol 1998; 40:326-334Medline, Google Scholar
16. Squire LR, Zola-Morgan S: The medial temporal lobe memory system. Science 1991; 253:1380-1386Crossref, Medline, Google Scholar
17. Duara R, Margolin RA, Robertson-Tchabo EA, London ED, Schwartz M, Renfrew JW, Koziarz BJ, Sundaram M, Grady C, Moore AM, Ingvar DH, Sokoloff L, Weingartner H, Kessler RM, Manning RG, Channing MA, Cutler NR, Rapoport SI: Cerebral glucose utilization, as measured with positron emission tomography in 21 resting healthy men between the ages of 21 and 83 years. Brain 1983; 106:761-775Crossref, Medline, Google Scholar
18. Kaye JA, May M, Atack JR, Daly E, Sweeney DL, Beal MF, Kaufman S, Milstien S, Friedland RP, Rapoport SI: Cerebrospinal fluid neurochemistry in the myoclonic subtype of Alzheimer’s disease. Ann Neurol 1988; 41:647-650Crossref, Google Scholar
19. Hachinski VC, Iliff LD, Zhilkha E, Du Boulay GH, McAllister VL, Marshall J, Russell RW, Symon L: Cerebral blood flow in dementia. Arch Neurol 1975; 32:632-637Crossref, Medline, Google Scholar
20. Schapiro MB, Haxby JV, Grady CL, Duara R, Schlageter NL, White B, Moore A, Sundaram M, Larson SM, Rapoport SI: Decline in cerebral glucose utilization and cognitive function with aging in Down syndrome. J Neurol Neurosurg Psychiatry 1987; 50:766-774Crossref, Medline, Google Scholar
21. Brinkman SD, Sarwar M, Lewin LHS, Morris HH: Quantitative indexes of computed tomography in dementia and normal aging. Radiology 1981; 138:89-92Crossref, Medline, Google Scholar
22. Murphy DGM, DeCarli C, Schapiro MB, Rapoport SI, Horwitz B: Age-related differences in volumes of subcortical nuclei, brain matter, and cerebrospinal fluid in healthy men as measured with magnetic resonance imaging. Arch Neurol 1992; 49:839-845Crossref, Medline, Google Scholar
23. DeCarli C, Maisog J, Murphy DGM, Teichberg D, Rapoport SI, Horwitz B: Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr 1992; 16:274-284Crossref, Medline, Google Scholar
24. Watson C, Andermann F, Gloor P, Jones-Gotman M, Peters T, Evans A, Olivier A, Melanson D, Leroux G: Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging. Neurology 1992; 42:1743-1750Crossref, Medline, Google Scholar
25. Krasuski JS, Alexander GE, Horwitz B, Daly EM, Murphy DGM, Rapoport SI, Schapiro MB: Volumes of medial temporal lobe structures in patients with Alzheimer disease and mild cognitive impairment (and in healthy controls). Biol Psychiatry 1998; 43:60-68Crossref, Medline, Google Scholar
26. Haxby JV: Neuropsychological evaluation of adults with Down’s syndrome: patterns of selective impairment in non-demented old adults. J Ment Defic Res 1989; 33:193-210Medline, Google Scholar
27. Dunn LM, Dunn LM: Peabody Picture Vocabulary Test—Revised. Circle Pines, Minn, American Guidance Service, 1981Google Scholar
28. Braak H, Braak E, Bohl J: Staging of Alzheimer-related cortical destruction. Eur Neurol 1993; 33:403-408Crossref, Medline, Google Scholar
29. Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL: Alzheimer’s disease: cell specific pathology isolates the hippocampal formation. Science 1984; 225:1168-1170Crossref, Medline, Google Scholar
30. Pearlson GD, Harris GJ, Powers RE, Barta PE, Camargo EE, Chase GA, Noga JT, Tune LE: Quantitative changes in mesial temporal volume, regional cerebral blood flow, and cognition in Alzheimer’s disease. Arch Gen Psychiatry 1992; 49:402-408Crossref, Medline, Google Scholar
31. Jack CR, Petersen RC, O’Brien PC, Tangalos EG: MR-based hippocampal volumetry in the diagnosis of Alzheimer’s disease. Neurology 1992; 42:183-188Crossref, Medline, Google Scholar
32. Laakso MP, Soininen H, Partanen K, Helkala EL, Hartikainen P, Vainio P, Hallikainen M, Hanninen T, Riekkinen PJ Sr: Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer’s disease: correlations with memory functions. J Neural Transm Park Dis Dement Sect 1995; 9:73-86Crossref, Medline, Google Scholar
33. Convit A, deLeon MJ, Golomb J, George AE, Tarshish CY, Bobinski M, Tsui W, De Santi S, Wegiel J, Wisniewski H: Hippocampal atrophy in early Alzheimer’s disease: anatomic specificity and validation. Psychiatr Q 1993; 64:371-387Crossref, Medline, Google Scholar
34. Plassman BL, Welsh-Bohmer KA, Bigler ED, Johnson SC, Anderson CV, Helms MJ, Saunders AM, Breitner JCS: Apolipoprotein E epsilon 4 allele and hippocampal volume in twins with normal cognition. Neurology 1997; 48:985-989Crossref, Medline, Google Scholar
35. Huesgen CT, Burger PC, Crain BJ, Johnson GA: In vitro MR microscopy of the hippocampus in Alzheimer’s disease. Neurology 1993; 43:145-152Crossref, Medline, Google Scholar
36. Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E: Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology 1999; 52:1397-1403Crossref, Medline, Google Scholar
37. Golomb J, de Leon MJ, Kluger A, George AE, Tarshish C, Ferris SH: Hippocampal atrophy in normal aging: an association with recent memory impairment. Arch Neurol 1993; 50:967-973Crossref, Medline, Google Scholar
38. Murphy DGM, DeCarli C, McIntosh AR, Daly E, Mentis MJ, Pietrini P, Szczepanik J, Schapiro MB, Grady CL, Horwitz B, Rapoport SI: Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry 1996; 53:585-594Crossref, Medline, Google Scholar
39. Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, Loken WJ, Thornton AE, Acker JD: Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex 1997; 7:268-282Crossref, Medline, Google Scholar
40. Sullivan EV, Marsh L, Lim KO, Pfefferbaum A: Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiol Aging 1995; 16:591-606Crossref, Medline, Google Scholar
41. Bhatia S, Bookheimer SY, Gaillard WD, Theodore WH: Measurement of whole temporal lobe and hippocampus for MR volumetry: normative data. Neurology 1993; 43:2006-2010Crossref, Medline, Google Scholar
42. Laakso MP, Soininen H, Partanen K, Lehtovirta M, Hallikainen M, Hanninen T, Helkala EL, Vainio P, Riekkinen PJ Sr: MRI of the hippocampus in Alzheimer’s disease: sensitivity, specificity, and analysis of the incorrectly classified subjects. Neurobiol Aging 1998; 19:23-31Crossref, Medline, Google Scholar
43. Lezak MD: Neuropsychological Assessment, 3rd ed. New York, Oxford University Press, 1995Google Scholar
44. Jernigan TL, Press GA, Hesselink JR: Methods for measuring brain morphologic features on magnetic resonance images: validation and normal aging. Arch Neurol 1990; 45:404-408Google Scholar
45. Pfefferbaum A, Mathalon DH, Sullivan EV, Rawles JM, Zipursky R, Lim KO: A quantitative magnetic resonance imaging study of changes in brain morphology from infancy to late adulthood. Arch Neurol 1994; 51:874-887Crossref, Medline, Google Scholar
46. Fox NC, Warrington EK, Rossor MN: Serial magnetic resonance imaging of cerebral atrophy in preclinical Alzheimer’s disease (letter). Lancet 1999; 353:2125Crossref, Medline, Google Scholar
47. Jernigan TL, Bellugi U: Anomalous brain morphology on magnetic resonance images in Williams syndrome and Down syndrome. Arch Neurol 1990; 47:529-533Crossref, Medline, Google Scholar



