Family Study of Girls With Attention Deficit Hyperactivity Disorder
Abstract
OBJECTIVE: Because attention deficit hyperactivity disorder (ADHD) is relatively infrequent among girls, little is known about the causes of ADHD in girls. To help fill this gap in the literature, the authors assessed the familial transmission of ADHD in families ascertained through girls.METHOD: Interviewers who were blind to diagnosis administered structured psychiatric interviews to 140 girls with ADHD and their 417 first-degree relatives and to 122 girls without ADHD and their 369 first-degree relatives.RESULTS: The relatives of the ADHD girls had a significantly higher prevalence of ADHD, according to either the DSM-III-R or DSM-IV definition, than the relatives of the comparison girls. However, this did not differ from the prevalence the authors reported previously for families of boys with ADHD. Like the boys’ families, the relatives of the girl probands also had significantly higher prevalences of antisocial, mood, anxiety, and substance use disorders, although the prevalence of familial antisocial disorders was lower than had been observed in the boys’ families. There was no association between the DSM-IV subtypes of the probands and relatives.CONCLUSIONS: The familial transmission of ADHD and comorbid disorders generalizes to families of girls with ADHD. Neither proband gender nor subtype influences the familial transmission of ADHD.
Because of gender differences in attention deficit hyperactivity disorder (ADHD) and the limited amount of research on girls (1), the National Institute of Mental Health’s Conference on Sex Differences in ADHD concluded that inferences drawn from studies of boys with ADHD could not be routinely applied to girls with ADHD (2). One notable gap in our knowledge about girls with ADHD is in the literature on family studies. Whereas there have been many family studies of ADHD boys (3, 4), little is known about the transmission of ADHD and related disorders in families of ADHD girls. Extant studies provide conflicting data regarding sex effects on familial transmission, and to our knowledge, these studies have not looked at the familial transmission of psychiatric comorbidity or ADHD subtypes.
Family studies are well suited to clarifying the most conspicuous sex difference in ADHD: its lower prevalence among girls (1). In the “familial dose” model, this difference is accounted for by the assumption that girls, in comparison with boys, need more familial risk factors to develop ADHD (5, pp. 94–106). Because it is assumed that ADHD girls carry a higher dose of familial risk factors, then their relatives should also carry more and thus be at greater risk for ADHD than are the relatives of ADHD boys. This pattern has been observed in two family studies (6, 7) but not in others (8–10). Also, the results of these latter family studies, which suggest that ADHD is as familial in boys as it is in girls, concur with the findings from twin studies (11–14), which indicate that the heritability of ADHD does not differ by gender. Further work is needed to assess the “familial dose” model because its rejection would point ADHD researchers to nonfamilial environmental events as the cause of the differing prevalence of ADHD between genders.
Another gap in the ADHD literature stems from the near absence of studies that have examined the familial transmission of other disorders in ADHD families ascertained through girl probands. In our DSM-III-based family study of girls with attention deficit disorder (ADD) (10), their families were strikingly like the families of boys with ADHD in their high risks for antisocial, affective, and anxiety disorders. To our knowledge, other studies have not examined this issue.
Prior family studies of ADHD girls were limited in several respects. The number of ADHD girl probands was small, ranging from 12 to 33 with a mean of 21. Three of the studies used DSM-II criteria, and three used DSM-III criteria. Thus, we know of no large family studies of ADHD girls and none that have used either DSM-III-R or DSM-IV criteria. These studies were also limited by having assessed only a limited number of outcomes, primarily ADHD, in the relatives.
To remedy these limitations, we have conducted a large family study of ADHD and non-ADHD girl probands (15). In the first report on this study group (15) we showed that ADHD in girls was characterized by prototypical symptoms of the disorder, comorbid psychopathology, social dysfunction, cognitive impairments, and school failure. The only notable differences from our prior study of boys (16) were the prevalences of conduct and oppositional defiant disorders in ADHD girls, which were one-half of those reported for boys.
The present report focuses on the families of girls with ADHD and on tests of several hypotheses. Hypothesis 1 was the prediction that the familial transmission of ADHD we previously reported for families of boy probands (17) would be replicated. We predicted that the relatives of ADHD girls would be at higher risk for ADHD than would relatives of non-ADHD girls. We then sought to assess the familial transmission of the DSM-IV subtypes. Thus, hypothesis 2 was that relatives of girls with DSM-IV ADHD would be at higher risk for each DSM-IV subtype of ADHD than would relatives of non-ADHD girls.
According to hypothesis 3, that the DSM-IV subtypes of ADHD arise from separate familial risk factors, ADHD subtypes would “breed true” in families (e.g., relatives of inattentive probands would be at high risk for inattentive ADHD but not other subtypes).
Hypothesis 4 was the “familial dose” explanation for the low prevalence of ADHD in girls. We predicted that relatives of girl probands would have higher rates of ADHD than the rates we previously observed among relatives of boy probands (17).
Hypothesis 5 followed up on our report of psychiatric comorbidity in this study group (15). We reasoned that if comorbid disorders in girl probands had a familial etiology similar to that seen in our study of boy probands (17), then relatives of ADHD girls would be at higher risk for mood, antisocial, and anxiety disorders than would relatives of non-ADHD girls.
Method
Subjects
We studied two groups of girls: 140 patients with ADHD and 122 comparison subjects without ADHD. These groups had 417 and 369 first-degree biological relatives, respectively, who provided data. All of the ADHD girls met the full DSM-III-R diagnostic criteria for ADHD at the time of the clinical referral; at the time of recruitment they all had active symptoms of the disorder. Of these, 127 met the DSM-IV criteria for inattentive ADHD (N=37), hyperactive-impulsive ADHD (N=9), and combined-type ADHD (N=81). All subjects older than 12 gave written informed consent for participation. Parents gave written informed consent for participation of children under 12, and these children participated only if they assented to the study procedures.
We identified 63 girls with ADHD from lists of consecutive patients in the pediatric psychiatry clinic at the Massachusetts General Hospital. Another 77 girls with ADHD were identified from lists of children having evidence of ADHD in the computerized medical records of a health maintenance organization (HMO). Within each setting, we selected normal comparison subjects from lists of outpatients at pediatric medical clinics (Massachusetts General Hospital, N=55; HMO, N=67). The fractions of subjects selected from the hospital and HMO sources did not differ between the ADHD and comparison groups (χ2=0.0, df=1, p=0.99).
We selected potential ADHD families if evidence in the medical record and the results of an initial interview suggested a child might have ADHD. We selected potential comparison families if evidence in the medical record and the results of an initial interview suggested a child did not have ADHD. Among the subjects who were eligible to participate on the basis of our interview, 84% of the 167 potential ADHD families participated and 96% of the 127 potential comparison families participated. The families who did not participate were either ineligible or refused. These rates were statistically different (χ2=11.1, df=2, p=0.001).
Procedures
We used DSM-III-R-based structured interviews supplemented with questions for DSM-IV diagnoses. The psychiatric assessments of the girls used the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E) (18). Diagnoses were based on independent interviews with the mothers and direct interviews with the children. Children younger than 12 years of age were not interviewed directly. Parental diagnoses were based on direct interviews using the Structured Clinical Interview for DSM-III-R—Non-Patient Edition (19). To assess childhood diagnoses in the parents, we administered modules from the K-SADS-E. We assessed socioeconomic status with the Hollingshead-Redlich scale (20).
The interviewers were blind to proband diagnosis and ascertainment site. The direct interviews of the mothers and the direct interviews of the children were conducted by different raters. For about 90% of the families, the mother’s interview about her children occurred after the direct interview with the mother about herself. The raters were trained and supervised by board-certified psychiatrists (J.B., T.S.).
The psychiatrists making the diagnoses were board certified in both child and adult psychiatry and were blind to ascertainment group, ascertainment site, all data collected from family members, and all nondiagnostic data. As suggested by others (21), we diagnosed major depression only if the depressive episode was associated with marked impairment. We considered impairment to be severe enough for the diagnosis of depression if the episode led to either hospitalization or a persistent disruption in performance of a major role at home or work. We also examined a summary diagnosis of anxiety disorders; the subject had a positive diagnosis if he or she had two or more anxiety disorders and was rated negative otherwise. We did so because 1) our prior work has shown this measure to have high test-retest reliability (22) and 2) it allowed for direct comparisons with other studies in which we used this measure (17, 23).
Statistical Analyses
Our statistical analyses addressed the nonindependence of observations within families by adjusting variance estimates with Huber’s formula (24), a “theoretical bootstrap” that produces robust statistical tests. The method works by entering the cluster score (i.e., sum of scores within families) into the formula for the estimate of variance. The resulting p values are robust to distributional assumptions. Results of Yates-corrected chi-square tests are also presented for the benefit of future meta-analyses. All statistical tests were two-tailed and used the 0.01 level of statistical significance.
Results
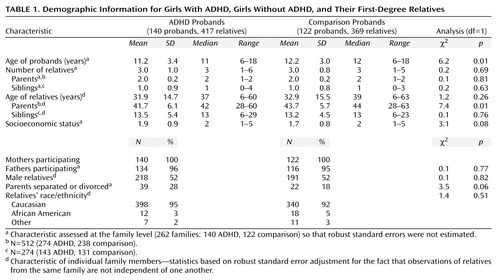
The ADHD and comparison families did not differ significantly in sex distribution of relatives, ethnicity, or number of relatives (Table 1). We found statistically significant differences in the ages of the probands and their parents. The proportion of adolescents was 60% among the comparison probands (N=73) and 46% among the ADHD probands (N=64) (χ2=5.2, df=1, p=0.02). We statistically controlled for all demographic differences in subsequent analyses.
As predicted from hypothesis 1, the rate of DSM-III-R ADHD was five times as high among the relatives of the ADHD girls (21%, N=87) as among the comparison relatives (4%, N=16) (χ2=35.9, df=1, p<0.001; Yates χ2=45.9, df=1, p<0.001). We also found a significantly higher prevalence of DSM-IV ADHD among the relatives of probands with DSM-IV-defined ADHD (24%, N=99) than among the comparison probands (7%, N=25) (χ2=32.1, df=1, p<0.001; Yates χ2=41.1, df=1, p<0.001).
To test hypothesis 2, we stratified the relatives by their subtype diagnoses and found that the higher risk for ADHD among the relatives of ADHD girls held for the inattentive and combined subtypes but not for the hyperactive-impulsive subtype. The respective rates in the relatives of the ADHD and non-ADHD girls were 10% (N=42) and 4% (N=14) for the inattentive subtype (χ2=13.6, df=1, p<0.001; Yates χ2=13.9, df=1, p<0.001), 11% (N=47) and 2% (N=8) for the combined subtype (χ2=22.3, df=1, p<0.001; Yates χ2=28.3, df=1, p<0.001), and 2% (N=10) and 1% (N=3) for the hyperactive-impulsive subtype (χ2=3.7, df=1, p=0.05; Yates χ2=3.2, df=1, p=0.07).
To test hypothesis 3, we analyzed the three-by-three table of proband subtype diagnosis by relative subtype diagnosis. Figure 1 presents these data and shows that the subtypes of the relatives were not significantly likely to be the same as the subtype of the probands, i.e., they did not breed true. To further examine this hypothesis, we reanalyzed our data after limiting the inattentive diagnosis to the 13 subjects with the DSM-IV inattentive subtype who had three or fewer hyperactive-impulsive symptoms. With this new definition, the prevalence of inattentive ADHD was 4% among the relatives of the probands with combined-type ADHD, 7% among the relatives of the hyperactive-impulsive probands, and 0% among the relatives of the inattentive probands (χ2=3.7, df=2, p=0.16; Yates χ2=2.6, df=2, p=0.27).
To test hypothesis 4, we analyzed the family data from the present study together with the data from our prior study of boy ADHD and non-ADHD probands (17). Among the relatives of the ADHD girl probands, we found DSM-III-R ADHD in 18% of the female relatives and 24% of the male relatives. For the relatives of the ADHD boys, these figures were 12% and 20%, respectively. These data show no main effect of proband gender (χ2=2.9, df=1, p=0.09), no main effect of the relative’s gender (χ2=2.9, df=1, p=0.09), and no interaction between the genders of the proband and relative (χ2=0.4, df=1, p=0.53).
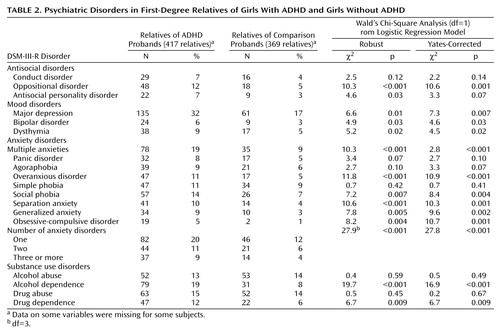
Consistent with hypothesis 5, Table 2 shows that the relatives of the girls with ADHD had significantly higher prevalences of mood, antisocial, substance use, and anxiety disorders than the relatives of the non-ADHD girls.
Discussion
The relatives of the ADHD girls had an elevated risk for ADHD, regardless of whether we used the DSM-III-R or DSM-IV definition. These findings support our first hypothesis and show that the familial transmission of ADHD—which has been so widely documented in families of ADHD boys—generalizes to families of ADHD girls. Familial transmission is a key validating criterion for any disorder (25). Thus, our finding that ADHD is familial in families ascertained through girl probands provides additional evidence for the validity of the diagnosis in girls.
Our second hypothesis received partial support: the relatives of ADHD girls had elevated prevalences of inattentive and combined-type ADHD but not hyperactive-impulsive ADHD. Although this could indicate that hyperactive-impulsive ADHD does not share familial determinants with inattentive and combined-type ADHD, other data argue against this interpretation. Namely, when we stratified the probands by DSM-IV subtype, the relatives of the hyperactive-impulsive probands had an elevated risk for DSM-IV ADHD, including the inattentive and combined subtypes. Thus, our results regarding the hyperactive-impulsive subtype are equivocal. Further, our inferences about this subgroup are clouded by the small number of hyperactive-impulsive subjects in our study, which has been the case in other studies of clinically referred subjects (26–30).
Our data refute hypothesis 3. Subtypes of ADHD did not breed true in these families. Also, the subtype data provide no evidence for a gradient of familial severity, i.e., no ADHD subtype was significantly more familial than the others. Thus, although the DSM-IV subtypes of ADHD have clinical implications (1, 29–31), they do not appear to influence the familial transmission of ADHD. Instead, it seems likely that the subtypes share the same pool of familial etiologic risk factors. This is consistent with findings from two twin studies that showed a significant overlap in the genetic contributions to symptoms of inattention and hyperactivity-impulsivity (32–34). Because familial risk factors cannot account for DSM-IV subtypes, researchers should turn to environmental risk factors to find an explanation for the variable expression of DSM-IV ADHD.
Our data also refute hypothesis 4, the prediction that relatives of girl probands would have higher rates of ADHD than relatives of boy probands (16). This finding is consistent with prior results (8, 11–14). Thus, the differing prevalences of ADHD between boys and girls cannot be attributed to any familial transmission model that posits that, compared with boys, girls require a greater “dose” of familial risk factors to express ADHD.
We found partial support for hypothesis 5: the relatives of the ADHD girls were at high risk for mood, antisocial, substance use, and anxiety disorders. Notably, despite the high levels of comorbidity and familial association between conduct disorder and ADHD seen in boys (35–37), we did not find an increased risk for conduct disorder among relatives of ADHD girls. These relatives, however, did have a significant increase in the risk for oppositional defiant disorder and showed an increased, but not significant, risk for antisocial personality disorder.
Our negative findings for conduct disorder are consistent with the low rate of conduct disorder (8%) we previously reported among the relatives of the girl probands (15) and with prior work showing ADHD girls to have a lower risk for conduct disorder (1). The present study shows that this gender difference in conduct disorder is not due to a failure to express an underlying familial predisposition to conduct disorder. If that were so, the family members should have had a significant risk for conduct disorder.
The low prevalence of conduct disorder in families of ADHD girls fits with our prior conclusions about two familial subtypes of ADHD: one related to conduct disorder and the other not (35, 36). In our prior work, the antisocial ADHD group was predominantly male, whereas the nonantisocial group had an even male-female ratio. From that we predicted that the familial link between ADHD and conduct disorder would be less evident in families ascertained through girl probands.
In relation to the comparison subjects, the relatives of the ADHD girls also had a significantly greater risk for depression. This agrees with findings from our meta-analyses of the ADHD-depression literature (38), which showed that children of depressed parents have an elevated risk for ADHD and that relatives of ADHD probands have an elevated risk for depression. Although our meta-analyses of the ADHD-bipolar literature (39) suggest a familial link between the two disorders, the positive association in the present study group did not attain statistical significance.
We also found an increased prevalence of several anxiety disorders among relatives of ADHD girls. This finding is consistent with the results in our prior family study of girls with DSM-III-defined ADD (10) and in our family studies of ADD boys (40) and ADHD boys (17). Consistent with findings in other studies, our data suggest a link between substance use disorders and ADHD (41). Notably, follow-up studies document a high risk for substance use disorders in adults who had ADHD as children (42), and family studies of substance use disorders (43) and of ADHD (44) have shown an association between the two disorders.
If, as our data suggest, the genetic contributions to ADHD are similar in boys and girls, this may indicate similarities in other biological features. Consistent with this idea are the findings of Sharp et al. (45). They found that, in addition to showing similarities in a wide range of clinical features, ADHD girls and boys did not differ in their clinical response to either methylphenidate or dextroamphetamine. In contrast, other studies suggest biological differences between ADHD boys and girls. For example, data we reported earlier (46) suggest that girls with ADHD may not have executive deficits, may be less vulnerable to such deficits, or may have a form of executive function deficits that differs from that for boys. On the other hand, a neuroimaging study by Ernst et al. (47) suggested significant brain dysfunction for ADHD girls but not boys. Clearly, more work is needed to determine if gender differences exist in the biological underpinnings of ADHD.
We must also consider the implications of the demographic differences seen between the ADHD and comparison families in this study. The ADHD probands and their parents were significantly younger than the comparison probands and their parents. This likely reflects the fact that ADHD has a relatively early onset. Also, although the difference did not attain statistical significance, the parents of the ADHD probands were more likely to be separated or divorced and to come from lower social strata. There are two possible explanations for these findings. Many of the parents had ADHD and associated psychopathology, disorders that may lead to marital discord and lower social attainment. It is also possible that the stresses of rearing an ADHD child could account for these findings. Further work is needed to clarify these issues.
The generalizability of our results is limited because the families were primarily Caucasian. We relied on maternal reports for children younger than 12, which may have led to a bias whereby mothers with ADHD overreported ADHD in their children. Another limitation is the fact that the rates of participation were higher for comparison than ADHD families. Although this may mean that fewer ADHD families were eligible, it is also possible that more ADHD families refused to participate. If so, that would further limit the generalizability of our results but would not confound case-control comparisons.
Despite these limitations, our results show that relatives of ADHD girls have an elevated risk for ADHD, mood, substance use, and anxiety disorders. The pattern of familial aggregation conforms to what is known about the families of ADHD boys, with one exception: antisocial disorders appear to be less familial in girls with ADHD. Our data also suggest that familial risk factors cannot account for either gender differences in prevalence or the clinical variability of DSM-IV subtypes. Further work is needed to better understand these features of ADHD and their implications for understanding the etiology of the disorder.
 |
 |
Received May 12, 1999; revisions received Aug. 23 and Dec. 13, 1999; accepted Jan. 18, 2000. From the Pediatric Psychopharmacology Unit, Child Psychiatry Service, Massachusetts General Hospital; and the Harvard Pilgrim Community Health Plan, Boston. No reprints are available. Address correspondence to Dr. Faraone, Pediatric Psychopharmacology Unit (ACC 725), Massachusetts General Hospital, Fruit St., Boston, MA 02114; [email protected] (e-mail). Supported by NIMH grant MH-41314 to Dr. Biederman.
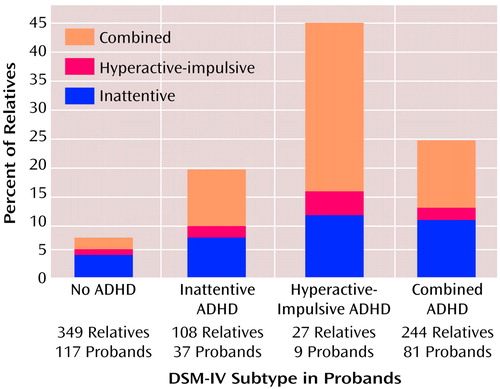
Figure 1. DSM-IV Subtypes of ADHD in First-Degree Relatives Stratified by ADHD Subtype of Girl Probandsa
aThe subtype of ADHD in the proband was not associated with the subtype in the relatives (chi-square analysis).
1. Gaub M, Carlson CL: Gender differences in ADHD: a meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry 1997; 36:1036–1046Google Scholar
2. Arnold LE: Sex differences in ADHD: conference summary. J Abnorm Child Psychol 1996; 24:555–569Crossref, Medline, Google Scholar
3. Faraone S, Biederman J: Is attention deficit hyperactivity disorder familial? Harvard Rev Psychiatry 1994; 1:271–287Google Scholar
4. Faraone S, Biederman J: Genetics of attention-deficit hyperactivity disorder. Child Adolesc Psychiatr Clin North Am 1994; 3:285–302Crossref, Google Scholar
5. Faraone SV, Tsuang D, Tsuang MT: Genetics and Mental Disorders: A Guide for Students, Clinicians, and Researchers. New York, Guilford, 1999Google Scholar
6. Pauls DL, Shaywitz SE, Kramer PL, Shaywitz BA, Cohen DJ: Demonstration of vertical transmission of attention deficit disorder (abstract). Ann Neurol 1983; 14:363Google Scholar
7. Kashani J, Chapel J, Ellis J, Shekim W: Hyperactive girls. J Operational Psychiatry 1979; 10:145–148Google Scholar
8. James A, Taylor E: Sex differences in the hyperkinetic syndrome of childhood. J Child Psychol Psychiatry 1990; 31:437–446Crossref, Medline, Google Scholar
9. Bhatia M, Nigam V, Bohra N, Malik S: Attention deficit disorder with hyperactivity among pediatric outpatients. J Child Psychol Psychiatry 1991; 32:297–306Crossref, Medline, Google Scholar
10. Faraone SV, Biederman J, Keenan K, Tsuang MT: A family-genetic study of girls with DSM-III attention deficit disorder. Am J Psychiatry 1991; 148:112–117Link, Google Scholar
11. Rhee SH, Waldman ID, Hay DA, Levy F: Sex differences in genetic and environmental influences on DSM-III-R attention-deficit/hyperactivity disorder. J Abnorm Psychol 1999; 108:24–41Crossref, Medline, Google Scholar
12. Gjone H, Stevenson J, Sundet J: Genetic influence on attention problems in a general population twin sample. J Am Acad Child Adolesc Psychiatry 1996; 45:588–596Crossref, Google Scholar
13. Silberg J, Rutter M, Meyer J, Maes H, Hewitt J, Simonoff E, Pickles A, Loeber R: Genetic and environmental influences on the covariation between hyperactivity and conduct disturbance in juvenile twins. J Child Psychol Psychiatry 1996; 37:803–816Crossref, Medline, Google Scholar
14. Goodman R, Stevenson J: A twin study of hyperactivity, I: an examination of hyperactivity scores and categories derived from Rutter teacher and parent questionnaires. J Child Psychol Psychiatry 1989; 30:671–689Crossref, Medline, Google Scholar
15. Biederman J, Faraone S, Mick E, Williamson S, Wilens T, Spencer T, Weber W, Jetton J, Kraus I, Pert J, Zallen B: Clinical correlates of attention deficit hyperactivity disorder in females: findings from a large group of pediatrically and psychiatrically referred girls. J Am Acad Child Adolesc Psychiatry 1999; 38:966–975Crossref, Medline, Google Scholar
16. Biederman J, Newcorn J, Sprich S: Comorbidity of attention deficit hyperactivity disorder with conduct, depressive, anxiety, and other disorders. Am J Psychiatry 1991; 148:564–577Link, Google Scholar
17. Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R, Spencer T, Norman D, Kolodny R, Kraus I, Perrin J, Keller MB, Tsuang MT: Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: patterns of comorbidity in probands and relatives psychiatrically and pediatrically referred samples. Arch Gen Psychiatry 1992; 49:728–738Crossref, Medline, Google Scholar
18. Orvaschel H, Puig-Antich J: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SADS-E), 4th revision. Pittsburgh, Western Psychiatric Institute and Clinic, 1987Google Scholar
19. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
20. Hollingshead AB: Four-Factor Index of Social Status. New Haven, Conn, Yale University, Department of Sociology, 1975Google Scholar
21. Gershon ES, Hamovit J, Guroff JJ, Dibble E, Leckman JF, Sceery W, Targum SD, Nurnberger JI Jr, Goldin LR, Bunney WE Jr: A family study of schizoaffective, bipolar I, bipolar II, unipolar, and normal control probands. Arch Gen Psychiatry 1982; 39:1157–1167Google Scholar
22. Faraone S, Biederman J, Milberger S: How reliable are maternal reports of their children’s psychopathology? one year recall of psychiatric diagnoses of ADHD children. J Am Acad Child Adolesc Psychiatry 1995; 34:1001–1008Google Scholar
23. Rosenbaum JF, Biederman J, Gersten M, Hirshfeld DR, Meminger SR, Herman JB, Kagan J, Reznick JS, Snidman N: Behavioral inhibition in children of parents with panic disorder and agoraphobia: a controlled study. Arch Gen Psychiatry 1988; 45:463–470Crossref, Medline, Google Scholar
24. Huber PJ: The behavior of maximum likelihood estimates under non-standard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability 1967; 1:221–233Google Scholar
25. Robins E, Guze SB: Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. Am J Psychiatry 1970; 126:983–987Link, Google Scholar
26. Lahey B, Applegate B, McBurnett K, Biederman J, Greenhill L, Hynd G, Barkley R, Newcorn J, Jensen P, Richters J, Garfinkel B, Kerdyk L, Frick P, Ollendick T, Perez D, Hart E, Waldman I, Shaffer D: DSM-IV field trials for attention deficit hyperactivity disorder in children and adolescents. Am J Psychiatry 1994; 151:1673–1685Google Scholar
27. Paternite C, Loney J, Roberts M: External validation of oppositional disorder and attention deficit disorder with hyperactivity. J Abnorm Child Psychol 1995; 23:453–471Crossref, Medline, Google Scholar
28. Morgan A, Hynd G, Riccio C, Hall J: Validity of DSM-IV ADHD predominantly inattentive and combined types: relationship to previous DSM diagnoses/subtype differences. J Am Acad Child Adolesc Psychiatry 1996; 35:325–333Crossref, Medline, Google Scholar
29. Eiraldi R, Power T, Nezu C: Patterns of comorbidity associated with subtypes of attention-deficit hyperactivity disorder among 6 to 12 year old children. J Am Acad Child Adolesc Psychiatry 1997; 36:503–514Crossref, Medline, Google Scholar
30. Faraone S, Biederman J, Webber W, Russell R: Psychiatric, neuropsychological, and psychosocial features of DSM-IV subtypes of attention-deficit/hyperactivity disorder: results from a clinically referred sample. J Am Acad Child Adolesc Psychiatry 1998; 37:185–193Crossref, Medline, Google Scholar
31. Wolraich M, Hannah J, Pinnock T, Baumgaetel A, Brown J: Comparison of diagnostic criteria for attention-deficit hyperactivity disorder in a county-wide sample. J Am Acad Child Adolesc Psychiatry 1996; 35:319–324Crossref, Medline, Google Scholar
32. Hudziak J, Heath A, Madden P, Reich W, Bucholz K, Slutske W, Bierut L, Neuman R, Todd R: Latent class and factor analysis of DSM-IV ADHD: a twin study of female adolescents. J Am Acad Child Adolesc Psychiatry 1998; 37:848–857Crossref, Medline, Google Scholar
33. Neuman R, Todd R, Heath A, Reich W, Hudziak J, Bucholz K, Madden P, Begleiter H, Porjesz B, Kuperman S, Hesselbrock V, Reich T: Evaluation of ADHD typology in three contrasting samples: a latent class approach. J Am Acad Child Adolesc Psychiatry 1999; 38:25–33Crossref, Medline, Google Scholar
34. Sherman D, Iacono W, McGue M: Attention deficit hyperactivity disorder dimensions: a twin study of inattention and impulsivity hyperactivity. J Am Acad Child Adolesc Psychiatry 1997; 36:745–753Crossref, Medline, Google Scholar
35. Faraone SV, Biederman J, Keenan K, Tsuang MT: Separation of DSM-III attention deficit disorder and conduct disorder: evidence from a family-genetic study of American child psychiatric patients. Psychol Med 1991; 21:109–121Crossref, Medline, Google Scholar
36. Faraone S, Biederman J, Garcia Jetton J, Tsuang M: Attention deficit disorder and conduct disorder: longitudinal evidence for a familial subtype. Psychol Med 1997; 27:291–300Crossref, Medline, Google Scholar
37. Lahey BB, Piacentini JC, McBurnett K, Stone P, Hartdagen S, Hynd G: Psychopathology in the parents of children with conduct disorder and hyperactivity. J Am Acad Child Adolesc Psychiatry 1988; 27:163–170Crossref, Medline, Google Scholar
38. Faraone SV, Biederman J: Do attention deficit hyperactivity disorder and major depression share familial risk factors? J Nerv Ment Dis 1997; 185:533–541Google Scholar
39. Faraone SV, Biederman J, Mennin D, Wozniak J, Spencer T: Attention deficit hyperactivity disorder with bipolar disorder: a familial subtype? J Am Acad Child Adolesc Psychiatry 1997; 36:1378–1387Google Scholar
40. Biederman J, Faraone SV, Keenan K, Knee D, Tsuang MT: Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1990; 29:526–533Crossref, Medline, Google Scholar
41. Levin FR, Kleber HD: Attention-deficit hyperactivity disorder and substance abuse: relationships and implications for treatment. Harvard Rev Psychiatry 1995; 2:246–258Crossref, Medline, Google Scholar
42. Hechtman L, Weiss G: Controlled prospective fifteen year follow-up of hyperactives as adults: non-medical drug and alcohol use and anti-social behaviour. Can J Psychiatry 1986; 31:557–567Crossref, Medline, Google Scholar
43. Wilens T, Biederman J, Kiely K, Bredin E, Spencer T: Pilot study of behavioral and emotional disturbances in the high-risk children of parents with opioid dependence. J Am Acad Child Adolesc Psychiatry 1995; 34:779–785Crossref, Medline, Google Scholar
44. Cantwell DP: Psychiatric illness in the families of hyperactive children. Arch Gen Psychiatry 1972; 27:414–417Crossref, Medline, Google Scholar
45. Sharp W, Walter J, Marsh W, Ritchie G, Hamburger S, Castellanos X: ADHD in girls: clinical comparability of a research sample. J Am Acad Child Adolesc Psychiatry 1999; 38:40–47Crossref, Medline, Google Scholar
46. Seidman L, Biederman J, Faraone S, Weber W, Mennin D, Jones J: A pilot study of neuropsychological function in ADHD girls. J Am Acad Child Adolesc Psychiatry 1997; 36:366–373Crossref, Medline, Google Scholar
47. Ernst M, Liebenauer L, King A, Fitzgerald G, Cohen R, Zametkin A: Reduced brain metabolism in hyperactive girls. J Am Acad Child Adolesc Psychiatry 1994; 33:858–868Crossref, Medline, Google Scholar



