Exploring the Social Brain in Schizophrenia: Left Prefrontal Underactivation During Mental State Attribution
Abstract
OBJECTIVE: Evidence suggests that patients with schizophrenia have a deficit in “theory of mind,” i.e., interpretation of the mental state of others. The authors used functional magnetic resonance imaging (MRI) to investigate the hypothesis that patients with schizophrenia have a dysfunction in brain regions responsible for mental state attribution. METHOD: Mean brain activation in five male patients with schizophrenia was compared to that in seven comparison subjects during performance of a task involving attribution of mental state. RESULTS: During performance of the mental state attribution task, the patients made more errors and showed less blood-oxygen-level-dependent signal in the left inferior frontal gyrus. CONCLUSIONS: To the authors’ knowledge, this is the first functional MRI study to show a deficit in the left prefrontal cortex in schizophrenia during a socioemotional task.
Deficits in social functioning are a hallmark of schizophrenia (DSM-IV). Studies (1, 2) have shown that patients with schizophrenia have deficits in performance on “theory of mind” tasks, i.e., requiring the interpretation of the mental states of others.
In the study reported here, a small group of patients with schizophrenia performed a task involving attribution of mental state. We believe that this is the first study to investigate neurophysiological correlates of theory of mind in patients diagnosed with schizophrenia. Using functional magnetic resonance imaging (MRI), we compared the brain activation of schizophrenic patients with that of healthy comparison subjects while they labeled the social/emotional expression of photographed eyes (3). We hypothesized a neurocognitive deficit in the patients.
Method
Five male, right-handed patients with schizophrenia (DSM-IV), aged 26 to 57 years (mean=36, SD=9), participated in the study. They had a mean education level of 11 years (SD=11), and their mean IQ according to the Quick Test (4) was 100 (SD=4). They had a mean duration of illness of 13 years (SD=7), and the mean age at onset of psychotic symptoms was 23 years (SD=3). The patients were receiving antipsychotic medication. Seven healthy comparison subjects with comparable ages, handedness, and education level also participated. Their mean age was 40 years (SD=11, range=26–58), they had a mean education level of 16 years (SD=3), and their mean IQ was 116 (SD=15). There was a significant group difference in IQ (U=5.5, p=0.04, Mann Whitney U test). Subjects with a history of substance abuse or neurological disorders were excluded. The study was approved by the local ethics committee, and the subjects gave written informed consent.
The subjects were asked to perform the “eyes” task of Baron-Cohen et al. (3). Subjects are required to choose one of two words (e.g., “concerned” or “angry”) that best describes the mental state reflected in photographed eyes. The control condition requires a decision regarding the gender of the same eyes. Six pairs of eyes (interstimulus interval, 5 seconds) were alternated five times in both conditions; performance was monitored by button presses with the right hand.
During this task, gradient-echo echoplanar MR images were acquired on a 1.5-T GE Signa system. In each of 14 noncontiguous planes parallel to the anterior-posterior commissure, 100 T2*-weighted MR images depicting blood-oxygen-level-dependent (BOLD) contrast were acquired with TE=40 msec, TR=3 sec, flip angle=90°, in-plane resolution=3.1 mm, slice thickness=5 mm, slice skip=0.5 mm. These images were coregistered on a corresponding 43-slice, high-resolution structural echoplanar image.
The methods used for functional MRI time series analysis have been described elsewhere in detail (5, 6). The standardized power of periodic signal change at the frequency of alternation between the control and activation conditions was estimated by fitting a sinusoidal regression model, by means of an iterated least squares procedure, to the movement-corrected time functional MRI series at each voxel. Power maps were registered in standard space and smoothed by a two-dimensional Gaussian filter with a standard deviation of 3 mm or 1 voxel. Generic brain activation maps of voxels demonstrating significant (p<0.0004) median power of response over all subjects in each group were generated in standard space by a permutation test procedure (5, 7). Between-group differences in activation were estimated by fitting a one-way analysis of covariance (ANCOVA) model at each voxel, with IQ as the covariate, to generate a map of the main effect of group at each voxel. This map was thresholded to generate a set of spatially contiguous three-dimensional clusters of suprathreshold voxel statistics, and the sum of suprathreshold voxel statistics in each cluster was tested against its sampled permutation distribution under the null hypothesis of zero group effect with a two-tailed p value of <0.01 (see reference 8 for details).
Results
The patients with schizophrenia made more errors in mental state attribution (mean=12.60, SD=5.03; N=5) than did the comparison subjects (mean=6.14, SD=3.84; N=7) (U=4.5, p=0.03, Mann-Whitney U test).
During the mental state attribution condition, the comparison group showed an increase in signal response, relative to the control condition, in the portions of the left inferior frontal gyrus reaching into the insula (Brodmann’s area 44/45/47; Talairach coordinates –52, 17, 4; 97 voxels) and into the medial frontal lobe (Brodmann’s area 45/9; –43, 7, 26; 33 voxels) and in the left middle (Brodmann’s area 21; –55, –47, 4; 35 voxels) and left superior (Brodmann’s area 22; –55, –31, 9; 15 voxels) temporal gyrus. Cluster-based ANCOVA (p<0.01; estimated number of false positives=9, observed number of suprathreshold clusters=20) (8) showed significantly less BOLD signal in the schizophrenic subjects than in the comparison group in the left middle/inferior frontal cortex bordering the insula (Brodmann’s area 9/44/45; –45, 14, 8; 173 voxels) (Figure 1).
Discussion
The neurocognitive network for normal attribution of mental state comprised the middle, inferior frontal, and middle temporal regions. The patients with schizophrenia showed less activation in the left middle/inferior frontal gyrus and insula than the comparison subjects and demonstrated impaired performance on the task.
The normative network found for mental state attribution is in line with previous findings (3). The frontal underactivation in schizophrenia is in line with functional and structural findings indicating a frontal dysfunction in this patient population (9, 10). To our knowledge, however, this is the first demonstration of hypofrontality in schizophrenia during social processing. A deficit in a strikingly similar left inferior frontal focus has also been shown in patients with autism, known to show theory of mind deficits (3). The integrity of the left prefrontal cortex seems thus to be crucial for intact theory of mind processing, as previously suggested (11, 12). A left frontal underactivation in schizophrenia during mental state attribution thus confirms our hypothesis of a socioemotional neurocognitive deficit.
Received Oct. 18, 1999; revisions received May 2 and June 7, 2000; accepted June 16, 2000. From the Institute of Psychiatry, King’s College. Address reprint requests to Dr. Sharma, Section of Cognitive Psychopharmacology, Institute of Psychiatry, De Crespigny Park, London SE5 8AF, U.K.; [email protected] (e-mail). Supported by a donation from Grosvenor Group Estates and Psychmed, Ltd., to Dr. Sharma and by a grant from the Wellcome Trust to Dr. Bullmore. The authors thank Drs. S. Baron-Cohen and Sally Wheelwright for details of the eyes task.
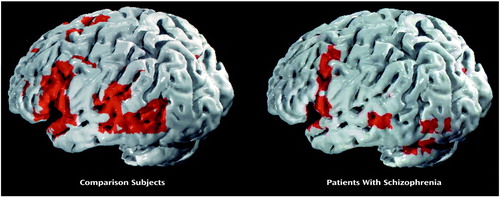
Figure 1. Generic Brain Maps of Activation in Comparison Subjects and Patients With Schizophrenia During Attribution of Mental State to Photographs of Eyesa
aView of the left hemisphere. Voxel-wise probability of type I error=0.0004.
1. Corcoran R, Mercer G, Frith CD: Schizophrenia, symptomatology and social inference: investigating “theory of mind” in people with schizophrenia. Schizophr Res 1995; 17:5–13Crossref, Medline, Google Scholar
2. Corcoran R, Cahill C, Frith CD: The appreciation of visual jokes in people with schizophrenia: a study of “mentalizing” ability. Schizophr Res 1997; 24:319–327Crossref, Medline, Google Scholar
3. Baron-Cohen S, Ring H, Williams S, Wheelwright S, Bullmore E, Brammer M, Andrew C: Social intelligence: the role of the amygdala. Eur J Neurosci 1999; 11:1891–1898Google Scholar
4. Ammons R, Ammons C: Quick Test. Missoula, Mont, Psychological Test Specialists, 1962Google Scholar
5. Brammer MJ, Bullmore ET, Simmons A, Williams SCR, Grasby PM, Howard RJ, Woodruff PW, Rabe-Hesketh S: Generic brain activation mapping in fMRI: a nonparametric approach. Magn Res Imaging 1997; 15:763–770Crossref, Medline, Google Scholar
6. Bullmore E, Brammer MJ, Rabe-Hesketh S, Curtis VA, Morris RG, Williams SC, Sharma T, McGuire PK: Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 1999; 7:38–48Crossref, Medline, Google Scholar
7. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
8. Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ: Global, voxel, and cluster tests, by theory and permutation for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 1999; 18:32–42Crossref, Medline, Google Scholar
9. Harrison P: The neuropathology of schizophrenia: a critical review of the data and their interpretation. Brain 1999; 122:593–624Crossref, Medline, Google Scholar
10. Weinberger D, Aloia M, Goldberg T, Berman K: The frontal lobes and schizophrenia. J Neuropsychiatry 1994; 6:419–427Crossref, Medline, Google Scholar
11. Frith CD: Brain mechanisms for having a “theory of mind.” J Psychopharmacol 1996; 19:9–15Crossref, Google Scholar
12. Goel V, Grafman J, Sadato N, Hallett M: Modeling other minds. Neuroreport 1995; 6:1741–1746Google Scholar



