Effects of Methylphenidate on Functional MRI Blood-Oxygen-Level-Dependent Contrast
Abstract
OBJECTIVE: The authors’ goal was to determine potential hemodynamic consequences of methylphenidate on functional magnetic resonance imaging (MRI) blood-oxygen-level-dependent (BOLD) contrast. METHOD: BOLD and perfusion changes were recorded from the motor cortex of six healthy subjects while they performed flexion-extension movements of the right index finger (finger tapping) at varying rates before and after oral methylphenidate administration. RESULTS: Functional MRI signals increased monotonically with faster movement rates. Subjects’ heart rates increased modestly after methylphenidate administration, but no changes in finger tapping performance or functional MRI signals were observed. CONCLUSIONS: Methylphenidate does not alter BOLD neural-hemodynamic coupling. Consequently, functional MRI can be used to map neural systems that subserve cognitive operations (e.g., attention and executive processes) in subjects taking methylphenidate.
Methylphenidate, a psychostimulant that acts primarily by releasing and inhibiting the reuptake of dopamine (1), has been shown to enhance cognitive performance in healthy subjects (2) and children with attention deficit hyperactivity disorder (ADHD) (3). The neuroanatomical sites and mechanisms by which methylphenidate alters neural circuitry during performance of cognitive tasks are not well understood. A functional magnetic resonance imaging (MRI) study by Vaidya et al. (4) demonstrated that methylphenidate affects frontostriatal activation differently in children with ADHD and healthy children during performance of response inhibition tasks.
Functional MRI does not measure neural activity directly but relies on the hemodynamic coupling between neural activity and regional changes in blood flow and oxygenation. Most functional MRI studies are based on endogenous blood-oxygen-level-dependent (BOLD) contrast. A number of drugs and other manipulations can potentially disrupt this hemodynamic coupling. For example, hypercapnia-related increases in resting blood volume reduce the activation-induced BOLD response in the primary motor cortex, although motor performance and, presumably, underlying neural activity remain unchanged (5). If the BOLD response is to be used to measure drug-induced changes in neural circuitry, it is imperative to demonstrate that the drug does not alter hemodynamic coupling as well or have direct vasoactive properties, either of which could lead to data misinterpretation.
To address this issue, we obtained brain images of healthy subjects before and after oral administration of methylphenidate. During each imaging series, the subject performed a repetitive finger tapping task at different rates in response to a metronome. We assume that contralateral primary motor cortex neural activity is constant with and without methylphenidate, leaving a relatively pure examination of hemodynamic coupling in the BOLD response. To better understand changes in blood perfusion, a pulsed arterial spin labeling technique was also acquired, interleaved with the BOLD technique.
Method
The study was approved by the institutional review boards of the Medical College of Wisconsin. Written informed consent was obtained from six healthy, right-handed volunteers (two men and four women; age range=20–45 years). Subjects performed flexion-extension movements of the right index finger (finger tapping) at rates of 1, 2, 3, 4, and 5 Hz in response to an auditory metronome. Each imaging series contained 10 alternating activation and rest epochs (24 seconds each). Tapping rate was constant within an activation epoch; each rate occurred twice in pseudo-random order within a series. Two series were obtained 10 and 0 minutes before oral administration of 20 mg of methylphenidate (0.2–0.4 mg/kg); six imaging series were collected every 10 minutes starting 40 minutes after methylphenidate administration.
Functional MRI was conducted on a 3-tesla Bruker Biospec scanner (Karlsruhe, Germany). A QUIPPS II pulsed arterial spin labeling technique (6) provided a measure of perfusion. Arterial spin labeling and BOLD time series were extracted by subtracting (control minus tag) or averaging (control plus tag divided by 2) consecutive images, respectively. These derived time series each contained 120 images (TR=4 seconds). An 8-mm-thick axial slice (3.75-mm2 resolution) centered 22 mm from the vertex of the brain was selected as an optimal site for detecting functional activity from the left primary motor cortex hand region. A T1-weighted image (0.9-mm2 resolution) was acquired to derive anatomical landmarks. Head motion was minimized during scanning with foam padding and during image registration after processing (7).
Cross-correlation (8) was used to identify three primary motor cortex voxels having the highest correlation with the activation/rest cycles. For these voxels, mean percent signal change was calculated as a function of tapping rate for each of the eight BOLD and arterial spin labeling imaging series. The total number of primary motor cortex activated voxels (maximum=16) was defined in a multiple regression model, where the addition of the regressor for each movement rate contributed significantly to the fit compared with a reduced model (t>3.02, df=118, p<0.003; Bonferroni-corrected p=0.05).
To assess possible region-specific effects of methylphenidate on perfusion, flow values were averaged over the entire time series for four regions (prefrontal, left and right sensorimotor, and parietal) with the pre- and postcentral sulci as landmarks. Interresponse intervals were recorded to verify subject performance. Mean heart rate was recorded during each imaging series.
Results
As expected (8), tapping rate resulted in monotonic increases in percent signal change within the three contralateral primary motor cortex voxels for BOLD response (A in Figure 1) (F=34.0, df=4, 20, p<0.0001) and arterial spin labeling (B in Figure 1) (F=14.5, df=4, 20, p<0.0001). No significant changes in BOLD response or arterial spin labeling were observed across imaging series; rate-by-series interaction effects were nonsignificant. The number of activated voxels increased significantly with tapping rate (C in Figure 1) (F=24.0, df=4, 20, p<0.0001). A significant change was observed across series (F=2.5, df=7, 35, p=0.04); post hoc analyses indicated no significant differences between the second baseline series (0 minutes) and any of the postmethylphenidate series. Regional perfusion indexes (D in Figure 1) did not change across series. Interresponse intervals (E in Figure 1) were unchanged, indicating that subjects performed the task with equivalent accuracy before and after methylphenidate. Heart rate (F in Figure 1) declined from the first to second baseline series and gradually increased during the postmethylphenidate series (F=2.2, df=7, 35, p<0.06).
Discussion
These data suggest that, when task performance is equated, a 20-mg oral dose of methylphenidate does not alter activation-induced BOLD or relative blood flow response changes. The superior contrast-to-noise ratio of BOLD response relative to arterial spin labeling is evident in the rate-response function of each measure (A versus B in Figure 1). Dopaminergic terminals are found in many cortical regions, including the prefrontal and motor regions (9), and have been shown to directly modulate local cerebral blood flow (10). Since methylphenidate raises local dopamine concentration (1), a global or local effect on cerebral perfusion or activation-flow coupling was possible. That no changes in either flow or BOLD signal were seen suggests that enhanced extracellular dopamine levels did not alter local neural-hemodynamic coupling. Our negative result could not be attributed to the small number of subjects because power exceeded 80% to detect a 0.2% fluctuation in the BOLD signal across imaging series.
Taken together, these results support the use of functional MRI to study the neural mechanisms and sites involved in methylphenidate-induced cognitive changes. The methods described in this paper may also serve as a model for demonstrating the validity of functional MRI in other pharmacological imaging investigations.
Received Dec. 13, 1999; revision received March 22, 2000; accepted May 3, 2000. From the Departments of Neurology; Psychiatry; Cell Biology, Neurobiology, and Anatomy; and the Biophysics Research Institute, Medical College of Wisconsin. Address reprint requests to Dr. Rao, Section of Neuropsychology, Medical College of Wisconsin, 9200 W. Wisconsin Ave., Milwaukee, WI 53226; [email protected] (e-mail). Supported by NIMH grants MH-57836 and MH-51358 (Dr. Rao); National Institute on Drug Abuse grant DA-09465 (Dr. Stein); and National Center for Research Resources grant RR-00058 (General Clinical Research Center, Medical College of Wisconsin).
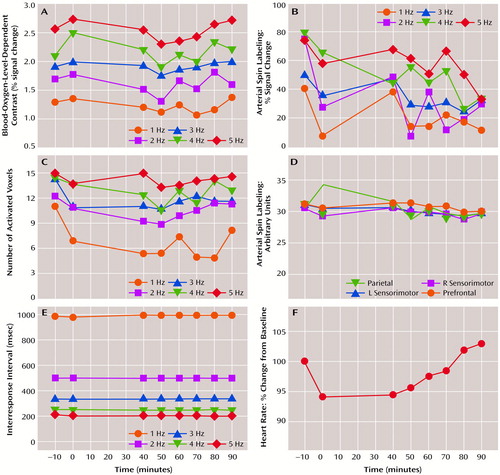
Figure 1. MRI, Performance, and Physiological Measures During Finger Tapping at Varying Rates in Six Healthy Subjects Before and After Methylphenidate Administration
1. Ross SB: The central stimulatory action of inhibitors of dopamine uptake. Life Sci 1979; 24:159–168Crossref, Medline, Google Scholar
2. Koelega HS: Stimulant drugs and vigilance performance: a review. Psychopharmacology (Berl) 1993; 111:1–16Crossref, Medline, Google Scholar
3. Barkley RA: A review of stimulant drug research with hyperactive children. J Child Psychol Psychiatry 1977; 18:137–165Crossref, Medline, Google Scholar
4. Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD: Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 1998; 95:14494–14499Google Scholar
5. Bandettini PA, Wong EC: A hypercapnia-based normalization method for improved spatial localization of human brain activation with fMRI. NMR Biomed 1997; 10:197–203Crossref, Medline, Google Scholar
6. Luh WM, Wong EC, Bandettini PA, Hyde JS: QUIPSS II with thin-slice TI1 periodic saturation: a method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn Reson Med 1999; 41:1246–1254Google Scholar
7. Cox RW: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
8. Rao SM, Bandettini PA, Binder JR, Bobholz J, Hammeke TA, Stein EA, Hyde JS: Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J Cereb Blood Flow Metab 1996; 16:1250–1254Google Scholar
9. Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH: The distribution of tyrosine hydroxylase-immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci 1987; 7:279–290Crossref, Medline, Google Scholar
10. Krimer LS, Muly EC III, Williams GV, Goldman-Rakic PS: Dopaminergic regulation of cerebral cortical microcirculation. Nat Neurosci 1998; 1:286–289Crossref, Medline, Google Scholar



