Genetic Influence on Laterality in Schizophrenia? A Twin Study of Neurological Soft Signs
Abstract
OBJECTIVE: This study explored the genetic basis of neurological soft signs in schizophrenia and addressed disturbed hemispheric lateralization. METHOD: The authors investigated neurological soft signs in 30 monozygotic twin pairs, 13 pairs discordant for schizophrenia or schizoaffective disorder and 17 healthy comparison twin pairs. RESULTS: The twins with schizophrenia showed higher total scores for neurological soft signs than did the comparison subjects. The total scores for neurological soft signs of the nonaffected discordant twins were significantly higher than those of the comparison twins. There was a significant difference between the nonaffected and affected discordant twins in total scores for neurological soft signs. In contrast to the comparison subjects, the nonaffected and affected twins of the discordant pairs showed a trend toward higher scores for neurological soft signs on the left body half. CONCLUSIONS: These results suggest that the occurrence of neurological soft signs and, more specifically, their lateralization to the left body half are genetically transmitted.
Minor motor and sensor abnormalities, or neurological soft signs, are frequently found in schizophrenia (1). Increased scores for neurological soft signs can also be demonstrated in nonaffected twins of pairs discordant for schizophrenia (2, 3) and in otherwise healthy first-degree relatives of schizophrenic patients (4), which suggests that neurological soft signs are at least in part genetically determined. While the question of hemispheric imbalances has gained increasing importance in the pathophysiology of schizophrenia (5), potential lateralization effects were not addressed in the studies just cited. Crow (5) even postulated that abnormalities of the cerebral asymmetry constitute the genetic basis of schizophrenia.
The aim of our study was to investigate whether neurological soft signs and, more specifically, the laterality phenomenon of the neurological soft signs are under genetic control in schizophrenia. We sought to answer these questions by examining neurological soft signs in identical healthy comparison twins and twins discordant for schizophrenia.
METHOD
Thirty monozygotic twin pairs participated in the study. The study group consisted of 13 pairs discordant for schizophrenia (N=10) or schizoaffective psychosis (N=3), according to ICD-10—established by the Schedules for Clinical Assessment in Neuropsychiatry (6)—and 17 healthy comparison pairs. Ten of the co-twins in the pairs discordant for schizophrenia did not show any signs of a psychiatric disorder. Two co-twins fulfilled the criteria for remitted bipolar disorder (F31.7), and one reported a single depressive episode in the past (F32.1). Six of the patients with schizophrenia received neuroleptic treatment with a mean chlorpromazine-equivalent dose of 140 mg/day (SD=237).
The comparison subjects had no personal or family histories of mental illness on the basis of the Family History Research Diagnostic Criteria (7). None of the subjects had a history of neurological disorder or head injury. The zygosity of all twins was determined by microsatellite analysis (8).
The discordant twins did not differ from the comparison subjects in age (mean=31.5 years, SD=10.1; mean=31.1 years, SD=9, respectively), sex (five women and 13 men; seven women and 17 men), and education (discordant-affected twins: mean=10.5 years, SD=1.6; discordant-nonaffected twins: mean=10.9, SD=1.8; and comparison subjects: mean=11.2, SD=2). The neurological soft signs were examined according to the Heidelberg scale (1), which consists of 16 items. Eleven of these items were scored for each body half separately.
Since twins do not constitute independent observations, statistical analyses were performed not on single subjects but on twin pairs by using a repeated measures design. A within-pair member variable was created for affected and nonaffected discordant twins. Each twin of each comparison pair was randomly assigned to one of two groups (comparison group A or B). Three two-way analyses of variance (ANOVAs) with scores for total, motoric, and sensory neurological soft signs as dependent variables were calculated. To assess hemispheric differences, a three-way ANOVA was calculated with group as the independent variable and member and body side (left versus right) as between-pair factors. All post hoc analyses were performed with the Newman-Keuls test.
After a complete description of the study design was given to the subjects, written informed consent was obtained.
RESULTS
A two-way ANOVA for the total score for neurological soft signs yielded a significant group-versus-member interaction (F=6.0, df=1, 28, p=0.02). The affected twins and nonaffected twins of the discordant pairs showed significantly higher total scores for neurological soft signs than did the comparison subjects. Additionally, total scores for neurological soft signs were higher in the affected than in the nonaffected twins of the discordant pairs, whereas the comparison twins did not differ from each other (figure 1). Similar group differences appeared when only motor items were included (group-by-member interaction: F=6.4, df=1, 28, p=0.02). Post hoc analyses showed significantly higher scores for motor neurological soft signs in the affected and in the nonaffected discordant twins than in the comparison subjects. The affected twins of the discordant pairs scored significantly higher than their nonaffected co-twins (figure 1). With respect to sensory items, no significant effects were revealed.
A three-way ANOVA showed a nearly significant group-by-side interaction (F=3.1, df=1, 28, p=0.08) due to nearly significant (p=0.08) higher scores for neurological soft signs on the left body side in the discordant twins (figure 1). The group-by-side-by-member interaction was not significant (F=1.5, df=1, 28, p=0.2). Similar results were obtained when we excluded all pairs in which at least one twin was left-handed (one discordant-affected, two discordant-nonaffected, and three comparison twins).
DISCUSSION
Two major findings emerged in this study. First, higher scores for neurological soft signs were recorded in affected and nonaffected twins of monozygotic twin pairs discordant for schizophrenia than in healthy comparison twins. This confirms that neurological soft signs occur more frequently in patients with schizophrenia than in healthy comparison subjects. Our finding that nonaffected twins of discordant pairs differed from comparison subjects in their scores for neurological soft signs suggests a genetic contribution because the nonaffected twins had the same genotype as the affected twins but never showed the symptoms of schizophrenia. In this respect, our findings corroborate the National Institute of Mental Health twin studies (2, 3). If we take into account that the affected twins of the discordant pairs showed higher scores for neurological soft signs than their co-twins, our results suggest that genetics may determine a set point for neurological soft signs around which dynamic state fluctuations may occur (1).
Second, both the affected and nonaffected twins of the discordant pairs showed a trend for higher scores for neurological soft signs on the left body half. In contrast, Torrey (9) reported a lateralization of neurological soft signs to the right body half, which suggests a left hemispheric dysfunction. However, he had investigated only sensory functions (graphesthesia and the face-hand test), whereas our scale predominantly included motor phenomena. Indeed, a detailed analysis showed that our findings are due to motor dysfunctions lateralized to the left body half. That motor abnormalities primarily involve left-side movements is also supported by Walker et al. (10), who analyzed childhood videotapes of patients who later developed schizophrenia and demonstrated a left-side lateralization of motor abnormalities. In an oxygen-15 positron emission tomography study, Liddle et al. (11) found that the disorganized subsyndrome of schizophrenia—which is associated with particularly increased scores for neurological soft signs (12)—is characterized by a right-sided hypoperfusion in the ventral prefrontal cortex and the right cingulate gyrus. However, motor neurological soft signs do not strictly correspond to disturbances of the contralateral hemisphere but also involve a disturbed coactivation of the ipsilateral hemisphere, as indicated by a functional magnetic resonance tomography study (13).
Thus, our findings are in line with an increasing number of reports suggesting that the notion of an isolated left hemispheric dysfunction in schizophrenia might be a simplification. In contrast, in more recent hypotheses, schizophrenia is conceptualized as a disturbance of distributed brain circuits (14). Our findings indicate that the disturbance of these fundamental integrative functions is genetically transmitted.
Received June 3, 1998; revisions received March 30 and June 7, 1999; accepted June 8, 1999. From the Department of Psychiatry, University of Heidelberg, Heidelberg, Germany; and the Department of Psychiatry, University of Jena, Jena, Germany. Address reprint requests to Dr. Niethammer, Psychiatrische Universitätsklinik, Voss-Str. 4, 69115 Heidelberg, Germany; [email protected] (e-mail). Supported by German Research Community (Deutsche Forschungsgemeinschaft) grant WE-1996/1-3 to Dr. Weisbrod. The authors thank Prof. Propping for determining zygosity and the following institutions for help in recruitment of twins: Pfalzklinik Landeck Klingenmünster (Prof. Steinberg); Psychiatrisches Krankenhaus Christophsbad Göppingen (Prof. Hermle); Psychiatrisches Krankenhaus Karlsruhe (Dr. Rave-Schwank); Rehabilitationskrankenhaus Langensteinbach (Dr. Claus); Universität Bonn (Prof. Maier); Zentralinstitut für Seelische Gesundheit Mannheim (Dr. Häfner-Ranabauer); Zentrum für Psychiatrie Wiesloch (Dr. Middelhoff and Prof. Ulmar).
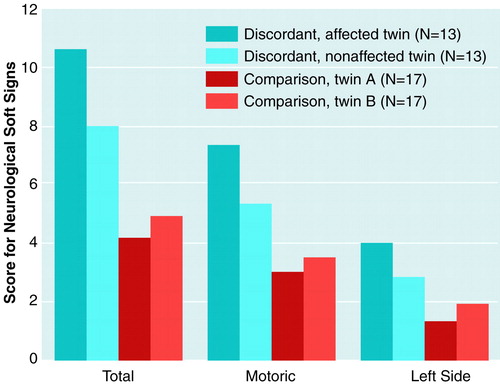
FIGURE 1. Scores for Neurological Soft Signs in Affected and Nonaffected Monozygotic Twins Discordant for Schizophrenia and in Comparison Twins
1. Schröder J, Niethammer R, Geider FJ, Reitz C, Binkert M, Jauss M, Sauer H: Neurological soft signs in schizophrenia. Schizophr Res 1992; 6:25–30Crossref, Google Scholar
2. Mosher LR, Pollin W, Stabenau JR: Identical twins discordant for schizophrenia. Arch Gen Psychiatry 1971; 24:422–430Crossref, Medline, Google Scholar
3. Torrey EF: Schizophrenia and Manic-Depressive Disorder. New York, Basic Books, 1994Google Scholar
4. Rossi A, de Cataldo S, di Michele V, Manna V, Ceccoli S, Stratta P, Cassachia M: Neurological soft signs in schizophrenia. Br J Psychiatry 1990; 157:735–739Crossref, Medline, Google Scholar
5. Crow TJ: Ontogeny and phylogeny of cerebral asymmetry—schizophrenia as a component of the variation generated in the evolution of language, in The Neurodevelopmental Basis of Schizophrenia. Edited by Waddington JL, Buckley PF. Austin, Tex, RG Landes, 1996, pp 153–165Google Scholar
6. Schedules for Clinical Assessment in Neuropsychiatry (SCAN). Geneva, World Health Organization, Division of Mental Health, 1992Google Scholar
7. Andreasen NC, Endicott J, Spitzer RL, Winokur G: The family history method using diagnostic criteria: reliability and validity. Arch Gen Psychiatry 1977; 34:1229–1235Google Scholar
8. Erdmann J, Nöthen MM, Stratmann M, Fimmers R, Franzek E, Propping P: The use of microsatellites in zygosity diagnosis of twins. Acta Genet Med Gemellol (Roma) 1993; 42:45–51Crossref, Medline, Google Scholar
9. Torrey EF: Neurological abnormalities in schizophrenic patients. Biol Psychiatry 1980; 15:381–388Medline, Google Scholar
10. Walker EF, Savole T, Davis D: Neuromotor precursors of schizophrenia. Schizophr Bull 1994; 20:441–451Crossref, Medline, Google Scholar
11. Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RSJ: Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160:179–186Crossref, Medline, Google Scholar
12. Schröder J, Tittel A, Stockert A, Karr M: Memory deficits in subsyndromes of chronic schizophrenia. Schizophr Res 1996; 21:19–26Crossref, Medline, Google Scholar
13. Schröder J, Wenz F, Baudendistel K, Schad LR, Knopp MV: Sensorimotor cortex and supplementary motor area changes in schizophrenia. Br J Psychiatry 1995; 167:197–201Crossref, Medline, Google Scholar
14. Andreasen NC: Understanding schizophrenia: a silent spring? (editorial). Am J Psychiatry 1998; 155:1657–1659Google Scholar



