Randomized Study of the Dopamine Receptor Agonist Piribedil in the Treatment of Mild Cognitive Impairment
Abstract
OBJECTIVE: Age-related decrease in dopamine D2 receptors is associated with cognitive decline in healthy elderly individuals. This study was an investigation of whether the dopamine receptor agonist piribedil improves global cognitive function in patients with mild cognitive impairment. METHOD: In a 90-day randomized double-blind study, treatment with piribedil was compared to placebo in 60 patients with clinically diagnosed mild cognitive impairment and a Mini-Mental State Examination (MMSE) score of 21 to 25. The primary outcome was change in MMSE score. RESULTS: Of the 30 patients randomly assigned to each treatment group, 19 (63.3%) of those taking piribedil and eight (26.7%) of those treated with placebo had increases in MMSE scores, to 26 or more. The response rate and the mean increase in MMSE scores were significantly greater with piribedil. CONCLUSIONS: Patients with mild cognitive impairment had improvement in global cognitive function when treated with the dopamine receptor agonist piribedil. The results support the role of age-related dopamine decline in cognitive impairment of the elderly.
Healthy elderly patients frequently complain of cognitive decline that hampers daily activity but does not meet the diagnostic criteria for dementia. Although these patients are at risk of Alzheimer’s disease, an aging process may be involved in the cognitive difficulty, and mild cognitive impairment is a widely cited concept used to describe such patients (1). In this regard, work using brain imaging techniques (2) has demonstrated an association between age-related decrease in dopamine D2 receptors and impaired performance on neurocognitive tasks involving the frontal lobe (such as executive function and response inhibition) by a mechanism that may involve reduced frontal and cingulate metabolism (3) in healthy individuals. Age-related decline in dopamine activity may therefore contribute to mild cognitive impairment and provide a rationale for testing specific pharmacotherapy. In this study, we examined whether the dopamine receptor agonist piribedil improved short-term global cognitive function in patients with mild cognitive impairment.
Method
The institutional ethics committee of the National Institute of Mental Health and Neurosciences approved the study protocol. Consecutive outpatients or patients from old-age homes aged 60 years and over with complaints of gradual memory loss resulting in impaired performance of everyday tasks, but with adequate intellectual function, underwent a detailed clinical examination that included an interview of a first-degree relative. An ECG was done, and venous blood was drawn for biochemical tests.
Patients with dementia were excluded. The diagnosis of dementia was entirely clinical, based on DSM-IV criteria. Specifically, the patients had to have difficulty learning new information and recalling previously learned information and had to have at least one other cognitive deficit within the context of a stable consciousness. Further, relatives had to confirm a resulting alteration in the patient’s social or occupational functioning for a diagnosis of dementia to be made.
In addition to patients with dementia, those with a clinical history or signs of other disease that could cause dementia due to vascular or general medical conditions (DSM-IV) and those with previous intolerance to piribedil were excluded. The Mini-Mental State Examination (MMSE) was administered to the remaining patients, and those with a score of 21 to 25 were selected for the study (N=60). After a complete description of the study to the subjects, written informed consent was obtained.
After a placebo run-in period of 14 days (one tablet per day), the patients were reexamined. Those with no change in clinical status and an MMSE score of 21–25 were assessed for baseline characteristics and randomly assigned to receive either one tablet (50 mg) of piribedil or one tablet of placebo per day after breakfast, under double-blind conditions, for 90 days. The tablets of piribedil and placebo (prepared by the manufacturer) were identical in appearance and taste. Treatment of associated disease not specified as an exclusion criterion was allowed at the discretion of the neurologist (S.J.). The patients were reassessed by the same neurologist on the 30th, 60th, and 90th treatment days with a clinical examination and MMSE, a visual analogue scale assessing well-being (range=1–7), and a tablet count to determine compliance. The ECG and biochemical tests were repeated at the end of the study.
Change in MMSE score was the principal outcome variable. Response to treatment was defined as an improvement in the MMSE score to at least 26 (which is within one standard deviation of that expected of normal individuals) (4). The study group size was calculated to detect an increase in responders to piribedil from 15% (estimated response in the placebo group) to 55% (alpha=0.05, beta=0.2). At each assessment, response was analyzed on an intention-to-treat basis, and the numbers of responders were compared by a Yates-corrected chi-square test. Changes in continuous variables were compared by the t test. Significance was defined as a two-tailed value of p<0.05.
Results
One-half of the subjects (N=15) in both the piribedil and placebo groups were male; 26 (86.7%) and 30 (100.0%) of the piribedil and placebo subjects, respectively, had completed only high school, and four (13.3%) and none (0.0%) had graduated from college. At baseline, the mean durations of their cognitive impairment were 11.7 months (SD=11.5) and 10.8 months (SD=9.4), and their mean MMSE scores were 23.6 (SD=1.3) and 23.5 (SD=1.5).
After 1 month, the numbers of patients who responded to treatment with an improvement in MMSE score to 26 or more were 14 (46.7%) for piribedil and eight (26.7%) for placebo (χ2=1.76, df=1, p<0.50). After 2 months, the numbers were 17 (56.7%) versus nine (30.0%) (χ2=3.32, df=1, p<0.07). After 3 months, the numbers were 19 (63.3%) versus eight (26.7%) (χ2=6.73, df=1, p<0.01). The increase in mean MMSE score between baseline and 90 days was 1.23 for the patients who received piribedil, which was significantly greater than the increase for the patients who received placebo (Figure 1).
Subjective well-being assessed on the visual analogue scale improved or was unchanged after treatment (relative to baseline) in 24 (80.0%) of the patients receiving piribedil versus 20 (66.7%) of those receiving placebo. The changes in hemodynamic, electrocardiographic, and biochemical variables did not significantly differ between treatment groups. During the study one of the patients taking piribedil withdrew because of diarrhea, and two were lost to follow-up. Of the patients receiving placebo, six withdrew because of gastrointestinal symptoms and weakness. The reported side effects were mostly gastrointestinal. The mean level of compliance with medication after 90 days of treatment was 94.4% (SD=14.2) with piribedil (85 of 90 tablets) versus 91.1% (SD=15.9) with placebo (82 of 90 tablets).
Discussion
The elderly patients we studied had cognitive decline that interfered with everyday tasks. There was no clinically evident dementia. Although several clinical labels, including “age-associated memory impairment” and “mild cognitive impairment,” have been proposed for such patients, there is no consensus on diagnostic criteria, whether the cognitive impairment should be considered an isolated memory deficit or a prodrome of Alzheimer’s disease (1). Because of their clinical appearance and an MMSE score of 21 to 25, our patients probably represent the more severe category of mild cognitive impairment, rather than age-associated memory impairment, although there may be some overlap. Distinguishing patients on the basis of MMSE scores has not been validated in Indians. Although India’s educational standards are comparable to those in the United States, the effects of cultural differences are unknown. Since the study rationale was that an age-related decrease in D2 receptors may contribute to age-related cognitive decline by affecting frontal lobe function (2, 3), our focus was on demonstrating that the treatment improves global cognitive function rather than a specific domain.
At baseline, patient characteristics that could influence outcome were balanced between treatment groups. After 90 days’ treatment, twice as many patients had responded to piribedil as to placebo, and the mean MMSE score of the piribedil patients was also significantly greater. The treatment was well accepted.
The study has limitations. We did not perform neuropsychometric tests to differentiate mild cognitive impairment from age-associated memory impairment or to differentiate both of these conditions from preclinical dementia. The number of patients was small, and the results need replication.
The usefulness of the study is the finding that patients with mild cognitive impairment had significant global cognitive improvement in the short term when treated with the dopamine receptor agonist piribedil. The results also lend support to the etiologic hypothesis of dopamine insufficiency in age-related cognitive decline.
Received Oct. 20, 2000; revision received Feb. 22, 2001; accepted April 19, 2001. From the Department of Neurology, National Institute of Mental Health and Neurosciences. Address reprint requests to Dr. Nagaraja, Department of Neurology, National Institute of Mental Health and Neurosciences, Hosur Rd., Bangalore 560 029, India; [email protected] (e-mail). Supported by a financial grant and supply of study medications to the National Institute of Mental Health and Neurosciences, Bangalore, India, by Serdia Pharmaceuticals (India), Ltd., manufacturer of piribedil. The authors thank Dr. David Park, epidemiological consultant; Dr. Prashant Desai and Dr. Arundhati Datye for assistance in organizing the study; and Ms. Lavita D’Souza for secretarial assistance.
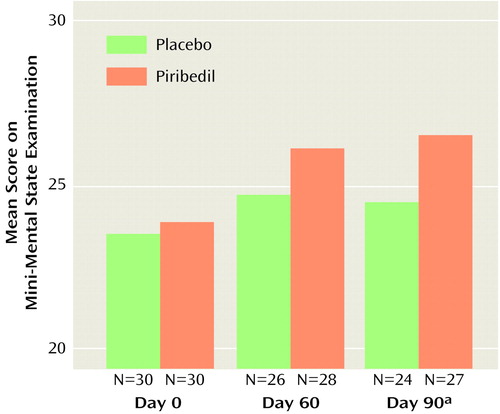
Figure 1. Effect of Piribedil and Placebo on Mini-Mental State Examination Scores of Patients With Mild Cognitive Impairment
aSignificant group effect on change from day 0 (t=2.83, df=49, p<0.01).
1. Ritchie K, Touchon J: Mild cognitive impairment: conceptual basis and current nosological status. Lancet 2000; 355:225-228Crossref, Medline, Google Scholar
2. Volkow ND, Gur RC, Wang G-J, Fowler JS, Moberg PJ, Ding YS, Hitzemann R, Smith G, Logan J: Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155:344-349Link, Google Scholar
3. Volkow ND, Logan J, Fowler JS, Wang G-J, Gur RC, Wong C, Felder C, Gatley SJ, Ding Y-S, Hitzemann R, Pappas N: Association between age-related decline in brain dopamine activity and impairment in frontal and cingulate metabolism. Am J Psychiatry 2000; 157:75-80Link, Google Scholar
4. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189-198Crossref, Medline, Google Scholar



