Nortriptyline Versus Fluoxetine in the Treatment of Depression and in Short-Term Recovery After Stroke: A Placebo-Controlled, Double-Blind Study
Abstract
OBJECTIVE: This study compared nortriptyline and fluoxetine with placebo in the treatment of depression and in recovery from physical and cognitive impairments after stroke. METHOD: A total of 104 patients with acute stroke enrolled between 1991 and 1997 entered a double-blind randomized study comparing nortriptyline, fluoxetine, and placebo over 12 weeks of treatment. The majority of patients were recruited from a rehabilitation hospital in Des Moines, Iowa, but other enrollment sites were also used. Both depressed and nondepressed patients were enrolled to determine whether improved recovery could be mediated by mechanisms unrelated to depression. Nortriptyline in doses of 25 mg/day gradually increased to 100 mg/day or fluoxetine in doses of 10 mg/day gradually increased to 40 mg/day or identical placebo were given over 12 weeks. Response to treatment of depression for individual patients was defined as a greater-than-50% reduction in scores on the Hamilton Rating Scale for Depression and no longer fulfilling diagnostic criteria for major or minor depression. Improved recovery for a treatment group was defined as a significantly higher mean score from baseline to end of the treatment trial, compared with patients treated with placebo, on measures of impairment in activities of daily living and levels of cognitive and social functioning. RESULTS: Nortriptyline produced a significantly higher response rate than fluoxetine or placebo in treating poststroke depression, in improving anxiety symptoms, and in improving recovery of activities of daily living as measured by the Functional Independence Measure. There was no effect of nortriptyline or fluoxetine on recovery of cognitive or social functioning among depressed or nondepressed patients. Fluoxetine in increasing doses of 10–40 mg/day led to an average weight loss of 15.1 pounds (8% of initial body weight) over 12 weeks of treatment that was not seen with nortriptyline or placebo. CONCLUSIONS: Given the doses of medication used in this study, nortriptyline was superior to fluoxetine in the treatment of poststroke depression. Demonstrating a benefit of antidepressant treatment in recovery from stroke may require the identification of specific subgroups of patients, alternative measurement scales, or the optimal time of treatment.
In 1984, the first controlled double-blind treatment study of poststroke depression was published (1). This study demonstrated that among patients who were an average of 6 months poststroke, 11 study completers receiving nortriptyline had significantly lower scores on the Hamilton Rating Scale for Depression at 4 weeks and 6 weeks after beginning treatment than 14 study completers given placebo. Since that time, at least three other double-blind randomized studies of treatment of poststroke depression have been reported (2–4).
The only double-blind treatment study of poststroke depression to examine the utility of selective serotonin reuptake inhibitors (SSRIs) in the treatment of this condition was by Andersen et al. (3) using citalopram. Results of efficacy and intention-to-treat analyses (i.e., analyses in which early dropouts were excluded or included, respectively) demonstrated better outcome as measured by the Hamilton depression scale or the Melancholia Scale at both 3 and 6 weeks among 33 patients treated with 20 mg/day of citalopram (or 10 mg/day for patients over age 66) than among 33 patients given placebo. Response to treatment was better in patients who were 7 or more weeks poststroke than among patients who were less than 7 weeks poststroke.
However, we are not aware of any studies that have compared a tricyclic antidepressant with an SSRI medication using double-blind controlled conditions. The relative safety of SSRI medications as well as their more favorable side effect profile has led to the widespread use of these medications in the treatment of depression, including poststroke depression. The efficacy of SSRIs in the treatment of major depression among geriatric patients was demonstrated by Tollefson et al. (5) in a double-blind study comparing fluoxetine in 335 patients with placebo in 336 patients. Overall response rates were 43.9% and 31.69%, respectively. The efficacy of SSRIs has also been demonstrated in patients with physical illnesses. Roose et al. (6) found that 61% of 41 patients with ischemic heart disease and depression improved after 6 weeks of treatment with paroxetine, compared with 55% of 40 patients treated with nortriptyline who had heart disease of similar severity.
Subgroups of geriatric patients, particularly those with physical illness, however, have sometimes suffered significant side effects when treated with SSRIs. In one study, seven of 15 medically ill and depressed patients over age 75 who were treated with fluoxetine lost more than 5% of their body weight (7). In contrast, only one of 68 patients in the same age group and with the same medical background who were treated with nortriptyline or desipramine lost more than 5% of his or her body weight. In addition, Roose et al. (8) found that elderly patients with heart disease and major depression, melancholic subtype, had a significantly lower response rate to fluoxetine (N=5 of 22) compared with nortriptyline (N=28 of 42). These studies suggest that in some elderly patients with medical illnesses, fluoxetine may have greater side effects or less efficacy than tricyclic antidepressants.
The study reported here was undertaken to examine the hypothesis that nortriptyline and fluoxetine would both be superior to placebo in the treatment of depression in elderly patients after stroke, although the fluoxetine-treated subjects could experience significant side effects of nausea or weight loss. Because a previous study had shown that active treatment with trazodone (N=7) had improved recovery in activities of daily living in a subgroup of depressed poststroke patients over 5 to 6 weeks of treatment (2), we examined treatment response in both depressed and nondepressed patients over 12 weeks. We included nondepressed patients (N=48) because we expected to see an effect on recovery in depressed patients and wanted to determine whether the improvement was related to recovery from depression or to a neurochemical effect that was independent of depression. We hypothesized that antidepressants would benefit recovery only in patients with poststroke depression.
METHOD
Patient Selection
Patients were enrolled in the treatment study between June 1991 and June 1997. The vast majority of enrolled patients were recruited from Younkers Rehabilitation Center of Iowa Methodist Medical Center in Des Moines, Iowa (N=89). A small number were recruited from the neurological service at The University of Iowa Hospitals and Clinics in Iowa City (N=1), the VA Medical Center in Iowa City (N=2), or the neurological service of the Ra�rrea Institute of Neurological Research in Buenos Aires, Argentina (N=12).
Initially, a total of 343 patients with an acute stroke who met inclusion criteria were asked to participate in a treatment study of poststroke depression. Inclusion criteria included acute stroke within 6 months of the onset of the study and age 18–85. Exclusion criteria included 1) any other significant medical illness that would threaten the patient’s life or recovery from stroke, 2) severe comprehension deficit that precluded a verbal interview (defined as failing part 1 of the Token Test [9]), 3) prior history of head injury, or 4) prior history of other brain disease with the exception of prior stroke (N=103 were excluded). Patients taking antidepressants other than fluoxetine at the time of enrollment were allowed to stop their antidepressants for a 2-week washout period before the study (N=3). In addition to requiring the patient’s informed consent to participate in the study, we required that the patient’s immediate family and treating physician agree to the patient’s participation. One hundred thirty patients did not participate because either the patient or a family member refused participation. Two patients died before assignment to a treatment group, and four dropped out before assignment.
Treatment Protocol
After providing a complete description of the study to the subjects, we obtained their written informed consent to participate. Patients were randomly assigned to either fluoxetine or nortriptyline treatment unless nortriptyline was contraindicated because of a cardiac conduction abnormality (e.g., bundle branch block) or a heart attack within 3 months before the study (N=8). Fluoxetine was contraindicated for patients who had an intracerebral hemorrhage (N=9) (one patient was randomly assigned fluoxetine before the diagnosis was made). Thus, 85% of the patients were randomly assigned to nortriptyline or fluoxetine. All patients were randomly assigned to either active or placebo medication.
Depressed patients were treated in a crossover design (i.e., 12 weeks of active treatment followed by 12 weeks of placebo treatment) so that all patients would receive active treatment with random assignment to either active or placebo treatment during the first 12 weeks. Although the original design was a crossover, data for a significant number of patients taking placebo (i.e., 31% response rate) became ineligible for analysis. Because of this development and because we wanted to include as many patients as possible in the treatment group, we used an independent groups design to analyze the data. Data from the first 12 weeks of treatment were used in the analysis for 17 of the 23 depressed patients assigned to fluoxetine treatment, 15 of the 16 patients assigned to nortriptyline treatment, and all 17 of the patients assigned to receive placebo. If a patient was assigned to receive placebo during the first 12 weeks of treatment and continued to meet the inclusion criteria for depression during the second 12 weeks of treatment, the data for that patient from the second 12 weeks were analyzed. Data for six patients who received fluoxetine and one patient who received nortriptyline were analyzed in this way.
Depression was defined as meeting DSM-IV criteria for major depressive disorder or minor depressive disorder with a Hamilton depression scale score of 12 or greater (based on criteria used in prior studies [10]). Nondepressed patients were assigned to 12 weeks of treatment with either fluoxetine, nortriptyline, or placebo with no crossover. Patients were seen at enrollment and at 3, 6, 9 and 12 weeks after beginning the medication. The doses of nortriptyline were 25 mg/day for the first week, 50 mg/day for weeks 2 and 3, 75 mg/day for weeks 3–6, and 100 mg/day for the final 6 weeks. Doses of fluoxetine were 10 mg/day for the first 3 weeks, 20 mg/day for weeks 4–6, 30 mg/day for weeks 7–9, and 40 mg/day for the final 3 weeks. Doses were decreased if side effects were severe. Because of severe side effects, doses were decreased in four patients treated with fluoxetine (in one patient due to anxiety, two due to gastrointestinal symptoms, and one due to insomnia) and four patients treated with nortriptyline (two patients due to blood levels over 150 ng/ml and two due to sedation). To maintain the blind, doses were decreased for equal numbers of placebo patients. The active and placebo pills were identical.
Assessment
Patients were asked about background and illness information at the initial study evaluaion. They were also weighed at the first and last evaluation, and their blood pressure and pulse were taken at each 3-week follow-up visit. The follow-up visits were conducted in the treating hospital or, most often, in the patient’s home or long-term-care facility. Psychiatric assessment included administration of a version of the Present State Examination modified to identify DSM-III-R, and later, DSM-IV symptoms of depression and anxiety disorder. The Present State Examination is a semistructured psychiatric interview whose reliability and validity in this population have been previously demonstrated (11). Although part of the study was done while DSM-III-R was in use, symptoms elicited in response to the Present State Examination were used at the time of the analyses to determine DSM-IV diagnoses for all patients. Initially, and at each 3-week evaluation, the 28-item form of the Hamilton depression scale was administered. We have previously demonstrated the reliability and validity of the Hamilton depression scale in patients with stroke (11, 12). Successful treatment response was defined as a greater-than-50% reduction in the Hamilton depression scale score and no longer fulfilling diagnostic criteria for major or minor depression.
Several other instruments were used in the assessments. The Hamilton Rating Scale for Anxiety was used to assess the severity of patients’ anxiety symptoms at each follow-up visit. In a previous publication, we demonstrated the reliability and validity of this scale in poststroke patients (13). The Johns Hopkins Functioning Inventory and the Functional Independence Measure, which measure activities of daily living, were also administered at each evaluation. The Johns Hopkins Functioning Inventory is a 10-item, 44-point scale on which higher scores indicate greater levels of impairment. The Functional Independence Measure is an 18-item, 72-point scale on which higher scores indicate less impairment (14). We have previously demonstrated the reliability and validity of the Johns Hopkins Functioning Inventory in this patient population (11, 15). The Mini-Mental State (16) is a brief cognitive examination. We have used this cognitive examination in prior studies and demonstrated its reliability and validity in this population (17, 19). The Social Functioning Exam (18) is a 28-item scale that assesses patients’ satisfaction with their social functioning before the stroke (asked at the in-hospital evaluation) or during the 2 weeks before the follow-up examinations. Scores on the Social Functioning Exam range from 0.00 to 1.00, with higher scores indicating greater severity of social impairment. The reliability and validity of this instrument has also been previously demonstrated (18, 20). Improved recovery on each of these scales was defined as a significantly greater improvement of score from baseline to end of treatment trial compared with placebo-treated patients.
Computerized Tomography
Computerized tomography (CT) or magnetic resonance scans were obtained from the treating acute hospital for each of the patients in the study. Scans were evaluated blind to any of the psychiatric findings for the anatomical location and size of the brain injury.
Statistical Analysis
Statistical analyses utilized repeated measures analysis of variance for ratings on scales with continuous measurement. Where appropriate, Huhyn-Feldt corrected p values are shown. Important analyses were also analyzed using an analysis of covariance, controlling for baseline ratings. Post-hoc comparisons, where indicated, utilized the Duncan statistic. The Duncan statistic does not provide a precise p value, but the p value is always less than 0.05 when the Duncan statistic is cited with a significant finding.
In addition, nonparametric repeated analyses were performed. This nonparametric analysis, based on suggestions by Cliff (21), used tau as an index of trajectory for each patient’s course during the study. Values of tau approaching –1.0 indicated that the ratings steadily improved (lower Hamilton depression scores) over the five measurement times, values near zero indicated a near-random pattern, and a large positive value indicated steady worsening. A Kruskal-Wallis test was then used to compare the three groups for significantly different trajectories. Post-hoc tests for these comparisons were performed with Wilcoxon scores (i.e., Mann-Whitney tests [22]) and corrected according to Tukey’s honestly significant difference (with p<0.05, two-tailed) criterion to account for multiple comparisons.
For discrete variables, chi-square tests were used except when cell sizes were prohibitively small, in which case Fisher’s exact tests were used.
RESULTS
Patient Characteristics
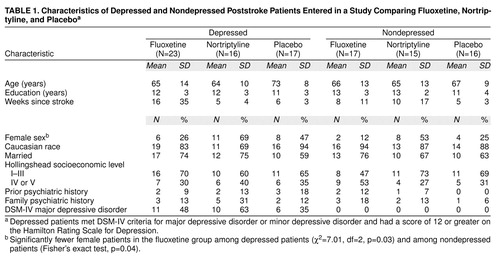
The background characteristics of the patients included in the intention-to-treat group are shown in table 1. Ninety-two patients were enrolled in Iowa, and 12 in Argentina. Approximately half of the patients with depression had major depressive disorder (table 1). The only significant difference among the three treatment groups of depressed and nondepressed patients was that the fluoxetine groups contained significantly more male patients than the nortriptyline or placebo groups.
The neurological and CT scan findings for the depressed and nondepressed groups are shown in table 2. There were no significant differences between treatment groups except that among the nondepressed patients there were more patients with hemorrhage in the nortriptyline group than in the fluoxetine and placebo groups (patients with hemorrhage were excluded from the fluoxetine group) and among the depressed patients the fluoxetine group had fewer patients with paresis of at least one limb than the nortriptyline or placebo groups (70% of the fluoxetine group versus 100% of the nortriptyline and placebo groups).
Intention-to-Treat Analysis
Of the nine withdrawals in the depressed fluoxetine group, six dropped out within the first 3 weeks, two dropped out between weeks 4 and 6, and one dropped out between weeks 7 and 9 (three complained of gastrointestinal symptoms, and six refused treatment). Of the depressed nortriptyline patients, one dropped out during the first 3 weeks, one dropped out between weeks 7 and 9, and one dropped out between weeks 10 and 12 (two had medical deterioration, and one refused treatment). In the depressed placebo group, four patients dropped out during the first 3 weeks (one patient died due to pulmonary embolus, one deteriorated medically, and two refused treatment).
In the nondepressed fluoxetine group, four patients dropped out—two during the first 3 weeks, and two between weeks 4 and 6. (One complained of gastrointestinal symptoms, two deteriorated medically, and one refused treatment.) Of the nondepressed nortriptyline group, two patients dropped out—one during the first 3 weeks and one during weeks 4–6. (One refused treatment, and one had sedation.) One of the nondepressed placebo patients dropped out between weeks 7 and 9. (The patient developed a rash.) Combining the dropout rates for both depressed and nondepressed patients, the dropout rate was significantly greater in the fluoxetine group than in the nortriptyline and placebo groups (χ2=4.10, df=1, p=0.04).
A repeated measures analysis of variance comparing Hamilton depression scale scores for all depressed patients entered in the fluoxetine, nortriptyline, and placebo groups demonstrated a significant time-by-treatment interaction (F=3.45, df=8, 212, p=0.004). A post-hoc analysis of group mean depression scores using the Duncan statistic indicated no intergroup difference at the initial evaluation between the nortriptyline and fluoxetine groups, although the placebo group had a significantly lower mean score than the nortriptyline group (p<0.05). At 12 weeks, the nortriptyline group had a significantly lower mean Hamilton depression scale score than the fluoxetine group, and the placebo group also had a significantly lower mean score than the fluoxetine group (Duncan statistic, p<0.05). The nonparametric analysis of trajectories also revealed a significant difference between the groups (Kruskal-Wallis χ2=8.00, df=2, p<0.02). Follow-up tests with Tukey’s honestly significant difference criterion indicated that the nortriptyline group had more consistent trajectory for improvement on Hamilton depression scale scores than the fluoxetine group.
Because placebo-treated patients had lower Hamilton depression scale scores than the nortriptyline-treated patients at the initial evaluation, we repeated the analyses covarying for baseline scores. This analysis demonstrated the same time-by-treatment interaction (F=3.24, df=6, 156, p=0.01). Post-hoc analysis showed that patients treated with nortriptyline had a significantly greater decline in Hamilton depression scale scores than either placebo- or fluoxetine-treated patients at 12 weeks (figure 1). There were no differences between fluoxetine and placebo. The successful treatment rate was 10 of 16 (63%) for nortriptyline, two of 23 (9%) for fluoxetine, and four of 17 (24%) for placebo.
Efficacy Analysis
A repeated measures analysis of variance of mean Hamilton depression scale scores comparing the depressed patients in the three treatment groups who completed the 12-week study (14 patients in the fluoxetine group, 13 in the nortriptyline group, and 13 in the placebo group) demonstrated a significant time-by-treatment interaction (F=3.65, df=8, 148, p=0.001). Post-hoc analysis demonstrated no significant intergroup differences during the initial evaluation. The nortriptyline group, however, had a significantly lower mean Hamilton depression scale score than the fluoxetine group at 12 weeks (Duncan statistic, p<0.05). The placebo group also had a significantly lower mean score than the fluoxetine group at 12 weeks (Duncan statistic, p<0.05). Nonparametric analysis of the trajectories provided similar results. There were overall differences in trajectories among the three treatment groups (Kruskal-Wallis χ2=8.07, df=2, p<0.02). Follow-up tests with Tukey’s honestly significant difference criterion indicated that nortriptyline was associated with more consistently improving trajectories compared with either the fluoxetine or placebo groups. To account for the lower scores of the placebo group at the initial evaluation, repeated measures analysis of variance covarying for baseline scores was done. The analysis revealed a significant time-by-treatment interaction (F=3.58, df=6, 108, p=0.005). Although there were no differences between fluoxetine and placebo at any time point, nortriptyline was superior to placebo and fluoxetine at 12 weeks of treatment and to placebo at 9 weeks of treatment (Duncan statistic, p<0.05).
The successful treatment rate was 10 of 13 (77%) for nortriptyline, two of 14 (14%) for fluoxetine, and four of 13 (31%) for placebo. The overall rate of response was significantly different than a random distribution (χ2=11.70, df=2, p=0.003). The response rate was significantly higher in the nortriptyline group than in either the fluoxetine (Fisher’s exact test, p=0.002) or placebo groups (Fisher’s exact test, p=0.05), and the response rates in the fluoxetine and placebo groups were not significantly different from each other.
Treatment Effects on Anxiety and Impairment Scores
Scores for the Hamilton depression and anxiety scales as well as the impairment scales before and after treatment for the fluoxetine, nortriptyline, and placebo groups are shown in table 3. Among the depressed patients, repeated measures analysis of variance comparing the nortriptyline, fluoxetine, and placebo groups on the Hamilton anxiety scale revealed a significant time-by-treatment interaction (F=2.14, df=8, 13, p=0.04). Although no significant post-hoc differences between groups were found at any time point, the nortriptyline group had a mean 2.9-point decline in the Hamilton anxiety scale score, the fluoxetine group had a mean 3.2-point increase in the Hamilton anxiety scale score, and the placebo group’s mean score did not change.
Analysis of scores on the impairment scales for depressed patients revealed a significant time-by-treatment interaction for the Functional Independence Measure (F=2.42, df=8, 14, p=0.02). Post-hoc analysis covarying for baseline scores revealed that improvement in Functional Independence Measure scores was significantly greater in the nortriptyline group than in the fluoxetine group at 9 and 12 weeks (Duncan statistic, p<0.05) and in the placebo group compared with the fluoxetine group at 12 weeks (table 3). Thus, the fluoxetine group did not show the same amount of recovery in activities of daily living as the nortriptyline or placebo groups.
Comparison of the depressed fluoxetine, nortriptyline, and placebo groups on other impairment measures demonstrated a significant time effect for results on the Johns Hopkins Functioning Inventory (F=11.80, df=4, 148, p=0.0001) but no time-by-treatment interaction. There were no significant treatment or time effects for results on the Mini-Mental State or the Social Functioning Exam.
Nondepressed Patients
Analysis of data for the nondepressed patients showed no significant treatment effects and no significant time-by-treatment interactions. The initial and 12-week scores for all scales are shown in table 3. There were significant time effects for the Functional Independence Measure (F=6.30, df=4, 144, p=0.0001) and the Johns Hopkins Functioning Inventory (F=3.80, df=4, 144, p=0.006) but no significant intergroup differences. Thus, the efficacy analysis of the nondepressed patients failed to demonstrate a significant effect of treatment over 12 weeks.
Potentially Confounding Variables
Because five of the nortriptyline-treated patients, three of the fluoxetine-treated patients, and one placebo-treated patient in the depressed group were treated in Argentina, we reanalyzed the results after removing the data for these patients. Using only the Iowa patients, analysis of Hamilton depression scale scores in either the intention-to-treat or the efficacy analysis showed a significant treatment-by-time interaction (F=2.22, df=8, 176, p=0.028, for the intention-to-treat analysis; F=2.72, df=8, 112, p=0.009, for the efficacy analysis). Assessment of differences at each time point demonstrated for both analyses that the nortriptyline-treated group had significantly lower scores than the fluoxetine-treated group at 12 weeks (Duncan statistic, p<0.05). There were also no significant effects of removing these patients on the treatment outcome for Hamilton anxiety scale scores, Functional Independence Measure scores, Johns Hopkins Functioning Inventory scores, Mini-Mental State scores, and Social Functioning Exam scores.
Five of the fluoxetine-treated patients who completed the study and one who dropped out were given active treatment during the second 3-month period, but only one of the nortriptyline-treated patients received active treatment during that period. To control for this potentially confounding variable, we repeated the intention-to-treat and efficacy analyses using only first 3-month or only second 3-month data for the fluoxetine group. Significant treatment-by-time interactions were found for both the first 3-month data and the second 3-month data (F=3.90, df=8, 128, p=0.004, first 3 months; F=2.57, df=8, 112, p=0.01, second 3 months). In both analyses, nortriptyline-treated patients had a significantly lower Hamilton depression scale score than fluoxetine-treated patients at 9 weeks and 12 weeks for the first 3 months and at 12 weeks for the second 3 months (Duncan statistic, p<0.05).
Because the fluoxetine-treated patients showed an increase in Hamilton depression scale scores between week 3 and week 12, we analyzed weight change and dose of fluoxetine in these patients to determine whether gastrointestinal side effects or doses over 20 mg of fluoxetine might explain the increased scores. To eliminate the effect of depression on weight, we analyzed weight change in nondepressed patients. Of the seven nondepressed treated with fluoxetine for whom we had data, six had lost more than 10 lb during the 3 months of treatment (mean=14.0 lb, SD=5.65), whereas none of the eight nortriptyline-treated patients (mean gain=7.6 lb, SD=16.49) or the six placebo-treated patients (mean gain=5.0 lb, SD=4.62) had lost weight (Fisher’s exact test, p=0.002). When depressed and nondepressed patients were included in the analysis of weight change, 10 of 12 fluoxetine-treated patients had lost 10 lb or more (mean=15.1 lb, SD=8.07 lb, or 8% of initial body weight), whereas only two of 13 nortriptyline-treated patients and one of 11 placebo-treated patients had lost 10 lb or more (Fisher’s exact test, p=0.0004). These results indicate that during the initial 12 weeks of treatment, fluoxetine induced a significant weight loss in the majority of these elderly stroke patients. There was, however, no significant correlation between the amount of weight loss and change in Hamilton depression scale scores among the depressed or nondepressed patients.
Furthermore, we examined pulse and blood pressure before treatment and at the 12-week follow-up. The mean pretreatment vital signs for the depressed nortriptyline, fluoxetine, and placebo groups were pulse 78 bpm, blood pressure 126/74 mm Hg; 72 bpm, 110/80 mm Hg; and 79 bpm, 133/74 mm Hg, respectively. After 12 weeks of treatment the vital signs were 87 bpm, 134/80 mm Hg; 73 bpm, 132/78 mm Hg; and 74 bpm, 133/73 mm Hg, respectively. There was a significant change in pulse (F=4.94, df=2, 26, p=0.02), and the increase in the nortriptyline group was significantly greater than that in the placebo group (Duncan statistic, p<0.05) but not significantly different than the pulse change in the fluoxetine group. There were no significant intergroup differences in pre- to posttreatment blood pressure. Among the nondepressed patients, we found a similar mean increase of four beats in pulse in both the nortriptyline and fluoxetine groups and an increase of 11 beats in the placebo group (F=4.21, df=2, 27, p=0.01). There were no changes in blood pressure among the nondepressed patients.
We also reanalyzed our data for the first 6 weeks of treatment when all patients were receiving a maximum dose of 20 mg/day of fluoxetine. Repeated measures analyses covarying for baseline scores in the intention-to-treat analysis revealed a significant time-by-treatment interaction (F=3.47, df=2, 53, p=0.04); the nortriptyline group had a significantly greater decrease in mean Hamilton depression scale score than either the fluoxetine or placebo groups at 6 weeks (Duncan statistic, p<0.05). Thus, the 40-mg dose of fluoxetine was not responsible for the failure of the SSRI to produce the same response to treatment as nortriptyline.
We next examined results by controlling for the increased number of male patients in the fluoxetine group. All groups were balanced for comparable male-female ratios. Intention-to-treat and efficacy analyses demonstrated a time-by-treatment interaction (F=2.59, df=8, 160, p=0.01), with the nortriptyline-treated group having significantly lower Hamilton depression scale scores than the fluoxetine- and placebo-treated groups at 12 weeks of treatment (Duncan statistic, p<0.05).
DISCUSSION
This study compared the efficacy of fluoxetine and nortriptyline in both depressed and nondepressed poststroke patients for antidepressant effect as well as influence on recovery from physical, cognitive, and social impairment. Nortriptyline was significantly more effective than fluoxetine in the treatment of depression and anxiety symptoms and in improving recovery in activities of daily living as measured by the Functional Independence Measure. Neither medication had a significant effect on recovery in activities of daily living as measured by the Johns Hopkins Functioning Inventory, cognitive function as measured by the Mini-Mental State, or social functioning as measured by the Social Functioning Exam.
Before discussing these findings, the limitations imposed by the methods used in this study should be acknowledged. The patients who participated in the study reflect the population of Iowa, which has a high proportion of high school- or college-educated persons and mainly consists of Caucasians. We do not know if the 130 patients who refused participation may have had different treatment outcomes, but we have no reason to suspect that they did. Our previous treatment study was done with lower socioeconomic class, predominantly African American, inner-city patients (1). However, the results from this study may not be generalized to all stroke populations. Second, our inability to detect significant improvements in impairments after antidepressant treatment may have been limited by the instruments we used to determine change in severity of impairment. Thus, the Mini-Mental State may be an insensitive measure of subtle changes in cognitive function, and the scales used to measure activities of daily living scales (the Johns Hopkins Functioning Inventory and the Functional Independence Measure) may not be sufficiently sensitive to measure more subtle changes in these functions. A more detailed neuropsychological battery or a more fine-grained assessment of functional capacity might have demonstrated significant improvements in patients who were successfully treated for depression. Third, the severity of depression found among poststroke patients is not generally as severe as depression among patients with primary depression (i.e., those with no known neuropathology). The treatment groups’ initial scores on the 28-item Hamilton depression scale—with means ranging from 17.5 to 22.5—suggest relatively mild to moderate depression, and only half of the depressed subjects had major depressive disorder. Treatment response to fluoxetine may have been more marked in a more severely depressed patient group. Ackerman et al. (23), however, found that elderly depressed patients treated with fluoxetine were more likely to respond if they had a mild to moderate depression, and Roose et al. (8) found lower response rates for fluoxetine than for nortriptyline in severe melancholic depression. The fact that fluoxetine doses were increased by 10 mg/day every 3 weeks probably prevented a steady state from being achieved. Finally, there could have been problems with compliance in the fluoxetine-treated patients. Medication containers were checked, however, and patients assured the interviewers of their compliance.
Despite these limitations, the study findings have some important implications. This study and previous studies (1, 10), using very different populations of stroke patients, have confirmed the finding that patients with poststroke depression respond well to nortriptyline if they are able to take the medication. In addition, this study is the first we are aware of to have compared a tricyclic antidepressant with an SSRI antidepressant for poststroke depression, and it included the largest group of patients ever given antidepressants in a double-blind treatment study after stroke. Although there is a substantial literature demonstrating that primary depression (i.e., no known neuropathology) in elderly patients responds to fluoxetine treatment, the literature on the efficacy of fluoxetine in elderly patients with physical illness is less clear. Evans et al. (24) found that depressed elderly patients with physical illness responded better to fluoxetine than placebo, and Roose et al. (8) reported similar findings with paroxetine. Brymer and Winograd (7), however, as indicated in the introduction, found that fluoxetine may cause weight loss, nausea, and anorexia in depressed, medically ill geriatric patients, and Goldstein et al. (25) found that among 671 medically stable geriatric patients treated for major depression, weight loss of 5% or greater occurred more frequently in patients treated with fluoxetine (20 mg/day) than with placebo, particularly if their baseline body mass index was high. The fact that nine of 23 fluoxetine patients in the present study dropped out, whereas only three of 16 nortriptyline patients dropped out, suggests that some significant side effects, such as weight loss with nausea or diarrhea, perhaps at the 30 or 40 mg/day doses, may have been the reason for the high dropout rate and/or lack of improvement in mood scores. Schneider et al. (26) found that 286 depressed women over age 60 who did not receive estrogen replacement therapy had no better response to fluoxetine than placebo.
A surprising finding from this study was that neither depressed or nondepressed patients treated with nortriptyline or fluoxetine showed significantly greater improvement in their stroke-associated impairments than patients treated with placebo. This finding is in contrast to those of Reding et al. (2) and Gonzalez-Torrecillas et al. (27), who found significant improvements in activities of daily living or Mini-Mental State score with either trazodone, fluoxetine, or nortriptyline treatment. The Gonzalez-Torrecillas study was unblinded, and patients were treated an average of 4 weeks following the stroke. In the Reding study, significantly greater improvement in the Barthel activities of daily living score was found only among patients treated with trazodone who had a positive dexamethasone suppression test. The median time since stroke in the present study was about 6 weeks, suggesting that some delay in the onset of treatment may have adversely affected the likelihood of improvement in impairment as a result of antidepressants, and we did not perform a dexamethasone suppression test. It is also possible that 12 weeks was not enough time to demonstrate the effect of antidepressant treatment on recovery from impairment. Previous findings of improved recovery associated with antidepressant or amphetamine treatment (2, 28) suggest that some treatments or some subgroups of patients may benefit from pharmacologic treatment during their recovery from stroke. Future research is needed to pursue this important issue of augmenting recovery from stroke.
Received Jan. 19, 1999; revisions received May 17 and July 21, 1999; accepted Sept. 9, 1999From the Department of Psychiatry, University of Iowa; and the Ra�rrea Institute of Neurological Research, Buenos Aires, Argentina. Address reprint requests to Dr. Robinson, Department of Psychiatry, University of Iowa, 200 Hawkins Dr., 2887 John Pappajohn Pavilion, Iowa City, IA 52242; [email protected] (e-mail). Supported in part by NIMH grants MH-40355, MH-52879, and MH-53592; and grants from the Ra�rrea Institute of Neurological Research and Fundaciò¬è±¥z Companc. Eli Lilly and Company supplied the fluoxetine and its placebo. The University of Iowa Pharmacy supplied the nortriptyline and its placebo. The authors thank Charles F. Denhart, M.D., as well as the Younker Rehabilitation Center of the Iowa Methodist Medical Center in Des Moines, Iowa, and the Neurology Departments of the University of Iowa Hospitals and the VA Medical Center in Iowa City for allowing the authors to assess patients for inclusion in the study.
 |
 |
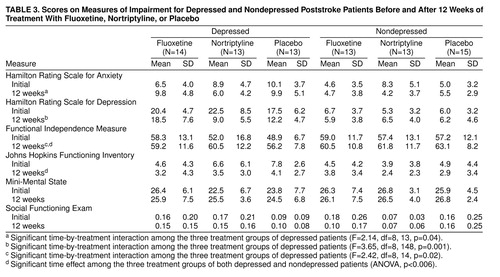 |
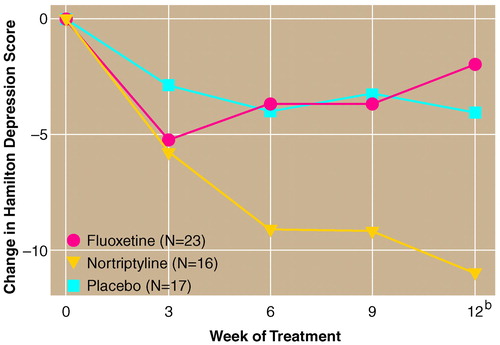
FIGURE 1. Change in Depression Scores for Depressed Poststroke Patients Entered in a Study Comparing Fluoxetine, Nortriptyline, and Placeboa
aSignificant time-by-treatment interaction (F=3.45, df=8, 212, p=0.004).
bSignificantly greater change in patients treated with nortriptyline than in those taking fluoxetine or placebo, (post-hoc tests with Duncan’s statistic, p<0.05).
1. Lipsey JR, Robinson RG, Pearlson GD, Rao K, Price TR: Nortriptyline treatment of post-stroke depression: a double-blind treatment trial. Lancet 1984; 1:297–300Crossref, Medline, Google Scholar
2. Reding JJ, Orto LA, Winter SW, Fortuna IM, DiPonte P, McDowell FH: Antidepressant therapy after stroke: a double-blind trial. Arch Neurol 1986; 43:763–765Crossref, Medline, Google Scholar
3. Andersen G, Vestergaard K, Lauritzen L: Effective treatment of poststroke depression with the selective serotonin reuptake inhibitor citalopram. Stroke 1994; 25:1099–1104Google Scholar
4. Lauritzen L, Bendsen BB, Vilmar T, Bendsen EB, Lunde M, Bech P: Post-stroke depression: combined treatment with imipramine or desipramine and mianserin: a controlled clinical study. Psychopharmacology (Berl) 1994; 114:119–122Crossref, Medline, Google Scholar
5. Tollefson GD, Bosomworth JC, Heiligenstein JH, Potvin JH, Holman S: A double-blind, placebo-controlled clinical trial of fluoxetine in geriatric patients with major depression. Int Psychogeriatr 1995; 7:89–104Crossref, Medline, Google Scholar
6. Roose SP, Laghrissi-Thode F, Kennedy JS, Nelson JC, Bigger JT Jr, Pollock BG, Gaffney A, Narayan M, Finkel MS, McCafferty J, Gergel I: Comparison of paroxetine and nortriptyline in depressed patients with ischemic heart disease. JAMA 1998; 279:287–291Crossref, Medline, Google Scholar
7. Brymer C, Winograd C: Fluoxetine in elderly patients: is there a cause for concern? J Am Geriatr 1992; 40:902–905Google Scholar
8. Roose SP, Glassman AH, Attia E, Woodring S: Comparative efficacy of selective serotonin reuptake inhibitors and tricyclics in the treatment of melancholia. Am J Psychiatry 1994; 151:1735–1739Google Scholar
9. DeRenzi E, Faglioni P: Development of a shortened version of the Token Test. Cortex 1978; 14:41–49Crossref, Medline, Google Scholar
10. Robinson RG, Parikh RM, Lipsey JR, Starkstein SE, Price TR: Pathological laughing and crying following stroke: validation of measurement scale and a double-blind treatment study. Am J Psychiatry 1993; 150:286–293Link, Google Scholar
11. Robinson RG, Starr LB, Kubos KL, Price TR: A two year longitudinal study of post-stroke mood disorders: findings during the initial evaluation. Stroke 1983; 14:736–744Crossref, Medline, Google Scholar
12. Robinson RG, Szetela B: Mood change following left hemispheric brain injury. Ann Neurol 1981; 9:447–453Crossref, Medline, Google Scholar
13. Starkstein SE, Fedoroff JP, Price TR, Leiguarda R, Robinson RG: Anosognosia in patients with cerebrovascular lesions: a study of causative factors. Stroke 1992; 23:1446–1453Google Scholar
14. Ottenbacher KJ, Mann WC, Granger CV, Tomita M, Hurren D, Charvat B: Inter-rater agreement and stability of functional assessment in the community-based elderly. Arch Phys Med Rehabil 1994; 75:1297–1301Google Scholar
15. Robinson RG, Kubos KL, Starr LB, Rao K, Price TR: Mood changes in stroke patients: relationship to lesion location. Compr Psychiatry 1983; 24:555–566Crossref, Medline, Google Scholar
16. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method of grading the cognitive state of patients for the clinician. J Psychiatry Res 1975; 12:189–198Crossref, Medline, Google Scholar
17. Robinson RG, Bolla-Wilson K, Kaplan E, Lipsey JR, Price TR: Depression influences intellectual impairment in stroke patients. Br J Psychiatry 1986; 148:541–547Crossref, Medline, Google Scholar
18. Robinson RG, Starr LB, Price TR: A two-year longitudinal study of post-stroke mood disorders: dynamic changes in associated variables over the first six months of follow-up. Stroke 1984; 15:510–517Crossref, Medline, Google Scholar
19. Starr LB, Robinson RG, Price TR: The Social Functioning Exam: an assessment of stroke patients. Soc Work Res Abstr 1983; 18:28–33Crossref, Google Scholar
20. Robinson RG, Bolduc PL, Kubos KL, Starr LB, Price TR: Social functioning assessment in stroke patients. Arch Phys Med Rehabil 1985; 66:496–500Medline, Google Scholar
21. Cliff N: Ordinal Methods for Behavioral Data Analysis. Mahway, NJ, Lawrence Erlbaum Associates, 1996Google Scholar
22. Conover WJ, Iman RL: Rank transformations as a bridge between parametric and nonparametric statistics. Am Statistician 1981; 35:124–129Google Scholar
23. Ackerman DL, Greenland S, Bystritsky A, Small GU: Characteristics of fluoxetine versus placebo responders in a randomized trial of geriatric depression. Psychopharmacol Bull 1997; 33:707–714Medline, Google Scholar
24. Evans M, Hammond M, Wilson K, Lye M, Copeland J: Placebo controlled treatment trial of depression in elderly physically ill patients. Int J Geriatr Psychiatry 1997; 12:817–824Crossref, Medline, Google Scholar
25. Goldstein DJ, Hamilton SH, Masica DN, Beasley CM Jr: Fluoxetine in medically stable, depressed geriatric patients: effects on weight. J Clin Psychopharmacol 1997; 17:365–369Crossref, Medline, Google Scholar
26. Schneider LS, Small GW, Hamilton SH, Bystritsky A, Nemeroff CB, Meyers BS: Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Am J Geriatr Psychiatry 1997; 5:97–106Crossref, Medline, Google Scholar
27. Gonzalez-Torrecillas JL, Mendlewicz J, Lobo A: Repercussion of early treatment of post-stroke depression on neuropsychological rehabilitation. Int Psychogeriatr 1995; 7:547–560Crossref, Medline, Google Scholar
28. Crisostomo EA, Duncan PW, Propst M, Dawson D, Davis JN: Evidence that amphetamine with physical therapy promotes recovery of motor function in stroke patients. Ann Neurol 1988; 23:94–97Crossref, Medline, Google Scholar



