Clinical Outcome in a Randomized 1-Year Trial of Clozapine Versus Treatment as Usual for Patients With Treatment-Resistant Illness and a History of Mania
Abstract
OBJECTIVE: Case series and follow-up studies suggest that clozapine may have mood-stabilizing properties in addition to antipsychotic action in patients with schizoaffective disorder, bipolar type, and bipolar I disorder, but the generalizability of these findings is limited. This article describes a randomized, open study of clozapine add-on therapy versus treatment as usual for patients with treatment-resistant illness and a history of mania. METHOD: Thirty-eight patients meeting the DSM-IV criteria for schizoaffective or bipolar disorder that was deemed treatment-resistant were randomly assigned to clozapine add-on treatment (N=19) or treatment as usual (no clozapine) (N=19) and followed up for 1 year. Patients received monthly ratings on the Brief Psychiatric Rating Scale, Clinical Global Impression scale, Bech-Rafaelsen Mania Scale, Hamilton Depression Rating Scale, Scale for the Assessment of Positive Symptoms, Scale for the Assessment of Negative Symptoms, Abnormal Involuntary Movement Scale, and a 40-item side effect checklist. Differences between treatment groups were assessed according to a pattern-mix random-regression model. An additional analysis compared group differences in rating scale scores against relative time in the study. RESULTS: Significant between-group differences were found in scores on all rating scales except the Hamilton depression scale. Total medication use over 1 year significantly decreased in the clozapine group. No significant differences between groups in somatic complaints were noted. The subjects with nonpsychotic bipolar I disorder who received clozapine showed a degree of improvement similar to that of the entire clozapine-treated group. Clozapine dose was significantly higher for the patients with schizoaffective illness than for those with bipolar disorder. CONCLUSIONS: The results of this study support clozapine’s independent mood-stabilizing property. They demonstrate that clozapine use was associated with significant clinical improvement relative to treatment as usual.
Clozapine’s antipsychotic efficacy in treatment-resistant schizophrenia is well-documented in randomized controlled studies (1, 2). Long-term follow-up with clozapine is associated with decreased hospitalization, improvement in clinical symptoms, and measurable social and functional gains as compared with typical antipsychotic agents (3, 4).
Case series from Europe suggest antipsychotic action and mood-stabilizing properties for clozapine in schizoaffective disorder, bipolar type, and bipolar disorder (5–8). Recent U.S. studies extend these observations (9, 10). In an early report of improvement in patients with treatment-resistant illness (patients with a history of mania over 26 months of unstructured clinical observation), McElroy and colleagues (11) observed ∼85% improvement with add-on clozapine treatment. More recently, uncontrolled trials in difficult-to-treat patients support the potential of clozapine as a mood stabilizer (12–19) (see reference 20 for a review).
While results from these early reports appear favorable, absence of control groups and varying definitions of response to treatment make across-study comparisons difficult. Most of these initial studies relied on unstructured evaluation of response and provided limited long-term follow-up. None of these studies included a randomly selected control group.
The present study was a prospective, randomized, open 1-year study of clozapine add-on therapy versus treatment as usual (no clozapine) in 38 patients with a history of mania. All patients had well-defined resistance to or intolerance of a combination of mood stabilizers and, if psychotic, antipsychotic medication as well.
METHOD
Recruitment proceeded through local advertising, advocacy groups, psychiatrists and staff at local public health facilities, and an ongoing treatment optimization study (21). Included were patients with a history of mania by DSM-IV criteria who were diagnosed with either schizoaffective disorder, bipolar type, or bipolar I disorder. Other inclusion criteria were the following: having had a mood episode within the preceding year, age between 18 and 65 years, ongoing illness symptoms, and a documented history of treatment resistance or intolerance.
Treatment resistance was defined as persistent symptoms despite simultaneous, adequate treatment with two mood stabilizers (lithium, valproate, carbamazepine) at standard therapeutic levels. If the patient also had psychotic symptoms, documented failure of treatment with a typical antipsychotic in addition to mood stabilizers was required. Inability to meet these criteria because of intolerance of medications was also sufficient to warrant classification as treatment-resistant.
Many of the patients referred for treatment in the early 1990s had had no prior exposure to an anticonvulsant. These potential study patients (N=28) were entered in a treatment optimization protocol (21) offering open treatment with lithium; if symptoms persisted despite therapeutic serum levels, divalproex sodium was added, and if the patient was psychotic, a typical antipsychotic was given. Patients with persistent symptoms or intolerance of these medications (N=13) were referred to the present study.
Patients with onset of illness after age 40 were excluded on the basis of evidence indicating that late-onset bipolar illness often results from secondary causes (22). Also excluded were women who were pregnant, breast-feeding, or not using contraception; patients with unstable general medical conditions, current active alcohol or substance abuse, and severe personality disorders; and those for whom clozapine was contraindicated.
After a thorough description of the study, written informed consent was obtained from each subject. Structured Clinical Interview for DSM-III-R (23) evaluations were completed; the diagnoses reported are consistent with DSM-IV criteria. Patients were randomly assigned to clozapine add-on therapy or treatment as usual (no clozapine) and stratified according to diagnoses of schizoaffective disorder, bipolar type, or bipolar I disorder. Stratification was done to ensure balance between the experimental and comparison groups and because some reports suggest a differential response to clozapine between patients with schizoaffective disorder, bipolar type, and those with bipolar I disorder (11). Moreover, we believed such stratification might contribute to the limited data available on the response to treatment of these groups of patients (24).
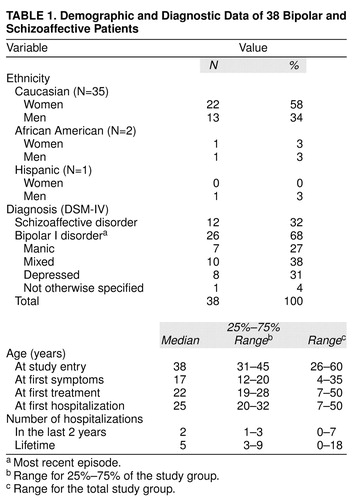
Demographic features (gender, age, race, marital status) and history of illness (head injury, medical illness, seizures, substance abuse, age at first symptoms, age at first treatment, age at first hospitalization, and number of psychiatric hospitalizations in the last 2 years) did not distinguish between the treatment groups (Table 1). There was an average of 16 years since first treatment, and most patients had been hospitalized within the previous 2 years.
Of 41 patients who signed consent forms, two (one randomly assigned to clozapine and one to treatment as usual) did not return for baseline or subsequent visits. A third (treatment as usual) completed baseline assessments but did not return for a second visit. The 38 patients eligible for evaluation (i.e., those with at least one postbaseline visit) all met DSM-IV criteria for schizoaffective disorder, bipolar type (N=12), or bipolar I disorder (N=26).
More than one-half of the study group (N=21, 16 of whom were women) met the DSM-IV criteria for rapid cycling in the preceding year. Fifteen patients met criteria for a lifetime history of mixed manic episodes, and 27 for psychotic symptoms. Twenty-one patients (55%) had a history of alcohol or drug abuse. Notably, 11 patients with bipolar I disorder had no history of psychotic symptoms when affectively ill.
Treatment Procedures
Baseline visits preceded initiation of clozapine treatment. All clozapine-treated patients had clozapine added to their ongoing medications, except for those receiving carbamazepine (N=2), which was tapered off before clozapine treatment was started. For treatment-as-usual patients, additional effort by study psychiatrists to optimize medication included using carbamazepine, calcium channel blockers, and various combinations of medications (supervised by T.S.). For both groups, ECT was available if clinical symptoms persisted despite changes in medication. Efforts were made to simplify and minimize medications for patients in both groups once they were stabilized. All medication changes were made without reference to clinical rating scales.
Patients in treatment as usual who, despite every effort, had partial or no improvement, or who showed clinical improvement but had intolerable side effects (N=9), were ultimately offered clozapine therapy. At that time, they were removed from the treatment-as-usual group (i.e., they were treated as dropouts in the following analyses).
Patients’ adherence to their regimens was evaluated at each visit. Self-reports indicated a high level of adherence. Blood levels of lithium, carbamazepine, and divalproex were monitored, on average, at 14% of all visits in both groups.
At baseline and every 2 weeks for 3 months thereafter, patients were administered the 18-item Brief Psychiatric Rating Scale (BPRS) (25), the Clinical Global Impression (CGI) scale (26), the Bech-Rafaelsen Mania Scale (27), the 24-item Hamilton Depression Rating Scale (28), the Scale for the Assessment of Positive Symptoms (SAPS) (29), the Scale for the Assessment of Negative Symptoms (SANS) (30), and the Abnormal Involuntary Movement Scale (AIMS) (31). Patients completed a 40-item somatic complaint questionnaire that was reviewed by a clinician at each visit. This checklist included a range of somatic complaints: nausea, headache, drowsiness, dizziness, tremor, change in weight, etc.
Study nurses who were not blind to treatment group administered the rating scales. Reliability among raters was assessed, and the intraclass correlation was 0.92.
After the first 3 months, the rating scale battery was administered monthly for a total of 1 year from study enrollment. Hospitalization did not exclude patients from continuing in the study; rating scale scores were obtained regardless of inpatient or outpatient status. Patients could be seen between study visits as clinically needed.
Analyses
In the pattern-mixture random-regression model (32, 33), subjects are classified according to the pattern of missing data: dropouts or completers. Although subjects with missing data were used, this does not eliminate the possibility of bias, especially that resulting from subject attrition. In other words, subjects who dropped out may have been systematically different from study completers. More treatment-as-usual subjects (N=9 of 19, 47%) than clozapine subjects (N=3 of 19, 16%) dropped out of the study. To assess the effect of dropouts on the comparison of clozapine and treatment as usual, we used the pattern-mixture random-regression model.
A random-regression model assesses the pattern of missing data in relation to treatment effect. In the usual random-regression model, terms are defined for group (clozapine or treatment as usual), time (log of months), and group-by-time interaction (which should be significant if, over time, clozapine subjects are doing better than treatment-as-usual subjects). If dropouts differ from completers in terms of treatment effect (as seen in the group-by-time interaction), then the pattern-by-group-by-time interaction will be significant. Our pattern-mixture model used the above terms and included terms for pattern (dropout or completer), pattern-by-time interaction, pattern-by-group interaction, and pattern-by-group-by-time interaction. We used the pattern-mixture approach for scores on the BPRS, the CGI, the Bech-Rafaelsen Mania Scale, the Hamilton depression scale, the SAPS, and the SANS.
We also evaluated the rate of change in the 18-item BPRS score as a clinically relevant assessment of clinical improvement. This was calculated from the slope of the linear regression line of each subject’s BPRS score at each visit and time (months from baseline to visit) and constructed by fitting a line to the actual BPRS scores for each patient. For example, a linear regression slope of –1.5 implies that the subject’s BPRS score was improving, on average, by 1.5 points per month during the time in the study. The natural log of months from baseline was used to give a more nearly linear relationship of BPRS score to time. Results were analyzed with the use of the Mann-Whitney U test with chi-square approximation and a p value of 0.05 to indicate significance. This analytic approach was used for all symptom ratings.
Additional between-group analyses compared both total numbers of medications used and somatic complaints. The Mann-Whitney U test with chi-square approximation was also used to calculate these results.
RESULTS
Overall patient retention was high. Three (16%) of the 19 clozapine patients discontinued—stating inability to tolerate the medication—between 4 and 8.3 weeks after study entry (mean=6.67 weeks, SD=2.21). As mentioned earlier, in the treatment-as-usual group, nine of the patients discontinued treatment 8–32 weeks after study entry (mean=16.96 weeks, SD=7.02) because of limited improvement or intolerable side effects. Before discontinuing treatment as usual, all nine patients were offered a calcium channel blocker (verapamil) or ECT after the more usual medication trials, including triple-mood-stabilizer therapy (lithium, divalproex, and carbamazepine). Given our impression of the relative advantage of clozapine therapy, we felt ethically bound to offer patients this option when all other efforts had failed to produce a reasonable, sustained degree of clinical improvement. Of these nine treatment-as-usual patients, one selected ECT, and three chose verapamil before electing to begin clozapine. The remaining five patients accepted clozapine therapy immediately.
The primary hypothesis was that global improvement in mood stabilization would result with clozapine use and be reflected by change in BPRS scores. The pattern-mix random-regression analysis found significant differences between the clozapine and treatment-as-usual groups on the BPRS and all measures except for the Hamilton depression scale (Table 2).
Figure 1 shows slope differences in BPRS scores over 1 year, or until the last visit, between the clozapine and treatment-as-usual groups. The downward trend in the clozapine group indicates improvement, whereas there was worsening in the treatment-as-usual group. The comparison between the slope means of these two groups was significant. The mean rates of change are slightly different from those in the pattern-mix analysis because the slope analysis does not take patient dropout into account.
When response was defined as a 30% improvement from baseline in BPRS score, 65% of the clozapine subjects met this criterion by 3 months, and 82% by 6 months. Forty-eight percent of the treatment-as-usual subjects achieved this degree of improvement by 3 months, and 57% by 6 months.
The nine patients (two with schizoaffective disorder, bipolar type, and seven with bipolar I disorder) who were removed from treatment as usual and offered clozapine had a significant difference in BPRS scores over 1 year of clozapine treatment from their scores at termination of treatment as usual (Mann-Whitney U=3.94, df=1, p<0.05). Those who improved significantly in treatment as usual, but were offered clozapine, had ongoing intolerable side effects from the usual treatment (Figure 1). For example, one patient had remission with maintenance ECT but developed severe headaches and memory complaints after 4.5 months. These complaints improved and the patient continued in a stable mood when the transition to clozapine was made.
Eleven patients with bipolar I disorder (10 in the clozapine group and one in treatment as usual) had no history of psychotic symptoms. The 10 patients receiving clozapine showed a rate of improvement similar to that of the entire clozapine group, with a mean BPRS score slope of –3.96 per natural log month, while the whole group of 19 clozapine patients had a BPRS score slope of –4.20.
After the initial titration period, the mean peak clozapine dose was 355 mg/day (SD=248, range=50–900). By diagnostic subgroup, the mean peak daily dose for bipolar I disorder was 234 mg/day (SD=114, range=50–403), and for schizoaffective disorder, bipolar type, 623 mg/day (SD=260, range=300–900); the difference was significant (Mann-Whitney U=7.09, df=1, p=0.008).
While a decrease in medications (including antipsychotics [typical and atypical], lithium, anticonvulsants, antidepressants, benzodiazepines, and side effect medications) during the first 6 months was observed in the clozapine group, the drop in medications was significant by 1 year, in comparison with the treatment-as-usual group (Mann-Whitney U=9.60, df=1, p=0.002).
Using a 40-item self-report, we evaluated somatic complaints at each visit to see whether the clozapine group experienced more side effects during the study. When presence (scored as 1) or absence (scored as 0) of a somatic complaint was assessed, there was no difference between the two groups (Mann-Whitney U=26.99, df=19, p=0.11). When relative severity of a given complaint was assessed (scale of 0–3), the difference in change in side effects between groups was not significant (Mann-Whitney U=2.14, df=1, p=0.14), but the median rating relative to the baseline rating increased by 1 in the clozapine group and decreased by 2 in the treatment-as-usual group. No significant difference in AIMS scores between the two treatment groups was found (Mann-Whitney U=1.67, df=1, p=0.20). No patient developed agranulocytosis during the study.
DISCUSSION
In this 1-year randomized, open trial of clozapine add-on therapy for treatment-resistant patients with a history of mania, clozapine use was associated with significant clinical improvement relative to treatment as usual (no clozapine).
Using either change in slope of rating scale scores or pattern-mix random-regression analysis, we found significant differences across all measures comparing clozapine patients with treatment-as-usual patients except for Hamilton depression scale score (Table 2). Given the naturalistic study design, including the option of antidepressants or other treatments in both groups, these differences are more striking. In particular, clozapine was a strong antimanic and anti-mood-lability agent, but in most patients it had limited antidepressant efficacy. Importantly, because of the 1-year duration of the study, we observed a sustained mood-stabilizing effect with clozapine add-on treatment.
In the classic study that proved clozapine’s efficacy for treatment-resistant schizophrenia (1), 30% improvement on the BPRS from baseline was used to define response. In the present clozapine add-on study, 65% of the subjects met this criterion by 3 months, and more than 80% by 6 months. An uncontrolled monotherapy clozapine study of treatment-resistant patients with a history of mania by Calabrese et al. (13) used 50% improvement in BPRS score as a marked response. While these investigators found that a number of patients had 50% improvement, at study entry all medications (an average of three to four) were discontinued for 7 days. The baseline BPRS was then administered before initiation of clozapine treatment. The researchers noted an average 10-point, or 40%, increase in BPRS score after the discontinuation of the medication and before the baseline BPRS at clozapine initiation. According to these response definitions, our study found 16% of clozapine patients improved over 3–6 months and almost 40% by 1 year. In addition, the study by Calabrese et al. did not use as strict treatment-resistance criteria, requiring only failure of one mood stabilizer in combination with an antipsychotic. Because of these differences, our study can not be directly compared to the work of Calabrese and colleagues.
The primary hypothesis was that clozapine could act as a mood stabilizer. The results taken as a whole support a primary mood-stabilizing property for clozapine. Our finding that the nonpsychotic clozapine subjects showed a degree of overall improvement similar to that of the psychotic subjects is consistent with case reports of clozapine’s effectiveness in nonpsychotic affective illness (16).
Patients entered the study while taking an average of four psychotropic medications, including antipsychotics, mood stabilizers, antidepressants, and benzodiazepines. The greater change in medication regimens noted in months 6–12 likely reflects the strategy of medication change used in our clinic: overlap, establish stabilization, and then taper medications previously resulting in only partial improvement. As clozapine patients stabilized, it was possible to simplify their medication regimen, leading to a significant between-group difference by 12 months. This result is ancillary support for the mood-stabilizing property of clozapine.
Side effects or somatic complaints were noted throughout the study. While somatic complaints increased for the clozapine patients, no significant difference between groups was found. Three clozapine patients discontinued because of side effects. Although sedation was reported, our clinical practice of giving clozapine in once-a-day doses appeared to mitigate some of these problems. Also, bipolar patients, and particularly nonpsychotic bipolar patients, appeared to stabilize on lower doses of clozapine and to have greater sensitivity to side effects. It is interesting that there was a significant difference in mean peak daily dose of clozapine between the patients with bipolar I disorder and those with schizoaffective disorder, bipolar type. These findings suggest that the antipsychotic dose was higher than the mood-stabilizing dose.
Most clinical improvement was obtained in the first 6 months, followed by continued remission in the second 6 months. While several earlier uncontrolled prospective studies support an acute antimanic property of clozapine (see Frye et al. [20] for a review), the present results clearly demonstrate both acute and persistent mood-stabilizing properties of clozapine.
This study had several limitations. Treatment was open, and evaluations were not blind, potentially biasing results in favor of the primary hypothesis. Because of the recurring nature of bipolar illness, spontaneous remission—especially in a high-intensity treatment setting—is a possible though remote likelihood, given the treatment resistance and long history of persistent illness of the subjects. Double-blind controlled studies, perhaps comparing clozapine with newer atypical agents, are needed to resolve this issue.
In sum, this study supports an acute and prophylactic mood-stabilizing property of clozapine. Importantly, patients who were treatment-resistant or intolerant of usual treatment options experienced significant and persistent clinical gains with clozapine add-on therapy.
Presented in part at the annual meeting of the Society of Biological Psychiatry, New York, May 1–5, 1996; the annual meeting of the American College of Neuropsychopharmacology, San Juan, Puerto Rico, Dec. 9–13, 1996; the Second International Conference on Bipolar Disorder, Pittsburgh, Pa., June 19–21, 1997; and the annual meeting of the Society for Neuroscience, New Orleans, Oct. 25–30, 1997. Received May 5, 1998; revision received Dec. 30, 1998; accepted Feb. 23, 1999. From the Department of Psychiatry, University of Texas Southwestern Medical Center. Address reprint requests to Dr. Suppes, Department of Psychiatry, University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75235-9070 Supported in part by Mental Health Connections, a partnership between Dallas County Mental Health Mental Retardation and the Department of Psychiatry of the University of Texas Southwestern Medical Center; a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, grants from the Forest E. Lattner Foundation and the Theodore and Vada Stanley Foundation, and NIMH grant MH-53799 (all to Dr. Suppes); and Novartis Pharmaceuticals for medication. The authors thank Chris Claeson for administrative and secretarial support, Drs. Barry Knezek, Lorenzo Triana, and David Missimo for clinical assistance, and nurses Starla Harrison and Broc Sanchez for their help.
 |
 |
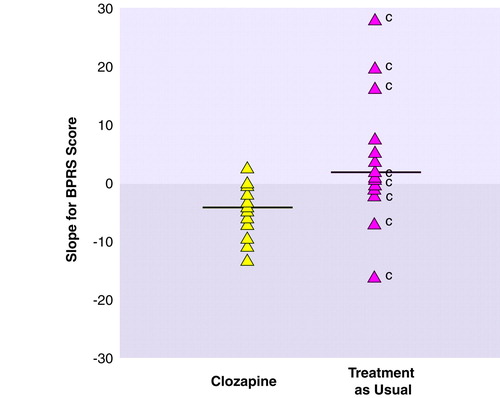
FIGURE 1. Slopesa for Mean BPRS Scores Over 1 Year or Until Last Visit for Patients Receiving Clozapine or Treatment as Usualb
aLinear regression slope of scores over time. A slope of –1.5 implies an average improvement in BPRS score of 1.5 points per month.
bThe horizontal lines represent mean slopes: –4.20 and 2.54 for the clozapine and treatment-as-usual groups, respectively (Mann-Whitney U=8.10, df=1, p=0.004).
Patient was removed from the group in order to receive clozapine therapy.
1. Kane J, Honigfeld G, Singer J, Meltzer H: Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
2. Marder SR, Van Putten T: Who should receive clozapine? Arch Gen Psychiatry 1988; 45:865–867Google Scholar
3. Meltzer H: Dimensions of outcome with clozapine. Br J Psychiatry 1992; 160(suppl 17):46–53Google Scholar
4. Meltzer HY, Okayli G: Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry 1995; 152:183–190Link, Google Scholar
5. Naber D, Lippig M, Grohmann R, Hippius H: Efficacy and adverse effects of clozapine in the treatment of schizophrenia and tardive dyskinesia—a retrospective study of 387 patients. Psychopharmacology (Berl) 1989; 99(suppl):S73–S76Google Scholar
6. Lindstrom LH: The effect of long-term treatment with clozapine in schizophrenia: a retrospective study in 96 patients treated with clozapine for up to 13 years. Acta Psychiatr Scand 1988; 77:524–529Crossref, Medline, Google Scholar
7. Leppig M, Bosch B, Naber D, Hippius H: Clozapine in the treatment of 121 outpatients. Psychopharmacology (Berl) 1989; 99(suppl):S77–S79Google Scholar
8. Naber B, Hippius H: The European experience with use of clozapine. Hosp Community Psychiatry 1990; 41:886–890Abstract, Google Scholar
9. McElroy SL, Keck PE, Strakowski SM: Mania, psychosis, and antipsychotics. J Clin Psychiatry 1996; 57(suppl 3):14–26Google Scholar
10. Tohen M, Zarate CA: Antipsychotic agents and bipolar disorder. J Clin Psychiatry 1998; 59(suppl 1):38–48Google Scholar
11. McElroy SL, Dessain EC, Pope HG, Cole JO, Keck PE, Frankenberg FR, Aizley HG, O’Brien S: Clozapine in the treatment of psychotic mood disorders, schizoaffective disorder, and schizophrenia. J Clin Psychiatry 1991; 52:411–414Medline, Google Scholar
12. Banov MD, Zarate CA, Tohen M, Scialabba D, Wines JD Jr, Kolbrener M, Kim JW, Cole JO: Clozapine therapy in refractory affective disorders: polarity predicts response in long-term follow-up. J Clin Psychiatry 1994; 55:295–300Medline, Google Scholar
13. Calabrese JR, Kimmel SE, Woyshville MJ, Rapport DJ, Faust CJ, Thompson PA, Meltzer HY: Clozapine for treatment-refractory mania. Am J Psychiatry 1996; 153:759–764Link, Google Scholar
14. Calabrese JR, Meltzer HY, Markovitz PJ: Clozapine prophylaxis in rapid cycling bipolar disorder (letter). J Clin Psychopharmacol 1991; 11:396–397Crossref, Medline, Google Scholar
15. Suppes T, McElroy S, Gilbert J, Dessain E, Cole J: Clozapine in the treatment of dysphoric mania. Biol Psychiatry 1992; 32:270–280Crossref, Medline, Google Scholar
16. Suppes T, Phillips K, Judd C: Clozapine treatment of nonpsychotic rapid cycling bipolar disorder: a report of three cases. Biol Psychiatry 1994; 36:338–340Crossref, Medline, Google Scholar
17. Kowatch RA, Suppes T, Gilfillan SK, Fuentes RM, Grannemann BD, Emslie GJ: Clozapine treatment for children and adolescents with schizophrenia and bipolar disorder: a clinical case series. J Child Adolesc Psychopharmacol 1995; 5:241–253Crossref, Google Scholar
18. Zarate CA, Tohen M, Banov M, Weiss MK, Cole JO: Is clozapine a mood stabilizer? J Clin Psychiatry 1995; 56:108–112Google Scholar
19. Zarate CA, Tohen M, Baldessarini RJ: Clozapine in severe mood disorders. J Clin Psychiatry 1995; 56:411–417Medline, Google Scholar
20. Frye MA, Ketter TA, Altshuler LL, Denicoff KD, Dunn RT, Kimbrell TA, Cora-Locatelli G, Post RM: Clozapine in bipolar disorder: treatment implications for other atypical antipsychotics. J Affect Disord 1998; 42:91–104Crossref, Google Scholar
21. Suppes T, Rush AJ, Kraemer H, Webb A: Treatment optimization for symptomatic patients with a history of mania using a treatment algorithm. J Clin Psychiatry 1998; 59:88–96Crossref, Google Scholar
22. Krauthammer C, Klerman G: Secondary mania: manic syndromes associated with antecedent physical illness or drugs. Arch Gen Psychiatry 1978; 35:1333–1339Google Scholar
23. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
24. Aubert JL, Rush AJ: Schizoaffective disorder, in DSM-IV Sourcebook, vol 2. Edited by Widiger TA, Frances AJ, Pincus HA, Ross R, First MB, Davis W. Washington, DC, American Psychiatric Press, 1996, pp 65–96Google Scholar
25. Overall JE, Gorham DR: Introduction—the Brief Psychiatric Rating Scale (BPRS): recent developments in ascertainment and scaling. Psychopharmacol Bull 1988; 24:97–99Google Scholar
26. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
27. Bech P, Bolwig TG, Kramp P, Rafaelsen OJ: The Bech-Rafaelsen Mania Scale and the Hamilton depression scale. Acta Psychiatr Scand 1979; 59:420–430Crossref, Medline, Google Scholar
28. Hamilton M: Standardized assessment and recording of depressive symptoms. Psychiatr Neurol Neurochir 1969; 72:201–205Medline, Google Scholar
29. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
30. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
31. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
32. Hedeker D, Gibbons RD: Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods 1997; 2:64–78Crossref, Google Scholar
33. Little RJA: Modeling the drop-out mechanism in repeated-measures studies. J Am Statistical Assoc 1995; 90:1112–1121Google Scholar



