Anxiolytic Activity of Atrial Natriuretic Peptide in Patients With Panic Disorder
Abstract
OBJECTIVE: Preclinical evidence exists for the anxiolytic activity of atrial natriuretic peptide, which is released during lactate-induced panic attacks. Atrial natriuretic peptide receptor modulation may have antipanic activity in patients with panic disorder. METHOD: The effects of 150 μg of atrial natriuretic peptide and placebo on panic attacks induced by cholecystokinin tetrapeptide (CCK-4) (25 μg) were studied in 10 panic disorder patients. The panicogenic activity of CCK-4 was measured with the Acute Panic Inventory. RESULTS: Panic attacks occurred in seven patients in the placebo condition and in two patients in the atrial natriuretic peptide condition. CCK-4 administration was accompanied by a significant increase in Acute Panic Inventory scores. Pretreatment with atrial natriuretic peptide resulted in significantly lower Acute Panic Inventory scores than pretreatment with placebo. CONCLUSIONS: The results support the antipanic activity of atrial natriuretic peptide. Nonpeptidergic atrial natriuretic peptide receptor ligands may be ultimately used to treat anxiety disorders.
Atrial natriuretic peptide is not only synthesized by atrial myocytes (1) and released into the circulatory system but is also found in the neurons of different brain regions in which specific atrial natriuretic peptide binding sites have been found (2). Atrial natriuretic peptide is released in patients with panic disorder when panic attacks are experimentally induced (3). This endocrine response possibly serves as a humoral feedback signal to mute overshooting anxiety. Preclinical data have given further evidence for the anxiolytic activity of atrial natriuretic peptide (4).
Cholecystokinin tetrapeptide (CCK-4) has enhanced panicogenic activity in patients with panic disorder (5). As with sodium lactate- and CO2-induced panic attacks, CCK-4-induced attacks are accompanied by hyperventilation and resemble the pattern of panic symptoms induced by CO2 administration (6). Furthermore, CCK-4-induced panic attacks are blocked by antipanic treatment (7) and can be used to study putative new treatment approaches. Therefore we studied the potential anxiolytic activity of atrial natriuretic peptide in patients with panic disorder during a CCK-4 challenge.
Method
Ten patients (five women and five men, mean age=36.0 years, SD=9.7) with a diagnosis of panic disorder but without a comorbid axis I disorder as assessed with the Structured Clinical Interview for DSM-IV, were studied. The patients had been free of medications for at least 10 days and had undergone thorough medical examinations to rule out other illnesses, drug intake, and lifestyles (with sleep deprivation, changes in the sleep/wake cycle) with effects that could interfere with our study. The protocol was approved by the local ethics committee for human experiments. After complete description of the study to the subjects, written informed consent was obtained.
All subjects were studied from 9:00 a.m. to 1:00 p.m. while lying in a supine position in a soundproof room on a single bed. Each subject received a bolus injection of 25 μg of CCK-4 (Clinalfa, Laufelfingen, Switzerland) dissolved in 10 ml of 0.9% saline solution at 11:00 a.m. In a double-blind, randomized study, 150 μg of atrial natriuretic peptide (Clinalfa, Laufelfingen, Switzerland) or 0.9% saline was administered from 10:40 until 11:10 a.m. (1 ml/min). The infusions were administered on separate days, with at least 2 days between experimental conditions. The Acute Panic Inventory (8) was administered at 10:40 a.m. (baseline) and 11:05 a.m. by a rater (A.S.) who was blind to the procedure; the maximum intensity of symptoms during the observation period was noted. To further characterize the possible effects of atrial natriuretic peptide, the anxiety subscore (afraid of dying, general fear) and the somatic subscore (palpitations, rapid breathing, nausea) of the Acute Panic Inventory are provided. In order for the rater to make a decision that a panic attack had occurred, an Acute Panic Inventory total score exceeding 20 and an increase of at least 14 points over the preinjection score (8, 9) were required.
The frequencies of panic attacks during the two treatment conditions were compared by using McNemar’s paired chi-square test. For statistical comparisons of mean Acute Panic Inventory scores and subscores between the saline and atrial natriuretic peptide conditions and between the two time points, a two-factorial multivariate analysis of variance (MANOVA) with a repeated measures design was performed. Treatment and time were the two within-subjects factors, each containing two levels. Hypothesis tests for the main and interaction effects of these factors were based on multivariate criteria, such as Wilks’s lambda and its approximated F value. When significant main or interaction effects were found, univariate F tests were performed in an analysis of variance (ANOVA) to locate the levels of the factor with significant differences in mean Acute Panic Inventory scores and its subscores that fell between the levels of the other factor. The contrasts (group mean differences) were tested for significance with approximated F values. Because of the small group size and a possible order effect, CCK-4-induced changes were normed (divided by the group mean) to their baseline values under each experimental condition. As a nominal level of significance, alpha=0.05 was accepted. To keep the type I error at 0.05 or less, all post hoc tests (tests with contrasts) were performed at a reduced level of significance (with an alpha adjustment according to the Bonferroni procedure).
Results
The rate of panic attack was two of 10 in the atrial natriuretic peptide and seven of 10 in the placebo condition. Although coadministration of atrial natriuretic peptide prevented the occurrence of CCK-4-induced panic attacks in five of the seven patients with CCK-4-induced panic attacks, this effect possessed only marginally statistical significance (p=0.06, McNemar’s test). MANOVAs revealed significant main effects of time (F=17.91, df=3, 7, p=0.001) and treatment (F=7.46, df=3, 7, p=0.01) and a significant interaction effect of time and treatment as well (F=8.05, df=3, 7, p=0.01). ANOVAs of Acute Panic Inventory scores yielded significant main effects of time (F=59.48, df=1, 9, p<0.001) and treatment (F=5.93, df=1, 9, p=0.04) and a significant interaction effect of time and treatment as well (F=26.37, df=1, 9, p=0.001). Since the mean Acute Panic Inventory scores before treatment did not differ from the scores after treatment because of norming, we can conclude that CCK-4 increased Acute Panic Inventory scores in both conditions significantly. However, the CCK-4-induced increase in the Acute Panic Inventory scores was significantly reduced by atrial natriuretic peptide (F=21.40, df=1, 9, p=0.001) (Figure 1).
Analysis of the subscores of the Acute Panic Inventory revealed a significant main effect of time (F=7.60, df=1, 9, p=0.02) and a significant interaction effect of time and treatment (F=14.19, df=1, 9, p=0.004) for the anxiety subscore and significant main effects of time (F=24.08, df=1, 9, p=0.001) and treatment (F=18.43, df=1, 9, p=0.002) and a significant interaction effect of time and treatment for the somatic subscore (F=11.41, df=1, 9, p=0.008) as well. Atrial natriuretic peptide administration reduced the CCK-4-induced increase in anxiety (F=6.55, df=1, 9, p=0.03) and also in somatic symptoms (F=27.03, df=1, 9, p=0.001).
Discussion
The major finding of this exploratory study in patients with panic disorder was that atrial natriuretic peptide had antipanic activity in five of seven patients with CCK-4 induced panic attacks. However, most possibly owing to the small group size, this effect failed to reach statistical significance. In addition, the CCK-4-induced increase in the Acute Panic Inventory scores was significantly reduced by the administration of atrial natriuretic peptide. Subscore analysis of the Acute Panic Inventory scores indicated that atrial natriuretic peptide decreases CCK-4-induced somatic as well as anxiety symptoms, providing further evidence for the anxiolytic activity of atrial natriuretic peptide in patients with panic disorder.
We know of no current evidence that peripheral atrial natriuretic peptide crosses the blood-brain barrier in physiologically significant amounts. A passive, nonsaturable transport of about 1%–2% of an administered dose, however, may be of significance if pharmacological amounts of the peptide are given (10). Since it is known that peptides exert their effects in the nanomolar range, a low amount of atrial natriuretic peptide administered peripherally may be sufficient to exert effects by means of central receptors. In addition, atrial natriuretic peptide may affect central receptors in brain regions that are not fully protected by the blood-brain barrier. Furthermore, atrial natriuretic peptide may exert effects by means of peripheral receptors: besides a direct depressant action on sympathetic nerve function (11), a suppression of adrenaline release and a stimulation of sympathoinhibitory afferent vagal activity from the cardiopulmonary baroreceptor system may be involved in the observed anxiolytic activity of peripherally administered atrial natriuretic peptide. However, at the moment, the site of action of atrial natriuretic peptide is unknown and central as well, as peripherally mediated effects may be involved in its anxiolytic activity.
In summary, our findings give clinical evidence for the anxiolytic activity of atrial natriuretic peptide in patients with panic disorder. Limited by our study’s small group size, our results call for further studies on the potential anxiolytic and antipanic activity of atrial natriuretic peptide. Together with preclinical data (4), our results suggest that trials with nonpeptidergic atrial natriuretic peptide receptor ligands may ultimately lead to new anxiolytic drugs.
Received Sept. 18, 2000; revisions received Jan. 8 and Feb. 13, 2001; accepted March 8, 2001. From the Max Planck Institute of Psychiatry. Address reprint requests to Dr. Ströhle, Max Planck Institute of Psychiatry, Kraepelinstr. 10, 80804 Munich, Germany; [email protected] (e-mail). The authors thank G. Gajewsky for technical assistance and A. Yassouridis, Ph.D., for statistical advice.
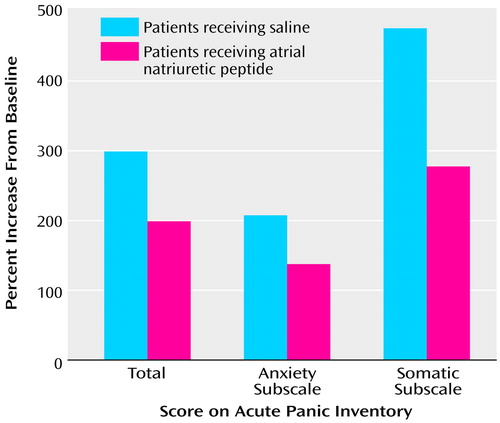
Figure 1. Percentage Increases From Normed Baseline in Acute Panic Inventory and Anxiety and Somatic Subscale Scores After Cholecystokinin Tetrapeptide Administration in 10 Panic Disorder Patients, by Pretreatment With Saline (Placebo) or Atrial Natriuretic Peptidea
aPatients received infusion of 150 μg of atrial natriuretic peptide or 0.9% saline beginning at baseline, 20 minutes before receiving a bolus injection of 25 μg of cholecystokinin tetrapeptide.
1. De Bold AJ: Atrial natriuretic factor: a hormone produced by the heart. Science 1985; 230:767-770Crossref, Medline, Google Scholar
2. Imura H, Nakao K, Itoh H: The natriuretic peptide system in the brain: implications in the central control of cardiovascular and neuroendocrine functions. Front Neuroendocrinol 1992; 3:217-249Google Scholar
3. Kellner M, Herzog L, Yassouridis A, Holsboer F, Wiedemann K: Possible role of atrial natriuretic hormone in pituitary-adrenocortical unresponsiveness in lactate-induced panic. Am J Psychiatry 1995; 152:1365-1367Google Scholar
4. Ströhle A, Jahn H, Montkowski A, Liebsch G, Boll E, Landgraf R, Holsboer F, Wiedemann K: Central and peripheral administration of atriopeptin is anxiolytic in rats. Neuroendocrinology 1997; 65:210-215Crossref, Medline, Google Scholar
5. Bradwejn J, Koszycki D, Shriqui C: Enhanced sensitivity to cholecystokinin tetrapeptide in panic disorder. Arch Gen Psychiatry 1991; 48:603-610Crossref, Medline, Google Scholar
6. Bradwejn J, Koszycki D: Comparison of the panicogenic effects of cholecystokinin 30-33 and carbon dioxide in panic disorder. Prog Neuropsychopharmacol Biol Psychiatry 1991; 15:237-239Crossref, Medline, Google Scholar
7. Bradwejn J, Koszycki D: Imipramine antagonism of the panicogenic effects of cholecystokinin tetrapeptide in panic disorder patients. Am J Psychiatry 1994; 151:261-263Link, Google Scholar
8. Dillon DJ, Gorman JM, Liebowitz MR, Fyer AJ, Klein DF: Measurement of lactate-induced panic and anxiety. Psychiatry Res 1987; 20:97-105Crossref, Medline, Google Scholar
9. Ströhle A, Holsboer F, Rupprecht R: Increased ACTH concentrations associated with cholecystokinin tetrapeptide-induced panic attacks in patients with panic disorder. Neuropsychopharmacology 2000; 22:251-256Crossref, Medline, Google Scholar
10. Ermisch A, Rühle HJ, Kretzschmar R, Baethmann A: On the blood-brain barrier to peptides: specific binding of atrial natriuretic peptide in vivo and in vitro. Brain Res 1991; 554:209-216Crossref, Medline, Google Scholar
11. Seier FE, Kellner M, Yassouridis A, Heese R, Strian F, Wiedemann K: Autonomic reactivity and hormonal secretion in lactate-induced panic attacks. Am J Physiol 1997; 272:H2630-H2638Google Scholar



