Discovering Light Effects on the Brain
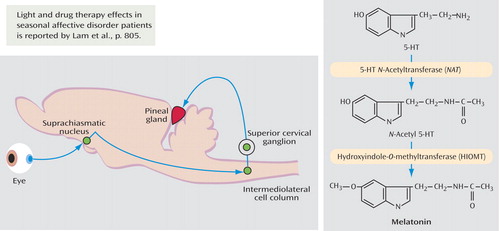
Circadian rhythms in the pineal gland were initially noted by Wilbur Quay in 1963. Axelrod and his colleagues showed it to be a true circadian rhythm, maintained during constant dark periods, but dramatically modulated by light. As seen in the diagram above, the neuronal pathway regulating melatonin synthesis in the rodent pineal gland connects 1) the retina to the suprachiasmatic nucleus, 2) the suprachiasmatic nucleus, through the intermediolateral cell column, to the superior cervical ganglion, and 3) the superior cervical ganglion to the pineal gland. Serotonin levels vary diurnally with the light/dark cycle: high levels in daylight, varying inversely with melatonin, which shows high levels in darkness. The biochemical link between serotonin and melatonin includes two enzymes that convert serotonin to melatonin (above): the first acetylating serotonin to form N -acetyl serotonin (5-HT N -acetyltransferase), and the other methylating N -acetyl serotonin to melatonin (hydroxyindole- O -methyltransferase). The molecular mechanisms of this process have been characterized as the following: nighttime release of noradrenaline in the mammalian pineal gland (which activates β-adrenergic receptors to stimulate adenylate cyclase activity and elevate cAMP) up-regulates the activity of 5-HT N -acetyltransferase and subsequently increases melatonin levels in a circadian fashion. The molecular signal is mediated by the expression of an inducible cAMP early repressor (ICER) factor that participates in an autoregulatory negative feedback process for melatonin synthesis at 5-HT N -acetyltransferase. An additional fundamental property of nighttime melatonin synthesis by the pineal gland is its sensitivity to the length of night, conveying not only the day/night transition to the animal but also seasonal information. The mechanism of this involves ICER factor, the levels of which reflect not only light and dark but also the duration of the darkness. Adaptation of an organism to a changing environment, mediated in part by these processes, is an essential feature of physiological regulation. Conversion of the day/night rhythm into hormonal oscillations governs human and animal metabolism. Melatonin is synthesized rhythmically in the pineal gland in response to signals originating from the biological clock in the suprachiasmatic nucleus. The molecular mechanisms involved in rhythmic synthesis of melatonin have been largely described and involve the rhythmically expressed ICER factor, which creates an autoregulatory loop that controls the amplitude of 5-HT N -acetyltransferase oscillations. Thus, the transcription factor (ICER) modulates the oscillatory levels of its target hormone (melatonin). The effect of altering the light/dark cycle, especially during seasons of long darkness with the goal of altering mood, is thought to be mediated to some degree by these molecular mechanisms.