[3H]cAMP Binding Sites and Protein Kinase A Activity in the Prefrontal Cortex of Suicide Victims
Abstract
OBJECTIVE: The cAMP-dependent enzyme protein kinase A phosphorylates intracellular proteins upon activation and thereby plays a major role in mediating various physiological functions in the brain. To examine the role of this enzyme in suicidal behavior, the authors examined the catalytic and regulatory activities of protein kinase A in the postmortem brain of suicide victims. METHOD: Brain tissues were collected from 17 suicide victims and 17 nonpsychiatric comparison subjects. Regulatory activity was determined by examining [3H]cAMP binding to protein kinase A, while catalytic activity was determined by enzymatic assay in the presence (total activity) and the absence (endogenous activity) of cAMP in the membrane and cytosol fractions of the prefrontal cortex. RESULTS: The number (Bmax) of [3H]cAMP binding sites to protein kinase A was significantly lower in the suicide victims without any changes in affinity in either the membrane or cytosol fractions of the prefrontal cortex. Further, significantly less protein kinase A activity, both in the presence and the absence of cAMP, was seen in the membrane and cytosol fractions of the prefrontal cortex of suicide victims; however, the difference in total protein kinase A activity was much more pronounced. CONCLUSIONS: The results suggest that cAMP binding to the regulatory subunits of protein kinase A, as well as the phosphotransfer catalytic activity of protein kinase A, are lower in the prefrontal cortex of suicide victims than in nonpsychiatric comparison subjects, which may be of clinical relevance in the pathophysiology of suicidal behavior.
Suicide is a major public health concern. Although there have been several studies that have suggested that suicide is associated with neurobiological abnormalities, the precise molecular mechanisms associated with suicidal behavior remain unclear. With this respect, several lines of evidence suggest the involvement of neurotransmitter receptors (e.g., α2-adrenergic, β-adrenergic, and the serotonin [5-HT] receptors 5-HT1A, 5-HT2A, and 5-HT2C) in the pathophysiology of suicide. Greater agonist binding to α2-adrenergic receptors and a higher number of binding sites for β-adrenergic, 5-HT2A, and 5-HT2C receptors have been shown in the postmortem brain of suicide victims, while fewer 5-HT1A binding sites have also been reported (reviewed by Gross-Iseroff et al. [1]).
Previous studies have suggested that apart from the neurotransmitter receptors, the signaling cascade through which these receptors mediate their physiological responses may be involved in the pathophysiology of suicide. Receptors such as 5-HT2A and 5-HT2C are linked with the phosphoinositide signaling system, whereas 5-HT1A, α2-adrenergic, and β-adrenergic receptors are linked with the adenylyl cyclase–cAMP signaling system. In the phosphoinositide signaling system, our previous studies have shown less [3H]phorbol dibutyrate binding to protein kinase C (2) and lower catalytic activity of phosphoinositide-specific phospholipase C (3) in the prefrontal cortex of suicide victims. In addition, Pacheco and Jope (4) and Jope et al. (5) have reported an alteration in the overall phosphoinositide signaling system in the postmortem brain of suicide victims. These studies thus suggest an abnormality in the phosphoinositide signaling system in the postmortem brain of suicide victims. Contrary to this, the involvement of the adenylyl cyclase–cAMP signaling system in the pathophysiology of suicide has not been evaluated in greater detail. In this signaling system, receptor-mediated activation of Gsα or Giα proteins causes modulation of adenylyl cyclase, which in turn causes the conversion of ATP to cAMP. cAMP serves as a second messenger and activates the protein kinase A enzyme. Protein kinase A then phosphorylates various substrate proteins in cells, thereby mediating a variety of hormonal and physiological responses, including receptor down-regulation, desensitization, altered neurotransmitter release, and activation or repression of gene expression (6, 7). In the postmortem brain of suicide victims, higher levels of Gs and lower levels of Gi have been shown (8, 9), and lower levels of GTPγS-stimulated and forskolin-stimulated cAMP formation have been reported (10). Since protein phosphorylation mediated by protein kinase A is the major mechanism of signal transduction in this signaling pathway, studying the status of protein kinase A beyond the generation of cAMP provides direct evidence of abnormal cellular signaling at the level of functional response.
Protein kinase A exists as a tetramer holoenzyme that consists of two regulatory and two catalytic subunits (11, 12). The regulatory subunits regulate the activity of the catalytic subunits and have been recognized as the proteins that bind cAMP. In the holoenzyme state, protein kinase A exists in an inactive form. Following an increase in intracellular cAMP, the regulatory subunits bind to cAMP, which results in the dissociation of the holoenzyme into a dimeric regulatory and two monomers of catalytic subunits (13). The free catalytic subunits can then phosphorylate substrates or translocate into the nucleus by passive diffusion and phosphorylate nuclear substrates (14). Thus, both catalytic and regulatory subunits are important in facilitating protein kinase A-mediated functions.
In the present investigation, we evaluated the role of protein kinase A in suicidal behavior by examining both its regulatory and catalytic properties in the prefrontal cortex (Brodmann’s area 9) of suicide victims and nonpsychiatric comparison subjects. We elected to analyze prefrontal area 9 because of our long-standing interest in the dorsolateral prefrontal cortex. Previously, we have observed a higher number of binding sites for 5-HT2A receptors and abnormalities in the phosphoinositide signaling system in this brain area of suicide victims (2, 3, 15). Very recently, we have shown lower activation and expression of another phosphorylating enzyme, extracellular-regulated kinases, in this brain area of suicide victims (16). Besides these findings, this brain area has been shown to play a relevant role in mood regulation (17) and has been implicated in the pathophysiology of suicide and affective disorders in a number of other neurochemical studies (reviewed in reference 18).
Method
Subjects, Tissue Collection, Dissection, and Storage
We studied [3H]cAMP binding to protein kinase A and protein kinase A activity in the right hemisphere prefrontal cortex (Brodmann’s area 9) of suicide victims and nonpsychiatric comparison subjects. Brain tissues were collected from the Brain Collection Program of the Maryland Psychiatric Research Center, Baltimore, in collaboration with the Medical Examiner’s Office of the State of Maryland. Tissues were collected only after a family member gave informed consent. Tissue samples were obtained from 17 suicide victims and 17 nonpsychiatric comparison subjects who died of natural or accidental causes. Family permission was obtained from the closest next of kin in all cases. After the brain had been removed from the cranium, the area of interest was defined from Brodmann’s atlas. Gray and white matters were separated by gross dissection. The prefrontal cortex was defined as the gray matter from the most anterior 1-cm coronal slice of the cortex. The prefrontal cortex was further dissected according to Brodmann’s atlas. The dissected areas were chopped into approximately 3-mm cubes, thoroughly mixed, and stored at –80°C until analyzed. To make sure that tissues were taken from the same anatomical levels, before chopping the tissues, one section was prepared from each block and Nissl stained. The tissue sections were matched across the comparison and suicide groups. Brain samples were free of any neuropathological abnormalities or HIV antibodies. Toxicological data were obtained by the analysis of urine and blood samples from these subjects.
Diagnosis
All subjects in this study were diagnosed in the following manner: at least one family member, after giving written informed consent, underwent an interview based on the Diagnostic Evaluation After Death (19) and the Structured Clinical Interview for DSM-IV (SCID) (20). Family members gave permission for clinical records to be obtained from mental health treatment providers for all cases of suicide and when a comparison subject had a history of mental health treatment. An attempt was made to collect all the available records on each subject, with the appropriate data then extracted from the records and collated by using the Diagnostic Evaluation After Death. Two senior psychiatrists provided independent DSM-IV diagnoses. These diagnoses were compared and discrepancies were resolved by means of a consensus conference. Similarly, the nonpsychiatric comparison subjects were verified as having been free from mental illnesses by using these consensus diagnostic procedures. Data on suicide victims were collected and the circumstances of the suicide were determined by using the Diagnostic Evaluation After Death form during the same interview process. Subjects were considered to be suicide victims only if the manner of death was determined to be suicide by the medical examiner. For subjects diagnosed with major depressive disorder, symptoms were present in the days before death. The protocol for tissue sampling and retrospective assessments was approved by the institutional review board of the University of Maryland. This study was also approved by the institutional review board of the University of Illinois at Chicago.
Determination of Bmax and KD of [3H]cAMP Binding
Specific [3H]cAMP binding was performed as previously described (21). Brain samples were homogenized in 10 volumes of ice-cold buffer containing 20 mM of Tris-HCl (pH 7.4 at 25°C), 2 mM of ethylenediaminetetraacetic acid (EDTA), 25 mM of 2-mercaptoethanol,4-(2-aminoethyl) benzenesulfonyl fluoride, and 10 μg/ml of leupeptin. The homogenate was centrifuged at 100,000 g for 60 minutes. The supernatant (S1) was saved. The pellet was resuspended in the same buffer and centrifuged again at 100,000 g for 60 minutes. This supernatant (S2) was combined with S1 and used as the cytosol fraction; the pellet was homogenized in the same buffer and used as the membrane fraction. The protein content was determined in these two fractions according to the procedure of Lowry et al. (22) by using bovine serum albumin as the standard. [3H]cAMP binding was performed in triplicate in an incubation buffer containing 20 mM of phosphate buffer (pH 7.4 at 25°C), 2 mM of EDTA, and 15 mM of 2-mercaptoethanol (PEM buffer); [3H]cAMP (0.25–10 nM); membrane or cytosol fraction (∼25 μg protein); 0.25 mg of bovine serum albumin; and 1.5 mM 3-isobutyl-1-methylyxanthine in a total volume of 500 μl. The incubation was carried out at 25°C for 60 minutes and terminated by rapid filtration under vacuum by using a Brandel Cell Harvester (Biomedical Research and Development Laboratories Inc., Gaithersburg, Md.) followed by three washes with 2 ml of cold PEM buffer. The radioactivity retained on the filter was counted by using a liquid scintillation counter. Nonspecific binding was defined as the radioactivity bound in the presence of 5 μM cAMP. Bmax and KD were calculated by Scatchard plots by using the EBDA program (23).
Determination of Protein Kinase A Activity
Basal and cAMP-stimulated protein kinase A activity were determined in the membrane and cytosol fractions as reported previously (21). This procedure is based on the phosphorylation of a specific substrate, kemptide (Leu-Arg-Arg-Ala-Ser-Leu-Gly), which uses the transfer of γ-phosphate of (γ-32P) ATP by protein kinase A. Protein kinase A activity was determined in the presence and the absence of 100 μM cAMP. The phosphorylated substrate was separated from residual (γ-32P) ATP by spotting 20 μl of reaction mixture onto p81 phosphocellulose paper, washing with 75 mM H3PO4, and finally with acetone. Data are expressed as picomoles of 32P phosphate transferred to kemptide substrate/min/mg protein.
Statistical Analyses
The data analyses were performed by using the statistical software package SPSS 8.0 (SPSS, Chicago). All values reported are means and standard deviations. Statistical differences in Bmax and KD of [3H]cAMP binding to protein kinase A and in protein kinase A activity among suicide victims with and without depression and nonpsychiatric comparison subjects were evaluated by one-way analysis of variance, followed by Tukey’s multiple comparison procedure when significant main effects were present. Mean age, postmortem interval, Bmax and KD of [3H]cAMP binding to protein kinase A, and protein kinase A activity were compared between suicide victims and nonpsychiatric comparison subjects by using independent t test samples. Bonferroni corrections were used to test for statistically significant differences between two subject groups (a p value of 0.05 was divided by the number of groups examined to calculate appropriate p values for statistical significance). The relationships of protein kinase A activity and Bmax of [3H]cAMP binding with postmortem interval, age, and gender were determined by Pearson’s product-moment correlation analyses. Significance was set at an alpha level of ≤0.05. This was the baseline p before Bonferroni correction.
Results
Detailed clinical and demographic characteristics of the suicide victims and the nonpsychiatric comparison subjects are shown in Table 1. There were no significant differences in postmortem interval (t=–0.12, df=32, p=0.90) or age (t=1.34, df=32, p=0.19) between the two groups.
[3H]cAMP Binding to Protein Kinase A
The maximum number of binding sites (Bmax) and the apparent dissociation constant (KD) in both the membrane and cytosol fractions were determined by using different concentrations of [3H]cAMP (0.25–10 nM). Nonspecific binding was determined in the presence of 5 μM of cAMP. Figure 1 represents a typical saturation isotherm and a Scatchard plot of [3H]cAMP binding to the membrane and cytosol fractions of the prefrontal cortex of a nonpsychiatric comparison subject. It was observed that specific binding was saturable and exhibited a single class of binding site. Nonspecific binding was nonsaturable and linear with concentrations of 0.25–10 of nM [3H]cAMP. It was observed that Bmax of [3H]cAMP binding to protein kinase A was greater in the cytosol than in the membrane fraction.
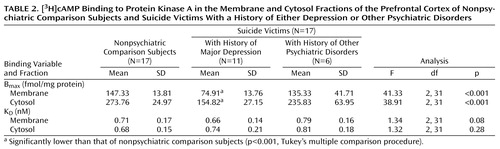
A comparison of Bmax and KD values of [3H]cAMP binding in the membrane and cytosol fractions of the prefrontal cortex between suicide victims and nonpsychiatric comparison subjects is given in Table 2. Bmax of [3H]cAMP binding to protein kinase A was significantly smaller in both the membrane (t=5.05, df=19.9, p<0.001) and cytosol (t=6.00, df=20.60, p<0.001) fractions of the prefrontal cortex of suicide victims than in the nonpsychiatric comparison subjects, whereas there were no significant between-group differences in KD values in either the membrane (t=0.04, df=32, p=0.97) or the cytosol (t=–1.42, df=32, p=0.17) fractions.
We then examined whether these effects were related to age, postmortem interval, or gender. However, no significant correlations were observed between Bmax of [3H]cAMP binding to protein kinase A and age (membrane: r=–0.06, p=0.74; cytosol: r=0.04, p=0.82), postmortem interval (membrane: r=–0.08, p=0.67; cytosol: r=–0.04, p=0.83), or gender (membrane: r=0.21, p=0.23; cytosol: r=0.26, p=0.12) (df=32 for all analyses).
We further examined if the lower Bmax of [3H]cAMP binding was generalized across all the suicide victims or was related to presence of a mental disorder. For this purpose, we divided the suicide victims into those who were diagnosed with major depression and those with other psychiatric disorders. Out of 17 suicide victims, 11 had a history of major depression. We observed that Bmax of [3H]cAMP binding to protein kinase A was significantly lower only in those suicide victims who had a previous history of major depression. It is of interest that Bmax of [3H]cAMP binding in the suicide victims with a history of other psychiatric disorders was only slightly lower (15%–20%) than that of the nonpsychiatric comparison subjects and was not significantly different (Table 2).
To rule out the possibility that the lower amount of [3H]cAMP binding was related to drug toxicology, we compared the membrane and cytosol Bmax values and found them to be similar in depressed suicide victims with drug toxicology (membrane: mean=76.85 fmol/min/mg protein [SD=16.65]; cytosol: mean=161.14 fmol/min/mg protein [SD=30.59]) and in those without drug toxicology (membrane: mean=71.50 fmol/min/mg protein [SD=7.18]; cytosol: mean=143.75 fmol/min/mg protein [SD=18.13]).
Protein Kinase A Activity
Total and endogenous protein kinase A activity in the prefrontal cortex of suicide victims and nonpsychiatric comparison subjects are presented in Table 3. We observed significantly less total and endogenous protein kinase A activity in the membrane fraction (total: t=6.0, df=20.6, p<0.001; endogenous: t=3.93, df=32, p<0.001) and cytosol fraction (total: t=6.42, df=17.5, p<0.001; endogenous: t=3.55, df=23.1, p=0.002) of the prefrontal cortex of suicide victims relative to that of nonpsychiatric comparison subjects. These changes in protein kinase A activity were much greater, however, in the presence of cAMP (35%–55%) than in the absence of cAMP (approximately 20%). Further, we did not observe in either the membrane or cytosol fractions any significant effects of age (total activity: r=0.21, p=0.23, and r=0.17, p=0.33, respectively; endogenous activity: r=–0.05, p=0.79, and r=0.11, p=0.53), postmortem interval (total activity: r=–0.06, p=0.72, and r=0.02, p=0.92; endogenous activity: r=–0.12, p=0.48, and r=–0.09, p=0.59), or gender (total activity: r=0.27, p=0.11, and r=0.30, p=0.08; endogenous activity: r=0.20, p=0.23, and r=0.25, p=0.15) (df=32 for all analyses).
Since we observed a lower Bmax of [3H]cAMP binding only in suicide victims with major depression, we sought to determine if protein kinase A activity followed a similar pattern of changes. As with Bmax of [3H]cAMP binding, both total and endogenous protein kinase A activity were significantly lower only in those suicide victims who had a previous history of major depression (Table 3). However, those suicide victims who were diagnosed with other psychiatric disorders had a slight but nonsignificant decrease (approximately 12%–20%) in both total and endogenous protein kinase A activity (Table 3).
In an analysis of drug toxicology effects, similar total and endogenous protein kinase A activity was seen in depressed suicide victims with drug toxicology (total activity: membrane mean=211.71 pmol/min/mg protein [SD=42.55], cytosol mean=440.71 pmol/min/mg protein [SD=16.41]; endogenous activity: membrane mean=96.85 pmol/min/mg protein [SD=11.50], cytosol mean=236.71 pmol/min/mg protein [SD=24.49]) and depressed suicide victims without drug toxicology (total activity: membrane mean=210.50 pmol/min/mg protein [SD=13.79], cytosol mean= 429.00 pmol/min/mg protein [SD=35.95]; endogenous activity: membrane mean=96.25 pmol/min/mg protein [SD=11.58], cytosol mean=267.00 pmol/min/mg protein [SD=7.48]).
Discussion
The present investigation reveals several interesting findings. For instance, the amount of [3H]cAMP binding was significantly lower in the membrane and cytosol fractions of the prefrontal cortex (Brodmann’s area 9) of suicide victims without any change in affinity. This lower binding was accompanied by lower total (in presence of cAMP) and endogenous (in absence of cAMP) protein kinase A activity. The lower level of protein kinase A activity was more pronounced in the presence of cAMP than in the absence of cAMP. We observed that the lower [3H]cAMP binding and protein kinase A activity was restricted to those suicide victims with a previous history of major depression rather than those with a history of other psychiatric disorders. These changes were not related to antidepressant toxicology or other confounding variables such as postmortem interval, age, or gender. Our study thus indicates that lower [3H]cAMP binding and protein kinase A activity is related to depression and that confounding variables—including antidepressant treatment—do not affect these measures. However, our study has some limitations. The numbers of subjects in each subgroup of suicide victims with other psychiatric disorders, as well as in the depressed group who showed positive antidepressant toxicology, were small. Therefore, it is premature to state that alterations in protein kinase A are specific to depression. It is interesting to mention that Shelton et al. (24) and Manier et al. (25) have found lower β-adrenergic receptor-stimulated protein kinase A activity in the fibroblasts of depressed patients, suggesting that depression may be associated with abnormalities in protein kinase A.
Although its status in depression remains unclear, protein kinase A has been studied extensively in affective disorders and in the mechanism of action of antidepressants. For example, Rahman et al. (26) reported lower [3H]cAMP binding in the cytosol fraction of various postmortem brain areas of subjects with bipolar affective disorder. In contrast, Fields et al. (27) reported higher protein kinase A activity in the temporal cortex of subjects with bipolar affective disorder. On the other hand, preclinical studies suggest that chronic treatment with imipramine, tranylcypromine, or ECT causes translocation of protein kinase A in the rat brain, resulting in greater protein kinase A activity in the particulate and less protein kinase A activity in the cytosol fraction (28). These effects are produced selectively by antidepressants and are not found with other psychotropic drugs. In addition, 5-HT and norepinephrine reuptake inhibitors increase the binding of cAMP to the regulatory subunit of protein kinase A (29–31). With regard to the CNS role of protein kinase A in depression, so far there is only one study to our knowledge that has examined protein kinase A in the postmortem brain of depressed suicide victims. In this study, Lowther et al. (32) found no significant change in [3H]cAMP binding. In contrast, our results indicate that not only [3H]cAMP binding to the regulatory subunit of protein kinase A but also total and endogenous protein kinase A activity are lower in the postmortem brain of suicide victims. The reason for this discrepancy is not clear but may tentatively be attributed to the brain areas studied. Whereas we studied Brodmann’s area 9, Lowther et al. (32) studied Brodmann’s areas 7, 10, and 21/22. Since we did not perform these studies in other brain areas, it is difficult to speculate whether these changes are restricted to Brodmann’s area 9 or are also present in other brain areas. An interesting observation by Lowther et al. (32) was that [3H]cAMP binding was significantly lower in those suicide victims who were treated with antidepressants. In our study, although the number of depressed suicide victims who showed positive drug toxicology was small, nonetheless the Bmax of [3H]cAMP binding and protein kinase A activity were similar among those depressed suicide victims with antidepressant toxicology present and those with no antidepressant toxicology.
Two other interesting observations were made in the present study: 1) the differences in [3H]cAMP binding and protein kinase A activity were present in both the membrane and cytosol fractions, and 2) protein kinase A activity was lower both in the presence and in the absence of cAMP. Upon activation by cAMP, protein kinase A translocates from the cytosol to the particulate fraction. It has been reported that the translocation of protein kinase A occurs through different compartments, with consequent phosphorylation of selective substrates (31). In our study, we did not find any translocation of protein kinase A, since lower [3H]cAMP binding as well as lower protein kinase A activity were seen in both the membrane and cytosol fractions. This suggests that abnormalities in cAMP-mediated functions are not localized to a certain compartment but rather are generalized. We presume that the observed lower [3H]cAMP binding occurred because of less abundance of regulatory subunits of protein kinase A available to bind the cAMP. Similarly, there are less catalytic subunits available; therefore less protein kinase A activity occurs even in the absence of cAMP. In this context, it is interesting to mention that lower [3H]cAMP binding (26) and greater protein kinase A activity (27) have been reported in the postmortem brain of subjects with bipolar affective disorder. These investigators have speculated that this opposite effect may occur because of the greater relative abundance of free catalytic subunits to regulatory subunits.
The mechanism of lower [3H]cAMP binding and protein kinase A activity in the prefrontal cortex of suicide victims is not clear at the present time. However, as we have mentioned, there is a strong possibility that there may be less expression of catalytic and regulatory subunits of protein kinase A, which may be related to the abnormalities upstream in the adenylyl cyclase–cAMP signaling system. A higher number of β-adrenergic and α2-adrenergic receptors (1), along with greater expression of Gsα and fewer Giα proteins (8, 9), have been shown in the postmortem brain of suicide victims, suggesting greater Gs- and Giα-mediated functions in suicidal behavior. In view of these observations, it is quite possible that an increase in cAMP levels due to higher levels of Gsα or lower levels of Giα protein may cause adaptive changes in the regulatory or the catalytic subunits, thereby reducing [3H]cAMP binding to protein kinase A and the enzyme’s activity as a compensatory event. In support of this notion, it has been reported that a sustained elevation in the intracellular levels of cAMP causes adaptive changes in both catalytic and regulatory subunits of protein kinase A (11, 12).
The relevance of impaired protein kinase A to the pathophysiology of depression/suicide remains to be elucidated; however, a decrease in the catalytic and regulatory properties of protein kinase A subunits may have important functional consequences, since it has been established that many biological responses are regulated by the state of the phosphorylation of specific substrate proteins, which in turn are involved in the regulation of cellular functions, such as neurotransmitter release (33), receptor desensitization (34), and gene expression (35, 36). Thus, a decrease in protein kinase A may result in significant impairments in various physiological functions in depression and suicidal behavior. At this juncture, it is difficult to relate our findings to Brodmann’s area 9, where we observed changes in protein kinase A, since the levels of cAMP differ from one brain area to other and even from cell to cell. Nonetheless, several neurochemical abnormalities have been reported in Brodmann’s area 9 of suicide victims, including abnormalities in serotonergic and adrenergic receptors (1). We and other investigators have also reported alterations in components of the phosphoinositide signaling system, such as protein kinase C (2), phospholipase C (3), and G proteins (9) in Brodmann’s area 9 of suicide victims. In most signaling pathways, protein phosphorylation represents a crossroad where extensive cross-talk occurs between various signaling systems (37). Given the alterations in multiple signaling system, it is quite possible that Brodmann’s area 9 may have physiological significance in suicidal behavior.
In conclusion, our observations of lower protein kinase A activity and [3H]cAMP binding in the prefrontal cortex of suicide victims indicate that abnormalities in the adenylyl cyclase–cAMP signaling system may be associated with the pathophysiology of suicide. Additional studies are required to delineate the physiological significance of these findings and whether these differences are mediated through receptors or are independently abnormal.
 |
 |
 |
Received Aug. 22, 2000; revisions received March 16 and June 6, 2001; accepted June 26, 2001. From the Psychiatric Institute, Department of Psychiatry, University of Illinois at Chicago; and the Maryland Psychiatric Research Center, Baltimore. Address reprint requests to Dr. Dwivedi, Psychiatric Institute, Department of Psychiatry, University of Illinois at Chicago, 1601 W. Taylor St., Chicago, IL 60612; [email protected] (e-mail). Supported by an NIMH Career Development Award (MH-01836) to Dr. Dwivedi and an NIMH grant (MH-48153) to Dr. Pandey. The authors thank John Smialek, M.D., and Dennis Chute, M.D., for their cooperation in the collection of brain samples; Ms. Terri U’Prichard for performing the psychological autopsies; Boris Lapidus, M.D., for the dissection; and Ms. Miljana Petkovich and Ms. Barbara Brown for their help in organizing the brain tissues.
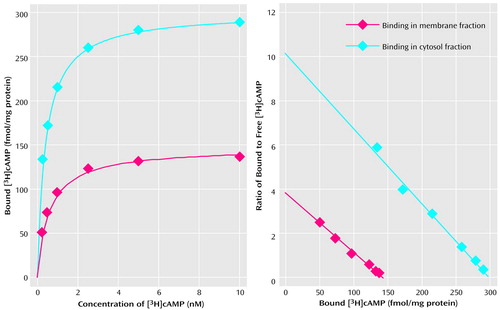
Figure 1. Saturation Curves and Scatchard Plot of [3H]cAMP Binding to Protein Kinase A in the Membrane and Cytosol Fractions of the Prefrontal Cortex of a Nonpsychiatric Comparison Subjecta
aEach point is the mean of triplicate determinations for different concentrations of [3H]cAMP (0.25–10 nM). Binding indices, membrane fraction: Bmax=142 fmol/mg protein, KD=0.37 nM; binding indices, cytosol fraction: Bmax=305 fmol/mg protein, KD=0.35 nM. A high correlation coefficient (r=0.99) was seen for binding indices in both fractions.
1. Gross-Iseroff R, Biegon A, Voet H, Weizman A: The suicide brain: a review of postmortem receptor/transporter binding studies. Neurosci Biobehav Rev 1998; 22:653-661Crossref, Medline, Google Scholar
2. Pandey GN, Dwivedi Y, Pandey SC, Conley RR, Roberts RC, Tamminga CA: Protein kinase C in the postmortem brain of teenage suicide victims. Neurosci Lett 1997; 228:111-114Crossref, Medline, Google Scholar
3. Pandey GN, Dwivedi Y, Pandey SC, Teas SS, Conley RR, Roberts RC, Tamminga CA, Reynolds CF III: Low phosphoinositide-specific phospholipase C activity and expression of phospholipase C β1 protein in the prefrontal cortex of teenage suicide subjects. Am J Psychiatry 1999; 156:1895-1901Abstract, Google Scholar
4. Pacheco MA, Jope RS: Phosphoinositide signaling in human brain. Prog Neurobiol 1996; 50:255-273Crossref, Medline, Google Scholar
5. Jope RS, Song L, Li PP, Young LT, Kish SJ, Pacheco MA, Warsh JJ: The phosphoinositide signal transduction system is impaired in bipolar affective disorder brain. J Neurochem 1996; 66:2402-2409Crossref, Medline, Google Scholar
6. Builder SE, Beavo JA, Krebs EG: Stoichiometry of cAMP and 1,N6-etheno-cAMP binding to protein kinase. J Biol Chem 1980; 255:2350-2354Medline, Google Scholar
7. Nestler EJ, Greengard P (eds): Protein Phosphorylation in the Nervous System. New York, John Wiley & Sons, 1984, p 398Google Scholar
8. Pandey GN, Dwivedi Y, Kumari R, Conley R, Roberts R, Tamminga C: G proteins in the postmortem brains of suicide victims. Abstr Soc Neurosci 1997; 23:1675Google Scholar
9. Pacheco MA, Stockmeier C, Meltzer HY, Overholser JC, Dilley GE, Jope RS: Alterations in phosphoinositide signaling and G-protein levels in depressed suicide brain. Brain Res 1996; 723:37-45Crossref, Medline, Google Scholar
10. Cowburn RF, Marcusson JO, Eriksson A, Wiehager B, O’Neill C: Adenylyl cyclase activity and G-protein subunit levels in postmortem frontal cortex of suicide victims. Brain Res 1994; 633:297-304Crossref, Medline, Google Scholar
11. Sibley DR, Benovic JL, Caron MG, Lefkowitz RJ: Phosphorylation of cell surface receptors: a mechanism for regulating signal transduction pathways. Endocr Rev 1988; 9:38-56Crossref, Medline, Google Scholar
12. Francis SH, Corbin JD: Structure and function of cyclic nucleotide-dependent protein kinases. Annu Rev Physiol 1994; 56:237-272Crossref, Medline, Google Scholar
13. Gettys TW, Corbin CJ: The protein kinase family of enzymes, in Receptor Phosphorylation. Edited by Moudgil VK. Boca Raton, Fla, CRC Press, 1989, pp 40-88Google Scholar
14. Wen W, Harootunian AT, Adams SR, Feramisco J, Tsien RY, Meinkoth JL, Taylor SS: Heat-stable inhibitors of cAMP-dependent protein kinase carry a nuclear export signal. J Biol Chem 1994; 269:32214-32220Medline, Google Scholar
15. Pandey GN, Dwivedi Y, Ren X, Rizavi H, Pandey S, Pesold C, Conley R, Roberts RC, Tamminga C: 5HT2A receptor protein and mRNA expression levels in the prefrontal cortex of teenage suicide victims. Abstr Soc Neurosci 2000; 26:2086Google Scholar
16. Dwivedi Y, Conley RR, Roberts RC, Tamminga CA, Pandey GN: Reduced activation and expression of ERK1/2 MAP kinase in the postmortem brain of depressed suicide subjects. J Neurochem 2001; 77:916-928Crossref, Medline, Google Scholar
17. George MS, Ketter TA, Post RM: Prefrontal cortex dysfunction in clinical depression. Depression 1994; 2:59-72Crossref, Google Scholar
18. Rajkowska G: Morphometric methods for studying the prefrontal cortex in suicide victims and psychiatric patients. Ann NY Acad Sci 1997; 836:253-268Crossref, Medline, Google Scholar
19. Salzman S, Clayton P, Winokur G: Diagnostic Evaluation After Death (DEAD). Rockville, Md, National Institute of Mental Health, Neuroscience Research Branch, 1983Google Scholar
20. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
21. Dwivedi Y, Pandey GN: Adrenal glucocorticoids modulate [3H] cyclic AMP binding to protein kinase A (PKA), cyclic AMP-dependent PKA activity, and protein levels of selective regulatory and catalytic subunit isoforms of PKA in rat brain. J Pharmacol Exp Ther 2000; 294:103-116Medline, Google Scholar
22. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with folin phenol reagent. J Biol Chem 1951; 193:265-275Medline, Google Scholar
23. McPherson GA: Analysis of radioligand binding experiments: a collection of computer programs for the IBM PC. J Pharmacol Methods 1985; 14:213-228Crossref, Medline, Google Scholar
24. Shelton RC, Manier DH, Sulser F: cAMP-dependent protein kinase activity in major depression. Am J Psychiatry 1996; 153:1037-1042Link, Google Scholar
25. Manier DH, Eiring A, Shelton RC, Sulser F: β-Adrenoceptor-linked protein kinase A (PKA) activity in human fibroblasts from normal subjects and from patients with major depression. Neuropsychopharmacology 1996; 15:555-561Crossref, Medline, Google Scholar
26. Rahman S, Li PP, Young LT, Kofman O, Kish SJ, Warsh JJ: Reduced [3H]cyclic AMP binding in postmortem brain from subjects with bipolar affective disorder. J Neurochem 1997; 68:297-304Crossref, Medline, Google Scholar
27. Fields A, Li PP, Kish SJ, Warsh JJ: Increased cyclic AMP-dependent protein kinase activity in postmortem brain from patients with bipolar affective disorder. J Neurochem 1999; 73:1704-1710Crossref, Medline, Google Scholar
28. Nestler EJ, Terwilliger RZ, Duman RS: Chronic antidepressant administration alters the subcellular distribution of cyclic AMP-dependent protein kinase in rat frontal cortex. J Neurochem 1989; 53:1644-1647Crossref, Medline, Google Scholar
29. Perez J, Tinelli D, Bianchi E, Brunello N, Racagni G: cAMP binding proteins in the rat cerebral cortex after administration of selective 5-HT and NE reuptake blockers with antidepressant activity. Neuropsychopharmacology 1991; 4:57-64Medline, Google Scholar
30. Mori S, Zanardi R, Popoli M, Garbini S, Brunello N, Smeraldi E, Racagni G, Perez J: cAMP-dependent phosphorylation system after short- and long-term administration of moclobemide. J Psychiatr Res 1998; 32:111-115Crossref, Medline, Google Scholar
31. Popoli M, Brunello N, Perez J, Racagni G: Second messenger-regulated protein kinases in the brain: their functional role and the action of antidepressant drugs. J Neurochem 2000; 74:21-33Crossref, Medline, Google Scholar
32. Lowther S, Katona CL, Crompton MR, Horton RW: Brain [3H]cAMP binding sites are unaltered in depressed suicides, but decreased by antidepressants. Brain Res 1997; 758:223-228Crossref, Medline, Google Scholar
33. Nichols RA, Sihra TS, Czernik AJ, Nairn AC, Greengard P: Calcium/calmodulin-dependent protein kinase II increases glutamate and noradrenaline release from synaptosomes. Nature 1990; 343:647-651Crossref, Medline, Google Scholar
34. Siegelbaum SA, Camardo JS, Kandel ER: Serotonin and cyclic AMP close single K+ channels in Aplysia sensory neurones. Nature 1982; 299:413-417Crossref, Medline, Google Scholar
35. Montminy MR, Gonzalez GA, Yamamoto KK: Regulation of cAMP-inducible genes by CREB. Trends Neurosci 1990; 13:184-188Crossref, Medline, Google Scholar
36. Yamamoto KK, Gonzalez GA, Biggs WHD, Montminy MR: Phosphorylation-induced binding and transcriptional efficacy of nuclear factor CREB. Nature 1988; 334:494-498Crossref, Medline, Google Scholar
37. Selbie LA, Hill SJ: G protein-coupled-receptor cross-talk: the fine-tuning of multiple receptor-signaling pathways. Trends Pharmacol Sci 1998; 19:87-93Crossref, Medline, Google Scholar



