Reduction of Trace but Not Delay Eyeblink Conditioning in Panic Disorder
Abstract
Objective: Individuals with panic disorder perceive panic attacks as unpredictable. Because predictability is fundamental to Pavlovian conditioning, failure to predict panic attacks could be due to a basic deficit in conditioning. The present study examined trace eyeblink conditioning in order to test the hypothesis that individuals with panic disorder are impaired in associative learning tasks that depend on declarative memory. Method: Delay and trace eyeblink conditioning were tested in separate experimental sessions in 19 individuals meeting DSM-IV criteria for panic disorder and 19 sex- and age-matched healthy comparison subjects. In the delay paradigm, a mild puff was delivered to the eye at the end of a 500-msec tone; in the trace paradigm, the puff was delivered after a 700-msec empty “trace” interval that followed the end of the tone. Results: Patients and comparison subjects showed similar rates of conditioned responses in the delay paradigm, but patients showed reduced rates of conditioned responses in the trace paradigm. Conclusions: These results suggest that individuals with panic disorder suffer from a deficit in declarative associative learning. Such a deficit points to impaired hippocampal function that may disrupt cognitive processing of internal and external cues predictive of a panic attack.
To be diagnosed with panic disorder, a person must experience recurrent unexpected panic attacks, that is, panic attacks not triggered by a cue. Panic disorder is also characterized in DSM-IV by intense persistent concerns or anticipatory anxiety about future panic attacks. This propensity to develop anxiety focused on the next potential panic attack is a distinguishing feature of panic disorder (1) . Underlying the symptom of anticipatory anxiety is a perceived sense that panic attacks are uncontrollable and unpredictable (2) .
Predictability is fundamental to Pavlovian conditioning, a process of contingency or associative learning (3) . Pavlovian conditioning is an adaptive associative process that enables organisms to learn to anticipate events, aversive or otherwise. It is conceivable that a basic deficit in forming appropriate associations prevents individuals with panic disorder from learning to associate predictive cues with panic attacks. Failure to predict dangers, such as panic attacks, could lead to a chronic state of anticipatory anxiety (4 , 5) . Preliminary evidence for this hypothesis comes from studies showing that, in humans, failure to condition properly and to learn to predict aversive stimuli results in enhanced anticipatory anxiety (5 , 6) and in heightened subjective expectancy of danger (7) .
The mechanism underlying associative learning can be tested using Pavlovian aversive conditioning experiments such as eyeblink conditioning, a well-defined associative learning paradigm (8 , 9) . During eyeblink conditioning, a puff of air to the eye (unconditioned stimulus) is paired repeatedly with a short-duration tone (conditioned stimulus), causing subjects to blink (unconditioned response). Following several tone puff pairings, an eyeblink response, constituting a conditioned response, develops subsequent to the tone presentation and prior to the delivery of the puff. The present study investigated associative learning deficits as a potential pathogenic mechanism for panic disorder using eyeblink conditioning.
In general, eyeblink conditioning studies have not supported the hypothesis of impaired Pavlovian conditioning in anxious subjects. If anything, anxiety has been associated traditionally with enhanced, not reduced, eyeblink conditioning (10) . However, enhanced eyeblink conditioning has been reported in individuals with high trait anxiety rather than patients with panic disorder, for whom there are no published data yet. Conditioning in pathological anxiety states may differ from conditioning in high trait anxiety. In addition, these studies have used delay eyeblink conditioning, a test of nondeclarative associative learning mediated by the cerebellum (8) . In delay eyeblink conditioning, the unconditioned stimulus (puff) is administered at the end of the conditioned stimulus (tone). Because delay eyeblink conditioning can occur in the absence of awareness, it may be an unsuitable model for associative learning processes related to panic attacks. While cues for panic attacks may exist in the absence of awareness (11) , unperceived cues may take the form of internal sensations, cognitions, or external triggers (1) . Trace eyeblink conditioning provides an excellent model for this type of declarative associative learning (9) .
Trace eyeblink conditioning differs from delay eyeblink conditioning in that the unconditioned stimulus (puff) follows an empty (trace) interval (e.g., 1000 msec) that separates the end of the conditioned stimulus (tone) from the onset of the unconditioned stimulus. Trace eyeblink conditioning is more complex than delay eyeblink conditioning because of the temporal gap between the conditioned stimulus and the unconditioned stimulus, which requires the formation of an abstract link between the two stimuli before learning can occur. Substantial evidence has implicated the hippocampus in encoding of the temporal “trace” association between the conditioned stimulus and the unconditioned stimulus for long trace intervals (8 , 12) . Indeed, clinical studies indicate reduced trace eyeblink conditioning in patients with temporal lesions with trace intervals that exceed 1000 msec (9 , 13 , 14) .
Trace eyeblink conditioning could provide valuable information regarding associative learning in panic disorder. Unlike the delay paradigm, the occurrence of conditioned eyeblink responses during trace eyeblink conditioning is critically dependent on subjects’ awareness of the tone puff contingency (as reported by Spence and Beecroft [10] but see also the study of LaBar and Disterhoft [15] ). Because it is a test of declarative learning, trace eyeblink conditioning may be a better model for expectancy-based associative learning required to experience a sense of predictability over one’s environment. We therefore hypothesized that individuals with panic disorder would exhibit reduced conditioned responses during trace eyeblink conditioning but not during delay eyeblink conditioning.
Method
Participants
Nineteen patients with panic disorder (13 women and six men; mean age=35.0 years [SD=9.1]) and 19 age- and sex-matched healthy comparison subjects (mean age=31.6 years [SD=9.0]) participated in the study. All patients had been free of medication for at least 4 weeks preceding the testing. Participants in the patient group met DSM-IV criteria for panic disorder according to the Structural Clinical Interview for DSM-IV Axis I Disorders (SCID). Comorbid diagnoses among the panic patients included current major depressive disorder (N=5), past major depressive disorder (N=4), and social anxiety disorder (N=5). Healthy comparison subjects had no current or past psychiatric diagnoses according to the SCID. Patients had significantly higher scores than did the healthy comparison subjects on the following measures: the state portion of the Spielberger State-Trait Anxiety Inventory (46.6 [SD=13.9] versus 26.6 [SD=4.8], respectively; t=6.0, df=36, p<0.0009), Beck Depression Inventory (11.3 [SD=2.1] versus 1.5 [SD=2.1]; t=4.2, df=36, p<0.0009), Beck Anxiety Inventory (15.6 [SD=11.2] versus 2.1 [SD=4.6]; t=0.51, df=36, p<0.0009), and Anxiety Sensitivity Index (16) (36.6 [SD=14.1] versus 13.2 [SD=7.3]; t=6.2, df=34, p<0.0009). After complete description of the study to the subjects, written informed consent was obtained.
Stimulus and Recording Apparatus
The conditioned stimulus was a 500-msec pure 1-kHz tone at 75 dB(A) delivered binaurally through headphones. The unconditioned stimulus was a 10-psi air puff (measured at the level of the air tank as opposed to the eye) with duration of 50 msec delivered to the right eye. The eyeblinks were assessed by recording right eye EMG activity with two miniature electrodes (impedance below 5 kW). The EMG was filtered (30–500Hz) and integrated with a 10-msec time constant.
Design
The eyeblink conditioning procedure was a part of a larger study conducted over two testing sessions separated by 1 week. In each of the two sessions, the eyeblink conditioning experiment was preceded by a mildly aversive procedure, either a threat experiment in which unpleasant sounds were anticipated (first session) or an aversive conditioning test in which mildly unpleasant shocks were administered (second session). During these tests, psychophysiological measures were recorded including the skin conductance response, the ECG, and the eyeblink reflex to startling stimuli. The eyeblink conditioning tests were given 40 minutes after these tests in a counterbalanced manner over the two sessions. Prior to the eyeblink conditioning tests, subjects were told that they would hear tones and receive puffs of air to one eye. They were asked to blink naturally. Awareness of the conditioned stimulus/unconditioned stimulus contingency was not assessed because assessing awareness after one task (e.g., delay paradigm) could have influenced the way subjects approached the second task (e.g., trace eyeblink). In addition, several reviews have emphasized the difficulties in assessing subjective awareness (17 , 18) .
Eyeblink Conditioning
For both types of conditioning, the experiment consisted of the delivery of four puff-alone (without tone) trials followed by five tone-alone (without puff) trials, followed by six acquisition blocks and two extinction blocks. Each acquisition block started with one tone-alone trial followed by nine tone/puff trials. Each extinction block consisted of 10 tone-alone trials. The interval between trials varied between 10 and 14 seconds.
Delay and trace eyeblink conditioning differed only with respect to the time of puff delivery following the tone in tone/puff trials during acquisition ( Figure 1 ). In the delay paradigm, the puff was delivered at the end of the tone (500 msec after tone termination). In the trace paradigm, a long trace interval was selected to ensure involvement of the hippocampus (13) . The puff was delivered 700 msec after tone termination resulting in a 1200-msec trace interval between the conditioned stimulus onset and unconditioned stimulus onset.
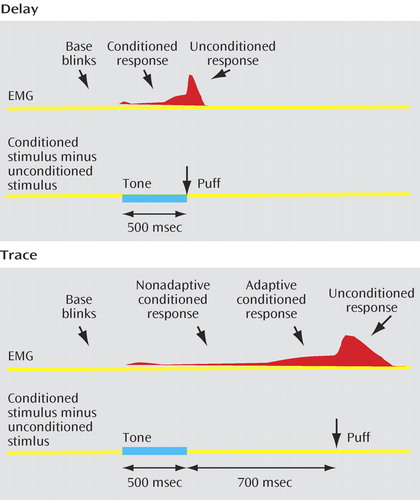
a In the delay paradigm, the puff (unconditioned stimulus) was delivered at the end of the 500-msec duration tone (conditioned stimulus). In the trace paradigm, the puff was delivered after a 700-msec empty “trace” interval following the tone. In both paradigms, the base rate of spontaneous blinks was calculated in the 400 msec that preceded tone onset. In the delay paradigm, blinks occurring in the 100–500 msec post tone onset were considered conditioned responses. In the trace procedures, blinks occurring in the 100–800 msec and 800–1200 msec post tone onset were considered nonadaptive and adaptive conditioned responses, respectively. The rate of alpha responses was calculated in the first 100 msec that followed the onset of the tone (not shown).
Data Analysis
One patient had only a partial recording of the extinction phase of the delay eyeblink conditioning due to technical problems. This subject’s data for this phase of the study were excluded from the analysis. Unconditioned blink response magnitudes to the puff were scored using the largest EMG peak that occurred 20–200 msec post puff onset during the initial puff-alone presentation.
For the analysis of delay eyeblink conditioning, a conditioned response was defined as a blink occurring 100–500 msec following tone onset ( Figure 1 ). The analysis of conditioned responses in a trace paradigm is complicated by the fact that the longer time interval between tone and puff provides a greater probability to record spontaneous or base blinks. Rate of spontaneous/base blinks were estimated by calculating the blink rate in the 400 msec that preceded the tone ( Figure 1 ). In addition, to minimize the potential impact of spontaneous blinks on the estimation of conditioned responses in the trace paradigm, we identified “adaptive” conditioned responses. An adaptive conditioned response, which occurs 300–400 msec before the onset of the puff, is elicited at an appropriate time to protect the eye from the puff (19) . Accordingly, blinks were counted as adaptive conditioned responses if their onset latency fell in the 400-msec period that preceded the puff (800–1200 msec posttone onset). Blinks with an onset occurring 100–800 msec following tone onset were considered as nonadaptive conditioned responses. Blinks in the first 100 msec after tone onset were classified as alpha responses (20) . Alpha responses did not differ between groups and are not further considered.
Statistics
The delay and trace data were analyzed separately. Preliminary analyses indicated no main gender effect or gender-by-group interaction and no effect of presentation order of the delay and trace eyeblink conditioning tests. Hence, gender and order were not included in subsequent analyses. Unconditioned blink magnitudes during the puff habituation period were averaged over the four trials and compared across groups using a t test. Base blink rates, nonadaptive conditioned responses (trace conditioning only), and adaptive conditioned responses were averaged within blocks. Because the window of analysis for nonadaptive conditioned responses was longer (700 msec) than that for base blinks (400 msec) or adaptive conditioned responses (400 msec) in the trace conditioning study, the rate of nonadaptive conditioned responses was expressed relative to a 400-msec window by multiplying the rate of nonadaptive conditioned responses by 400/700. This enabled us to use a similar metric to compare the different types of responses across windows of analysis. In order to analyze conditioned performance, the conditioned responses during delay conditioning and the nonadaptive and adaptive conditioned responses in the trace conditioning study were contrasted to the rate of spontaneous base blinks. The rate of conditioned responses was entered into separate ANOVAs examining the interactions of group (comparison subjects, patients), block (acquisition, extinction), and analysis window (base eyeblink, conditioned response); for the trace paradigm, learning was assessed by nonadaptive or adaptive conditioned responses. Evidence of conditioning was expected to be reflected by a significant main effect of analysis window or a significant analysis window-by-block interaction (due to greater conditioned response rates of blinks relative to base blinks). Greenhouse–Geisser epsilon corrections were implemented when appropriate to counter violations of the sphericity assumption underlying ANOVA with repeated measures.
Results
Delay Conditioning
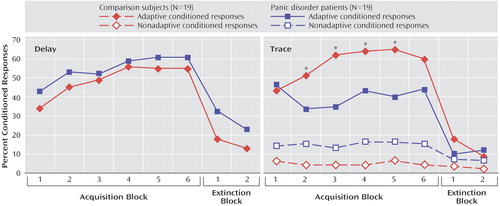
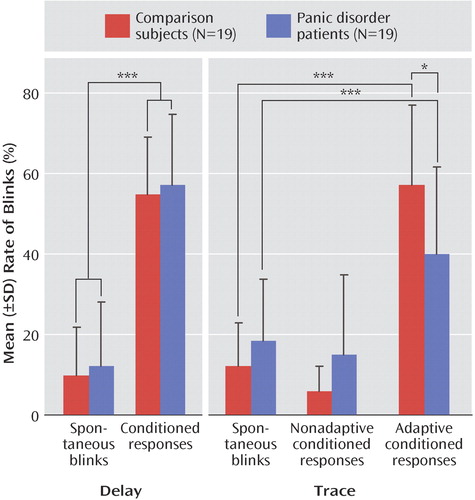
The magnitude of the unconditioned eyeblink response to the puff during the puff habituation phase did not differ between the comparison subjects (mean=61.7 μV, SD=38.2) and the panic disorder patients (mean=65.6 μV, SD=31.8). Figure 2 shows the rate of conditioned responses in the two groups during acquisition and extinction blocks. The mean rate of conditioned responses was greater than the base rate of blinks ( Figure 3 ), indicating that both groups conditioned with no difference between groups. This was statistically supported by a significant main effect of analysis window (F=106.9, df=1, 36, p<0.0009), with no significant group effect or group-by-analysis window interaction. The linear block effect (F=5.9, df=1, 36, p<0.02) and the linear block-by-analysis window interaction (F=18.1, df=1, 36, p<0.0009) were significant because of a progressive increase in the rate of conditioned responses over blocks.
*p<0.05.
*p<0.05. **p<0.0009.
During extinction, the group main effect was not significant, but both the analysis window main effect (F=16.7, df=1, 35, p<0.0009) and the group-by-analysis window interaction (F=7.6, df=1, 35, p<0.009) were significant. These results reflected higher rates of conditioned responses compared with base blinks, especially in the patients. Follow up tests showed no group difference in base blinks but a greater rate of conditioned responses in the patients that approached significance (F=3.7, df=1, 36, p=0.06), perhaps because of slower extinction in the patient groups.
Trace Conditioning
The magnitude of the unconditioned eyeblink response to the puff during the puff habituation phase did not differ significantly between the comparison subjects (mean=55.2 μV, SD=8.9) and the patients (mean=74.1 μV, SD=4.3). Figure 2 shows the rate of adaptive and nonadaptive conditioned responses in the two groups. Both groups showed higher rates of adaptive conditioned responses compared with base blink rates (analysis window: F=99.8, df=1, 36, p<0.0001 [ Figure 3 ]), indicating that both groups conditioned. However, the rate of adaptive conditioned responses was greater in the comparison subjects than in the patients (group-by-analysis window: F=11.8, df=1, 36, p<0.001). Follow up tests confirmed the higher rates of adaptive conditioned responses compared with base blink rates in both the comparison subjects (F=90.2, df=1, 36, p<0.0009) and the patients (F=21.4, df=1, 36, p<0.0009). However, there were more adaptive conditioned responses in the comparison subjects than in the patients (F=6.0, df=1, 36, p<0.02). This effect was due to the fact that both groups acquired conditioned responses similarly in the first block, but while the comparison subjects showed increasing rates of conditioned responses as conditioning proceeded (linear trend: F=7.2, df=1, 18, p<0.01), the patients did not, showing virtually the same rate of conditioned responses in the first and last acquisition blocks.
As mentioned in the “Statistics” section of the Method, the rate of nonadaptive conditioned response in the trace study was multiplied by 400/700 to express the data over a 400-msec window as for the base blink rates and the adaptive conditioned response (the mean raw rates, averaged over the six acquisition blocks, were 10.2% [SD=9.0] and 27.6% [SD=21.0] in the comparison and patient groups, respectively). For nonadaptive conditioned responses, there were only significant main effects of 1) group (F=6.0, df=1, 36, p<0.02) because of the overall higher blink rates among the patients relative to the comparison subjects, and 2) analysis window (F=5.5, df=1, 36, p<0.02), caused by higher rate of base blinks compared with nonadaptive blinks ( Figure 3 ). The group-by-analysis window interaction effect was not significant. Taken together, these results indicate higher rates of base blinks in the patients, with no evidence of conditioned responses in the nonadaptive conditioned response window.
During extinction, the analysis window main effect was significant (F=4.1, df=1, 36, p<0.04) because of a higher rate of adaptive conditioned responses compared with base blinks. Neither the group main effect nor the group-by-analysis window interaction were significant, suggesting similar extinction in the two groups.
Effect of Comorbidity
There was no difference between patients with and without comorbidity for any variable. Specifically, the patients with and without comorbidity showed similar rates of base blinks (0.22 [SD=0.21] and 0.14 [SD=0.20], respectively), nonadaptive conditioned responses (0.30 [SD=0.07] and 0.21 [SD=0.07]), and adaptive conditioned responses (0.40 [SD=0.28] and 0.40 [SD=0.26]) in the trace conditioning study. In addition, results in patients without comorbid disorders were compared to results in the comparison subjects. Results confirmed the main analysis. The rate of spontaneous blinks was similar in the two groups, but the overall rate of adaptive conditioned responses was greater in the comparison subjects than in the patients (F=4.5, df=1, 26, p<0.04).
Correlations
No significant correlations were found between the various measures of conditioning and scores on the State-Trait Anxiety Inventory, Anxiety Sensitivity Index, Beck Depression Inventory, or Beck Anxiety Inventory. The bivariate relation between base blinks and conditioned responses was examined to assess the potential influence of spontaneous blinks on the rate of conditioned responses. There was a positive correlation between base blink rates and the rate of conditioned responses in the delay eyeblink conditioning tests in both the comparison subjects (r=0.50, p<0.02) and the patients (r=0.60, p<0.007). There was also a positive correlation between base blink rates and the rate of adaptive conditioned responses in the trace eyeblink conditioning test in the patients only (r=0.50, p<0.02). These results suggest an influence of base blinks on the rate of conditioned responses, especially in the patients.
Discussion
This is the first eyeblink conditioning study in individuals with panic disorder. Consistent with our hypothesis of impaired declarative associative learning in panic disorder, the panic disorder patients showed impaired trace but not delay eyeblink conditioning. Such a deficit could play a role in the pathology of panic disorder underlying the perceived sense that panic attacks are unpredictable, thus promoting anticipatory anxiety.
The finding of impaired eyeblink conditioning in panic disorder emphasizes a distinct aspect of cognitive dysfunction in panic disorder relative to other cognitive models that stress the role of attentional and memory biases in anxiety disorders (21) . These models predict enhanced detection of and memory for threat-related materials among anxious individuals, including panic disorder (22) . Because of this attentional bias, one might expect individuals with panic disorder to learn quickly about signals that predict an aversive event during conditioning. However, it is important to note that the majority of these studies rely on threat-related verbal stimuli that are likely to engage cortically based processes involved in learning and memory. The current study investigated measures of learning less strongly related to cortically based processes, and no previous studies have used similar procedures in panic disorder patients. Given past work implicating perturbed hippocampal function in panic disorder (23 , 24) , one might expect anxious individuals to show poor learning in paradigms that require declarative memories of the conditioned stimulus (25) . The present results are consistent with this second interpretation.
Deficits in associative learning could increase anticipatory anxiety and the generalization of fear in individuals with panic disorder. Predictability is fundamental to conditioning. Prediction is a mitigating factor in response to aversive events, and lack of prediction is a causal factor in the experimental neurosis models of anxiety and depression (26) . Failure to condition could prevent individuals with panic disorder from recognizing predictive cues, leading to the feeling that panic attacks are unpredictable. Unpredictable aversive events are more debilitating than predictable aversive events because they lead to a chronic state of anticipatory anxiety and enhanced avoidance (5 , 27) . Likewise, impairment in declarative associative learning could also increase the generalization of fear. Fear conditioning leads to adaptive fear responses to the conditioned stimulus, but fear generalizes to the experimental context in individuals who do not learn to condition (5 , 6) . This could explain the tendency of individuals with panic disorder to avoid contexts associated with a panic attack.
The CA1 region of the hippocampus is a primary area of interest due to its role in complex sensory processing, such as trace eyeblink conditioning (28) , fear conditioning (29) , and anxiety (30) . It will be interesting to examine whether individuals with panic disorder also show deficits in another hippocampus-mediated conditioning task, context conditioning. Context conditioning, a form of conditioning in which aversive events become associated with places and situations, is an ideal model for agoraphobic avoidance (31) . Contexts are also critical to determine performance after extinction (32) .
The present results cannot be attributed to nonassociative processes. Both the unconditioned response magnitude and spontaneous blinks, which could be erroneously counted as a conditioned response, can affect the rate of conditioned responses. The unconditioned response did not differ between the two groups and the base rate of blinks was higher in the patients relative to the comparison subjects. One could argue that spontaneous blinks interfered with the ability of patients to generate conditioned responses. This is unlikely, since the rate of conditioned responses was positively correlated with the base blink rates in the patients. Thus, if anything, the rate of adaptive conditioned responses was overestimated in the patients.
The base rate of blinks was higher in the patients than in the comparison subjects in the trace paradigm. Since this effect was not found in the delay paradigm, it may be due to the failure to condition properly. Conditioning is an adaptive process that promotes appropriate responses timed with the unconditioned stimulus. One possibility is that deficits in conditioning lead to a more diffuse expression of these responses across time, resulting in less adaptive conditioned responses, more nonadaptive conditioned responses, and higher rates of base blinks. Consistent with this interpretation, we previously reported that subjects who condition during a fear conditioning experiment showed an appropriate fear response during the conditioned stimulus, whereas subjects who did not condition showed increased anxiety throughout the experiment (5) .
There were a number of limitations in this study. The eyeblink conditioning tasks were preceded by mildly stressful experiments, which could have affected performance (33) . This is unlikely, since stress impacts trace and delay eyeblink conditioning similarly (33) , but we found deficits in the patients in the trace paradigm but not in the delay paradigm. Nevertheless, the present findings should be replicated in the absence of explicit stressors. About half the patients had a comorbid disorder, including major depressive disorder, a disorder that has been associated with impaired eyeblink conditioning (34) . However, findings were not influenced by comorbidity. First, patients with and without comorbid disorders showed similar rates of conditioning. Second, when analyzed individually, panic disorder patients without comorbid disorders showed significantly reduced trace eyeblink conditioning relative to comparison subjects. Finally, major depressive disorder is also associated with deficits in delay eyeblink conditioning, not trace eyeblink conditioning (34) . We did not detect differences between men and women, but because of the small number of men in the study, any difference would have been difficult to detect. Even if the results did not generalize to men, the findings would be significant given the higher rate of panic disorder in women than men (35) .
The present eyeblink conditioning study found that panic patients had impaired associative learning in the trace paradigm but not in the delay paradigm. These results point to a cognitive dysfunction in declarative associative learning that could impact individuals with panic disorder’s awareness of cues associated with panic attacks, possibly leading to enhanced anticipatory anxiety and contextual fear. Longitudinal trace eyeblink conditioning studies in individuals at risk for panic disorder may help determine if such a dysfunction is a risk factor or a consequence of this disorder.
1. Barlow DH: Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. New York, Guilford, 2002Google Scholar
2. Barlow DH: Unraveling the mysteries of anxiety and its disorders from the perspective of emotion theory. Am Psychologist 2000; 55:1247–1263Google Scholar
3. Rescorla RA, Wagner AR: A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement, in Classical Conditioning II: Current Theory and Research. Edited by Black AH, Prokasy WF. New York, Appleton-Century-Crofts, 1972, pp 64–99Google Scholar
4. Seligman MEP, Binik YM: The safety signal hypothesis, in Operant-Pavlovian Interactions. Edited by Davis H, Hurwitz HMB. New York, Hillsdale, 1977, pp 165–187Google Scholar
5. Grillon C: Associative learning deficits increase symptoms of anxiety in humans. Biol Psychiatry 2002; 51:851–858Google Scholar
6. Grillon C, Morgan CA: Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol 1999; 108:134–142Google Scholar
7. Chan C, Lovibond P: Expectancy bias in trait anxiety. J Abnorm Psychol 1996; 105:637–647Google Scholar
8. Christian KM, Thompson RF: Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem 2003; 10:427–455Google Scholar
9. Clark RE, Squire LR: Classical conditioning and brain systems: the role of awareness. Science 1998; 280:77–81Google Scholar
10. Spence KW, Beecroft RS: Differential conditioning and level of anxiety. J Exp Psychol 1954; 48:399–403Google Scholar
11. Bouton ME, Mineka S, Barlow DH: A modern learning theory perspective on the etiology of panic disorder. Psychol Rev 2001; 108:4–32Google Scholar
12. Moyer JR Jr, Deyo RA, Disterhoft JF: Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci 1990; 104:243–252Google Scholar
13. McGlinchey-Berroth R, Carrillo MC, Gabrieli JD, Brawn CM, Disterhoft JF: Impaired trace eyeblink conditioning in bilateral, medial-temporal lobe amnesia. Behav Neurosci 1997; 111:873–882Google Scholar
14. Woodruff-Pak D: Eyeblink classical conditioning in HM: delay and trace paradigms. Behav Neurosci 1993; 107:911–925Google Scholar
15. LaBar KS, Disterhoft JF: Conditioning, awareness, and the hippocampus. Hippocampus 1998; 8:620–626Google Scholar
16. Reiss S, Peterson RA, Gursky DM, McNally RJ: Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther 1986; 24:1–8Google Scholar
17. LaBar KS, Disterhoft JF: Conditioning, awareness, and the hippocampus. Hippocampus 1998; 8:620–626Google Scholar
18. Lovibond PF, Shanks DR: The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. J Exp Psychol Anim Behav Process 2002; 28:3–26Google Scholar
19. Spence KW, Ross LE: A methodological study of the form and latency of eyelid responses in conditioning. J Exp Psychol 1959; 58:376–381Google Scholar
20. Gormezano I: Classical conditioning, in Experimental Methods and Instrumentation in Psychology. Edited by Sidowski JB. New York, McGraw-Hill, 1966, pp 385–420Google Scholar
21. Eysenck HJ: Anxiety: The Cognitive Perspective. Hillsdale, NJ, Erlbaum, 1992Google Scholar
22. Becker ES, Roth WT, Andrich M, Margraf J: Explicit memory in anxiety disorders. J Abnorm Psychol 1999; 108:153–163Google Scholar
23. Sakai Y, Kumano H, Nishikawa M, Sakano Y, Kaiya H, Imabayashi E, Ohnishi T, Matsuda H, Yasuda A, Sato A, Diksic M, Kuboki T: Cerebral glucose metabolism associated with a fear network in panic disorder. Neuroreport 2005; 16:927–931Google Scholar
24. Bisaga A, Katz JL, Antonini A, Wright CE, Margouleff C, Gorman JM, Eidelberg D: Cerebral glucose metabolism in women with panic disorder. Am J Psychiatry 1998; 155:1178–1183Google Scholar
25. Grillon C: Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry 2002; 52:958–975Google Scholar
26. Mineka S, Kihlstrom JF: Unpredictable and uncontrollable events: a new perspective on experimental neurosis. J Abnorm Psychol 1978; 87:256–271Google Scholar
27. Grillon C, Baas JM, Cornwell B, Johnson L: Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biol Psychiatry 2006; 60:752–759Google Scholar
28. Weiss C, Sametsky E, Sasse A, Spiess J, Disterhoft JF: Acute stress facilitates trace eyeblink conditioning in C57BL/6 male mice and increases the excitability of their CA1 pyramidal neurons. Learn Mem 2005; 12:138–143Google Scholar
29. Múnera A, Gruart A, Muñoz MD, Fernández-Mas R, Delgado-García JM: Hippocampal pyramidal cell activity encodes conditioned stimulus predictive value during classical conditioning in alert cats. J Neurophysiology 2001; 86:2571–2582Google Scholar
30. McNaughton N, Gray JA: Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord 2000; 61:161–176Google Scholar
31. Gorman JM, Kent JM, Sullivan GM, Coplan JD: Neuroanatomical hypothesis of panic disorder, revised. Am J Psychiatry 2000; 157:493–505Google Scholar
32. Bouton ME, King DA: Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J Exp Psychol Anim Behav Process 1983; 9:248–265Google Scholar
33. Beylin AV, Shors TJ: Stress enhanced excitatory trace eyeblink conditioning and opposes acquisition of inhibitory conditioning. Behav Neurosci 1998; 112:1327–1338Google Scholar
34. Greer TL, Trivedi MH, Thompson LT: Impaired delay and trace eyeblink conditioning performance in major depressive disorder. J Affect Disord 2005; 86(2–3):235–245Google Scholar
35. Grant BF, Hasin DS, Stinson FS, Dawson DA, Goldstein RB, Smith S, Huang B, Saha TD: The epidemiology of DSM-IV panic disorder and agoraphobia in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 2006; 67:363–374Google Scholar



