Symptoms, Cognitive Functioning, and Adaptive Skills in Geriatric Patients With Lifelong Schizophrenia: A Comparison Across Treatment Sites
Abstract
OBJECTIVE: Although many geriatric patients with schizophrenia have been referred to nursing home care, little is known about their characteristics. Across nursing home and chronic hospital settings, the authors directly assessed poor outcome geriatric patients with schizophrenia and contrasted their cognitive, symptomatic, and adaptive functioning to that of acutely admitted patients with a better outcome over the lifetime course of the illness. METHOD: The subjects were 97 chronically hospitalized patients with schizophrenia, 37 patients with chronic schizophrenia who lived in nursing homes, and 31 acutely admitted geriatric patients with schizophrenia. These patients were rated with the Positive and Negative Syndrome Scale, tested with a neuropsychological battery, evaluated with the Mini-Mental State examination, and rated on a scale of social and adaptive deficits, the Social Adaptive Functioning Evaluation scale. RESULTS: Each group of patients proved discriminable from the other two: nursing home patients displayed the most severe adaptive deficits, and acutely admitted patients were the least cognitively impaired. Cognitive impairment was the strongest predictor of adaptive deficits for all three groups, and negative symptom differences among the groups were smaller than differences in cognitive impairment. Nursing home patients had the least severe positive symptoms, and the acutely ill and chronic hospital patients did not differ on positive symptoms. CONCLUSIONS: Cognitive impairment is a predictor of both overall outcome and specific adaptive deficits. These data suggest that interventions aimed at cognitive impairment may have an impact on overall functional status. In comparison, positive symptom severity is less strongly correlated with overall adaptive outcome and is uncorrelated with specific deficits in adaptive skills. (Am J Psychiatry 1998; 155:1080–1086)
The first period of wholesale deinstitutionalization of chronic psychiatric patients occurred immediately after the introduction of chlorpromazine in the 1950s (1). After experiencing a partial reduction of their symptoms, thousands of long-stay patients were discharged into the community. Although the overall adaptive outcome of those discharged patients was clearly still impaired (2) and the living conditions of many discharged patients were poor (3), the majority of those patients with schizophrenia have lived outside psychiatric hospitals for the past 35 years (4). Despite this trend toward deinstitutionalization, a considerable number of geriatric patients with schizophrenia have never been discharged but, rather, have resided in chronic psychiatric hospitals for this entire period (5, 6).
A second wave of deinstitutionalization has been initiated in the past few years (7). Many states have begun the process of closing their state hospitals or converting them into exclusively forensic facilities. It is estimated that more than 200,000 patients with lifelong chronic schizophrenia have been referred to nursing homes as a part of this deinstitutionalization movement (8). It is not clear if such patients still have severe psychotic symptoms or manifest severe cognitive impairments, negative symptoms, and adaptive deficits (9–11). Since many nursing home patients are direct referrals from chronic psychiatric hospitals, most are poor outcome patients who would be expected to be quite similar to their chronically hospitalized counterparts (12).
Although some elderly individuals with a history of schizophrenia are apparently symptom free with little residual deficit in late life (13, 14), many patients with lifelong chronic schizophrenia have severe adaptive deficits, cognitive impairments, and psychotic symptoms in late life. Such patients may either spend extended periods in chronic care (15, 16) or experience extensive adaptive deficits while living in the community after deinstitutionalization (17). In two recent prospective studies, cognitive and adaptive deficits did not differentiate schizophrenic patients who were sent to nursing homes from those who were retained in long-term psychiatric care; rather, belligerence and hostility were the distinguishing factors between the two groups (18, 19). These data suggest that geriatric nursing home residents with lifelong schizophrenia may be very similar in typical symptomatic characteristics to residents of long-term psychiatric hospitals. In contrast to chronic schizophrenic patients who are lifelong residents of psychiatric care facilities, researchers have previously demonstrated that whether they are acutely ill or stable, geriatric patients with schizophrenia who dwell in the community manifest markedly less severe cognitive (20, 21) and adaptive impairment (22). Even when they are acutely psychotic, geriatric schizophrenic patients who have a good functional outcome and have lived their lives in the community appear to have no more serious deficits than do younger patients in cognitive functioning as measured by neuropsychological tests (23). No study to date has identified the specific characteristics of poor outcome patients referred to nursing homes and compared them 1) to other poor outcome patients who have remained in psychiatric hospitals and 2) to currently ill, better outcome patients with lifelong schizophrenia who have lived in the community. In this article, we present the results of such comparisons. We compared these patients on a full range of characteristics of schizophrenia, including positive and negative symptoms, cognitive impairments, adaptive functioning, and current treatments. To do so, we addressed several questions:
1. Are there specific characteristics that distinguish each group from the other two?
2. Are the relationships among clinical symptoms, cognitive impairments, and adaptive deficits the same in all three groups?
3. Which variables best identify geriatric patients with chronic illness and which variables are more typical of those who are community residents?
METHOD
Subjects
All subjects in this study were geriatric (more than 64 years old) patients with DSM-III-R diagnoses of schizophrenia with a nonelderly age (less than 45 years old) at onset, drawn from three different treatment sites. Ninety-seven of the patients were chronically hospitalized residents of a state psychiatric center. Investigators have previously reported on this group (6, 24, 25), who participated in a longitudinal study of the characteristics of late-life schizophrenia; we saw these patients again 50 to 60 months after the initiation of the longitudinal study. Another 37 patients were nursing home residents who had lifelong diagnoses of chronic schizophrenia and extended stays in chronic care hospitals; this group had been referred to nursing homes directly from chronic psychiatric hospitals at least 2 years earlier. A third group, all of whom were male, consisted of 31 acute admissions to the geriatric psychiatry unit at a veterans hospital. The last group of patients had been living in the community, either with relatives or in community residences with no treatment programs or professional staff on site, and had been referred to acute psychiatric care because of an exacerbation of their symptoms. We did not include in this study patients who had been referred directly from nursing homes to the acute psychiatric unit; we selected acutely ill patients who met the aforementioned entry criteria from 2 months of consecutive admissions to the acute psychiatric care unit of the veterans hospital. During the same 2-month period that we were recruiting acutely ill patients, we also identified, selected, and assessed patients from three different nursing homes. Acutely admitted patients provided written informed consent. Chronically institutionalized and nursing home patients assented to participation because the appropriate institutional review boards had granted a waiver of informed consent.
We diagnosed patients at all three settings by using a procedure similar to the one described previously for the chronically hospitalized patients (6, 24, 25). For each patient, we conducted a lifetime chart review, examined the history of psychiatric symptoms, and confirmed the diagnosis of schizophrenia. Clinical charts for the chronically hospitalized patients were available on site, and we included the nursing home patients only if we could obtain their lifetime clinical charts from their former psychiatric hospitals. The acute patients had been receiving long-term outpatient care at the hospital where they had been acutely admitted; we included new admissions only if their long-term clinical charts were available on site. We excluded any patient with a lifetime history of substance dependence or evidence of concurrent neurological conditions or unstable medical conditions. These concurrent neurological conditions included history of stroke, idiopathic Parkinson's disease, or evidence of rapid cognitive decline in the past year.
We used a lifetime chart-review procedure to generate diagnoses. In this procedure, a psychiatrist read the lifetime clinical chart in its entirety, interviewed the patient's caregivers (either in the hospital or at home), and interviewed the patient. Acutely ill patients were required to meet DSM-III-R criteria for schizophrenia only during the current episode; on the other hand, chronic patients in both nursing homes and hospitals had to meet more stringent lifetime diagnostic criteria. These criteria included 1) meeting DSM-III-R criteria for the first and current decades of illness and 2) not meeting at any time the full criteria for a disorder that would exclude a schizophrenia diagnosis. A reliability study of this procedure, based on 25 chronically ill cases reviewed by two psychiatrists, yielded a kappa reliability coefficient of 0.88 (p<0.001) for the diagnosis of schizophrenia (6). Descriptive characteristics of the patients are presented in table 1.
Assessments
Negative and positive symptoms. We assessed severity of schizophrenic symptoms by using the Positive and Negative Syndrome Scale (26). This is a 30-item scale with seven items measuring positive symptoms, seven measuring negative symptoms, and 16 measuring general aspects of psychopathology. In this study, we used the total scores on positive and negative subscales as our dependent measures. Interrater reliability of these scales in our patients was previously found (6) to be acceptably high, with intraclass correlations (ICCs) (N=30) ranging from a low of 0.86 to a high of 1.00 (all p values <0.001).
Cognitive and functional assessments. In this study, we used both global and specific measures of cognitive functioning. We studied the presentation of cognitive impairment in individuals ranging from community residents, outpatients with acute exacerbation, to patients in chronic care institutions with a 65-year history of continuous admissions to psychiatric care. Since we expected the levels of cognitive functioning to vary widely among these subjects, we required broad measures. We used the Mini-Mental State examination (27) as a global measure of cognitive functioning. For geriatric patients with chronic schizophrenia, the Mini-Mental State is reliable on interrater (ICC=0.99, N=35) (28) and test-retest (ICC=0.90, N=50) (29) levels, as well as stable over time (ICC=0.88 at 1 year, N=224) (29). Mini-Mental State scores range from 0 to 30 and comprise the results of assessments of registration, memory, orientation, praxis, and verbal skills (e.g., naming).
Cognitive battery of the Consortium to Establish a Registry for Alzheimer's Disease. This brief neuropsychological assessment battery was developed for the diagnosis and staging of Alzheimer's disease (30). As a result, it measures several crucial cognitive impairments that are present in dementia. Previous research with this battery has demonstrated that patients with Alzheimer's disease can be discriminated from patients with schizophrenia on a cross-sectional basis (25). Additional studies with the Consortium to Establish a Registry for Alzheimer's Disease battery demonstrated that schizophrenic patients have high test-retest stability; as a group, they do not decline at a 1-year follow-up on any of the measures in the battery (31).
1. Immediate and delayed recall of word list: on three separate learning trials, we presented a 10-item list of words to each subject. Immediately after each trial, the subjects had to perform a free recall of the list. After a delay, during which we administered the praxis examination described later in this article, we required a delayed recall of the word list. The dependent variables were 1) the total number of words correctly recalled over the three learning trials and 2) the proportion of words recalled on learning trial 3 and subsequently reproduced at the delayed recall.
2. Praxic drawings: we presented four drawings (circle, diamond, overlapping rectangles, cube) to each subject, who was instructed to copy them exactly. We scored the reproductions according to predetermined criteria; the dependent measure was the total score for the four drawings.
3. Modified Boston Naming Test (30): we presented subjects with 15 line drawings and asked them to name the objects depicted. Of the drawings, five represent objects with high frequency of occurrence in spoken English (e.g., a house), five represent objects of moderate frequency, and five represent objects of low frequency (e.g., tongs). The dependent variable was the total number of correct namings.
4. Category fluency: we instructed subjects to name as many different animals as possible in one minute. The dependent variable is the number of unique animals (e.g., dog, cat) named.
Social Adaptive Functioning Evaluation scale. Investigators developed this 17-item scale (32) to measure social-interpersonal, instrumental, and impulse control skills. This scale is designed to be rated by an evaluator after observation of and interaction with the subject, as well as an interview with the subject's caregiver. This scale has suitable reliability, with interrater reliabilities of the items all exceeding ICC=0.88 (N=60). The total score was the key dependent measure.
Medication dose and type. We recorded all antipsychotic medications administered to the patients. All patients were treated with either typical neuroleptic medication or risperidone. We converted typical antipsychotic medication dosages to chlorpromazine equivalents.
Data Analysis
We completed between-group comparisons by using a multivariate analysis of variance (MANOVA) with Rao's R (33) as the test statistic, followed by examination of the multivariate-corrected, one-way analyses of variance (ANOVAs), with treatment site (acute care, nursing home, chronic care) as the between-groups factor. We performed post hoc tests with the Scheffé procedure. We computed Pearson product-moment correlations between the different domains of functioning, and we used regression analyses to identify the specific predictors of adaptive functioning deficits as measured by scores on the Social Adaptive Functioning Evaluation scale. We computed these analyses for the group as a whole and repeated them for each of the three subgroups separately. We used discriminant function analyses to identify the best discriminators of the three groups of patients, comparing each group against the other two.
RESULTS
Between-Group Analyses
Demographic differences. Demographic characteristics of the patients are presented in table 1. ANOVAs revealed significant differences in age and education among the three groups. According to Scheffé post hoc follow-up tests, the nursing home patients were older than the other two groups, and the acutely admitted patients were better educated. There were no significant differences in age at first psychiatric admission across the three groups. We found significant differences in conventional neuroleptic, but not risperidone, dose; the acutely admitted patients were receiving significantly higher dosages of conventional antipsychotic medication than the other two groups were.
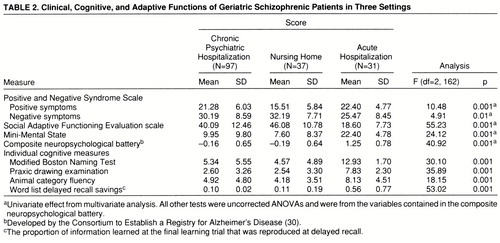
Cognitive, clinical, and adaptive symptoms. Scores on all of the cognitive, clinical, and adaptive functioning measures are presented in table 2. In previous studies (34, 35), the measures in the cognitive battery, other than the total scores on the Mini-Mental State, were standardized within the groups of schizophrenic patients and averaged into a single composite z score because of the high correlation between the different measures. In this study, we examined the pattern of intercorrelation between the cognitive measures in order to determine if standardization would be required. The lowest correlation between any two of the cognitive measures in any of the groups occurred between delayed-recall scores and Boston naming scores among the acutely ill patients: r=0.48, df=30, p<0.05. This correlation again suggests considerable overlap between the measures. During the standardization, we weighted the data from the inpatient group to reflect an N of 35 so that the different groups would contribute similarly to standardization. We used this single composite as a dependent measure.
The overall MANOVA was statistically significant (Wilks's lambda=0.35, Rao's R=21.75, df=10,316, p<0.001). The multivariate-corrected one-way ANOVAs revealed significant differences among groups for all five of the domains of functioning. According to post hoc Scheffé tests, the acutely ill and the chronically hospitalized patients had significantly higher severity scores than the nursing home patients on the positive symptom subscale of the Positive and Negative Syndrome Scale; furthermore, there were no differences between the severity scores of the acute and the chronic groups. For the other four variables, the pattern of between-groups differences was identical: in comparison with the acutely ill patients, nursing home and chronically hospitalized patients had significantly more severe negative symptoms, significantly lower scores on the Mini-Mental State, significantly greater adaptive impairment, and significantly more cognitive deficit on the composite neuropsychological measure.
Since the groups differed in age and educational status, we computed correlations between the five domains of functioning and these two demographic variables. For both age and level of education, we found significant correlations for Mini-Mental State scores, Social Adaptive Functioning Evaluation total scores, the composite cognitive battery, and Positive and Negative Syndrome Scale negative symptoms (all r values >0.30, all p values <0.05). As a result, we repeated the between-group ANOVAs for those four analyses with analysis of covariance (ANCOVA); age and education were the covariates. For all four variables, the covariate effect was significant, all F values >8.21, df=2,163, all p values >0.005. Only one of the four analyses was affected by the ANCOVA, with the between-groups differences in negative symptoms (Positive and Negative Syndrome Scale) reduced to a nonsignificant result (F=1.21, df=2,163, p=0.30).
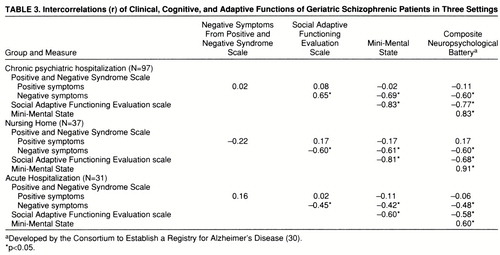
Correlational analyses. Correlations among the five cognitive, clinical, and functional dependent variables within each of the three subject groups are presented in table 3. The pattern of correlations was similar in each group: positive symptom severity was much less strongly related to all of the other four variables, which were all strongly intercorrelated. In each of the three groups, we used a regression analysis to predict adaptive impairments as measured by the Social Adaptive Functioning Evaluation scale; we entered simultaneously as predictors three of the four other variables—positive and negative symptoms (Positive and Negative Syndrome Scale) and the composite score for the cognitive battery. We did not enter the Mini-Mental State scores because of their high level of correlation and conceptual overlap with the composite score on the cognitive battery. For the chronic patients, the overall regression was significant, R2=0.70, F=75.59, df=3,93, p<0.001. We then repeated the analysis with a stepwise procedure to determine order of entry. All three variables entered the equation: the composite cognitive score accounted for 62% of the variance (F=153.94, df=1,95); negative symptoms accounted for an incremental 6% of the variance (F=23.03, df=2,94); and positive symptoms accounted for 2% of the variance (F=5.02, df=3,93). For the acute patients, the overall simultaneous regression was also significant (R2=0.22, F=4.01, df=3,27, p<0.05). When we repeated this analysis with stepwise entry, the only variable that entered the analysis was the composite cognitive functioning score (R2=0.21, F=7.01, df=1,29, p<0.02). Finally, in the regression analysis for the nursing home patients, the overall simultaneous entry analysis was also significant (R2=0.56, F=13.80, df=3,33, p<0.001). Two variables entered the equation in the stepwise analysis at p<0.05: cognitive functioning composite scores, accounting for 50% of the variance (F=34.74, df=1,35), and negative symptoms, accounting for 4% of the variance (F=3.76, df=2,34).
Between-Group Discrimination
As in the regression analyses, we did not enter the Mini-Mental State scores as a predictor because of their high overlap with the composite score from the cognitive battery. As we had also done in the regression analyses, we entered all variables simultaneously into the discriminant equation, which was then repeated with a stepwise procedure. In the first discriminant analysis, we compared the chronically hospitalized patients to the acutely admitted patients. The overall discriminant analysis was significant (F=38.69, df=4,124, p<0.001). Three variables entered the stepwise equation: total scores on the Social Adaptive Functioning Evaluation scale (F=81.37, df=1,127), followed by negative symptoms (F=28.71, df=2,126), followed by the cognitive composite score (F=9.82, df=3,126). Overall classification accuracy was 93%, with 96% of the chronic and 83% of the acute patients accurately classified. The second discriminant analysis compared the chronically hospitalized patients and the nursing home patients, also revealing a significant overall effect (F=9.45, df=4,130, p<0.001). Two variables entered the stepwise equation: positive symptoms (F=21.62, df=1,131) and the cognitive composite score (F=12.46, df=2,132). Correct classification was 79% overall, with 94% of the chronic patients correctly classified (21% improvement on chance) and 41% of the nursing home patients correctly classified (13% improvement on chance). Finally, when we compared nursing home patients and acute patients, the overall analysis was significant (F=43.01, df=4,64, p<0.001). Total scores on the Social Adaptive Functioning Evaluation scale were the only significant (F=58.19, df=1,67, p<0.001) predictor in the stepwise analysis, with correct classification accuracy at 95% overall. This accuracy was based on 100% classification accuracy in the nursing home group and 92% accuracy for the acute patients.
DISCUSSION
When geriatric patients with lifelong schizophrenia are compared across treatment sites, specific differences among the groups of patients can be identified. Acutely ill patients with a lifetime history of living in the community have less severe deficits in adaptive functioning, better cognitive functioning, and less severe negative symptoms than patients with a poor outcome. At the same time, among patients with schizophrenia, positive symptoms of acutely ill patients are no more severe than those of chronically hospitalized patients. Among poor outcome patients with unremitting illness, the major discriminator between those who are still residents of chronic psychiatric hospitals and those who have been previously referred to nursing homes is that among the former group, the severity of positive symptoms is elevated. Finally, the adaptive deficits seen in nursing home patients are so profound that they represent the only factor that independently discriminates nursing home patients from acutely ill patients. For patients with either persistent or episodic symptoms of schizophrenia, the severity of cognitive impairments was a stronger predictor of adaptive deficits than was the severity of either positive or negative schizophrenia symptoms. Thus, the correlation between cognitive deficit and adaptive deficit holds up, regardless of the relative severity of these different domains of schizophrenia, the outcome of the illness, or the site where the patients receive care. Consistent with earlier cross-sectional results (e.g., reference 36), these data suggest that adaptive-functioning deficit is correlated with overall functional status and that cognitive impairment is an important concurrent correlate of deficits in adaptive skills.
Before providing our interpretation of these results, we must present the limitations of the study. The acutely ill patients were all men who had a history of multiple psychiatric treatments prior to the current study. For the nursing home residents, the results may not generalize to patients who had not been chronically hospitalized before nursing home referral. Variations in education status and gender cannot be controlled with analysis of covariance because they are intrinsic features of better and worse outcome patients, although they do not appear to account completely for the cross-sectional group differences that we found. Another issue is the symptomatic status of the better outcome patients, who were examined while psychotic and admitted to inpatient psychiatric care. An assessment of more stable patients might have found different patterns of between-group differences. Finally, in a previous longitudinal study of patients referred to nursing home care (18), the results indicated that hostility and aggressiveness are powerful predictors of which patients will be difficult to refer from chronic psychiatric care to nursing homes.
In this study, the results underscore the importance of cognitive functioning in the outcome of schizophrenia. Cognitive impairment proved to be a correlate of the overall functional status of schizophrenic patients; the degree of impairment predicted whether the patients would require chronic treatment (in either nursing homes or psychiatric hospitals) or whether they would be able to live in the community. When the influence of other variables was statistically eliminated, the level of cognitive impairment also significantly discriminated between the poor outcome patients who were chronically hospitalized and those who lived in nursing homes. This difference is relatively small, however, and may not be clinically significant. In contrast, the magnitude of difference between the acutely admitted and the chronically hospitalized patients on the cognitive variables (scores on the Mini-Mental State and the components of the composite neuropsychological battery) was close to two standard deviations, whereas there was less difference between the two groups in negative symptoms and positive symptoms. Furthermore, negative symptoms did not differ across the groups overall when we considered covariate effects. When the correlates of adaptive functioning impairment, a key indicator of life success across neuropsychiatric conditions (37–39), are identified, the results are consistent regardless of overall functional status. These data suggest that outcome in geriatric patients with schizophrenia has the same pattern of predictors as in younger patients (40).
Although of all the variables studied, cognitive impairment accounts for more of the variance in adaptive skills deficits, there are clearly additional factors that are correlated with overall functional status. The most obvious is chronic institutionalization itself. While severe adaptive impairment may necessitate lengthy hospital stays, chronic institutionalization may, in turn, have a direct adverse effect on adaptive functioning. Previous investigators of community placement of chronically hospitalized patients have found improvements in some of the symptoms of the illness after discharge from chronic psychiatric care (15). All of the poor outcome patients in this study are also largely refractory to conventional antipsychotic treatment. Extended periods of severe and unremitting psychotic symptoms may have negative effects on the outcome of the illness (41), possibly through initiation of specific neurodegenerative processes (42). Although the direct effects of chronic institutionalization and treatment nonresponse are difficult to quantify, their impact cannot be ignored.
These data also make a methodological point about the assessment of cognition in patients with schizophrenia. It is a common belief that positive symptoms of schizophrenia interfere with the assessment of cognitive functioning. In this study, good outcome patients in a phase of acute exacerbation manifested positive symptoms equivalent in severity to those seen in chronically hospitalized patients; however, they performed better on cognitive measures by an average of one to two standard deviations. Thus, our finding of equal severity of positive symptoms in groups who differed markedly in their levels of cognitive impairment suggests that the cognitive impairments in the chronically hospitalized group are not likely to be a result of their severe positive symptoms alone.
A principal implication of these findings is that treatment of cognitive impairment in schizophrenia should be a focus of intervention (43, 44). While the existence of correlation between cognitive and adaptive deficits does not prove that remediation of cognitive functioning would improve adaptive functioning, specific cognitive deficits predict failure to benefit from interventions aimed at adaptive functioning (39). Researchers should test pharmacological and behavioral interventions aimed specifically at cognitive impairment for their ability 1) to directly affect adaptive impairment and 2) to enhance the effectiveness of behavioral interventions aimed specifically at deficits in adaptive skills. Such interventions may offer hope for altering the overall outcome of schizophrenia.
 |
 |
 |
Received July 25, 1997; revisions received Nov. 10, 1997, and March 2, 1998; accepted March 24, 1998. From the Departments of Psychiatry, Mount Sinai School of Medicine and Franklin Delano Roosevelt Veterans Administration Medical Center, Montrose, N.Y.; and the Pilgrim Psychiatric Center, West Brentwood, N.Y. Address reprint requests to Dr. Harvey, Department of Psychiatry, Box 1229, Mount Sinai School of Medicine, New York, NY 10029; [email protected] (e-mail). Supported by NIMH grant MH-46436 to Dr. Davidson and by the recruitment and assessment core of the Mount Sinai Mental Health Clinical Research Centers. The authors thank Janice McCrystal, Stephanie Bowler, Rita Ohsiek, Susan Frick, and Cynthia Blum for their contributions to this study.
1 Stiles PG, Culhane DP, Hadley TR: Old and new: a comparison of state psychiatric hospitals. Psychiatr Serv 1996; 47:866–868Link, Google Scholar
2 Hegarty JD, Baldessarini RJ, Tohen M, Waternaux C, Oepen G: One hundred years of schizophrenia: a meta-analysis of the outcome literature. Am J Psychiatry 1994; 151:1409–1416Link, Google Scholar
3 Talbott J: The care of the chronic mentally ill, deinstitutionalization, and homelessness in America. Psychiatrica Hungarica 1992; 7:615–626Google Scholar
4 Minkin EB, Stoline AM, Sharfstein SS: An analysis of the two-class system of care in private psychiatric hospitals. Hosp Community Psychiatry 1994; 45:975–977Abstract, Google Scholar
5 Fisher WH, Simon L, Geller JL, Penk WE: Case mix in the “downsizing” state hospital. Psychiatr Serv 1996; 47:255–262Link, Google Scholar
6 Davidson M, Harvey PD, Powchik P, Parrella M, White L, Knobler HY, Losonczy MF, Keefe RSE, Katz S, Frecksa E: Severity of symptoms in chronically institutionalized geriatric schizophrenic patients. Am J Psychiatry 1995; 152:197–207Link, Google Scholar
7 Lamb RH, Shaner R: When there are almost no state hospital beds left. Hosp Community Psychiatry 1993; 44:973–976Abstract, Google Scholar
8 Goldman HH, Feder J, Scanlon W: Chronic mental patients in nursing homes: reexamining data from the National Nursing Home Survey. Hosp Community Psychiatry 1986; 37:269–272Abstract, Google Scholar
9 Lamberti JS, Tariot PN: Schizophrenia in nursing home patients. Psychiatr Annals 1995; 25:441–448Crossref, Google Scholar
10 Burns BJ, Wagner HR, Taube JE, Magaziner J: Mental health service use by the elderly in nursing homes. Am J Public Health 1993; 83:331–337Crossref, Medline, Google Scholar
11 Meeks S, Walker J: Blunted affect, blunted lives? negative symptoms, ADL functioning, and mental health among older adults. Int J Geriatr Psychiatry 1990; 5:233–238Crossref, Google Scholar
12 Moak GA, Fisher WS: Geriatric patients and services in state hospitals: data from a national survey. Hosp Community Psychiatry 1991; 42:273–276Abstract, Google Scholar
13 Harding CM, Brooks GW, Ashikaga T, Strauss JS, Breier A: The Vermont longitudinal study of persons with severe mental illness, II: long-term outcome of subjects who retrospectively met DSM-III criteria for schizophrenia. Am J Psychiatry 1987; 144:727–735Link, Google Scholar
14 Harding CM, Zubin J, Strauss JS: Chronicity in schizophrenia: revisited. Br J Psychiatry 1992; 161(Oct suppl 18):27–37Google Scholar
15 Leff JP, Thornicroft G, Coxhead N, Crawford C: The TAPS Project, 22: a five-year followup of long stay psychiatric patients discharged to the community. Br J Psychiatry 1994; 165(suppl 25):13–17Google Scholar
16 Arnold SE, Gur RE, Shapiro RM, Fisher KR, Moberg PJ, Gibney MR, Gur RC, Blackwell P, Trojanowski JQ: Prospective clinicopathologic studies of schizophrenia: accrual and assessment of patients. Am J Psychiatry 1995; 152:731–737Link, Google Scholar
17 Bartels SJ, Mueser KT, Miles KM: Functional impairments in elderly patients with schizophrenia and major affective disorders living in the community: social skills, living skills, and behavior problems. Behavior Therapy 1997; 28:43–63Crossref, Google Scholar
18 White L, Parrella M, McCrystal-Simon J, Masiar S, Harvey PD, Davidson M: Characteristics of elderly psychiatric patients retained in a state hospital during downsizing: a prospective study with replication. Int J Geriatr Psychiatry 1997; 12:474–480Crossref, Medline, Google Scholar
19 Trieman N, Leff J: Difficult to place patients in a psychiatric closure programme: the TAPS Project 24. Psychol Med 1996; 26:765–774Crossref, Medline, Google Scholar
20 Palmer BW, Heaton RK, Paulsen JS, Kuck J, Braff D, Harris MJ, Zisook S, Jeste D: Is it possible to be schizophrenic and neuropsychologically normal? Neuropsychology 1997; 11:437–447Google Scholar
21 Jeste DV, Harris MJ, Krull A, Kuck J, McAdams LA, Heaton R: Clinical and neuropsychological characteristics of patients with late-onset schizophrenia. Am J Psychiatry 1995; 152:722–730Link, Google Scholar
22 Klapow JC, Evans J, Patterson TL, Heaton RK, Koch WL, Jeste DV: Direct assessment of functional status in older patients with schizophrenia. Am J Psychiatry 1997; 154:1022–1024Link, Google Scholar
23 Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris MJ, Jeste DV: Neuropsychological deficits in schizophrenics: relationship to age, chronicity, and dementia. Arch Gen Psychiatry 1994; 51:469–476Crossref, Medline, Google Scholar
24 Harvey PD, Lombardi J, Leibman M, Parrella M, White L, Powchik P, Mohs RC, Davidson M, Davis KL: Age-related differences in formal thought disorder in chronically hospitalized schizophrenic patients: a cross-sectional study across nine decades. Am J Psychiatry 1997; 154:205–210Link, Google Scholar
25 Davidson M, Harvey P, Welsh KA, Powchik P, Putnam KM, Mohs RC: Cognitive functioning in late-life schizophrenia: a comparison of elderly schizophrenic patients and patients with Alzheimer's disease. Am J Psychiatry 1996; 153:1274–1279Link, Google Scholar
26 Kay SR: Positive and Negative Syndromes in Schizophrenia. New York, Brunner/Mazel, 1991Google Scholar
27 Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
28 Harvey PD, Davidson M, Powchik P, Parrella M, White L, Mohs RC: Assessment of dementia in elderly schizophrenics with structured rating scales. Schizophr Res 1992; 7:85–90Crossref, Medline, Google Scholar
29 Harvey PD, White L, Parrella M, Putnam KM, Kincaid MM, Powchik P, Mohs RC, Davidson M: The longitudinal stability of cognitive impairment in schizophrenia: Mini-Mental State scores at one and two year follow-ups in geriatric inpatients. Br J Psychiatry 1995; 166:630–633Crossref, Medline, Google Scholar
30 Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C: The Consortium to Establish a Registry for Alzheimer's Disease (CERAD), part I: clinical and neuropsychological assessment of Alzheimer's disease. Neurology 1989; 39:1159–1165Crossref, Medline, Google Scholar
31 Harvey PD, Lombardi J, Leibman M, Parrella W, White L, Mohs RC, Davidson M: Performance of chronic schizophrenic patients on cognitive neuropsychological measures sensitive to dementia. Int J Geriatr Psychiatry 1996; 11:621–627Crossref, Google Scholar
32 Harvey PD, Davidson M, Mueser K, Parrella M, White L, Powchik P: The Social Adaptive Functioning Evaluation: an assessment measure for geriatric psychiatric patients. Schizophr Bull 1997; 23:131–146Crossref, Medline, Google Scholar
33 Rao CR: An asymptotic expansion of the distribution of Wilks lambda. Bull Int Statistical Institute 1951; 33:177–181Google Scholar
34 Harvey PD, Lombardi J, Leibman M, White L, Parrella M, Powchik P, Davidson M: Cognitive impairment and negative symptoms in schizophrenia: a prospective study of their relationship. Schizophr Res 1996; 22:223–231Crossref, Medline, Google Scholar
35 Harvey PD, Sukhodolsky D, Parrella M, White L, Davidson M: The association between cognitive and adaptive deficits in geriatric chronic schizophrenic patients. Schizophr Res 1997; 27:211–218Crossref, Medline, Google Scholar
36 Perlick D, Mattis S, Stastny P, Teresi J: Neuropsychological discriminators of long-term inpatient or outpatient status in chronic schizophrenia. J Neuropsychiatry Clin Neurosci 1992; 4:428–434Crossref, Medline, Google Scholar
37 Heaton RK, Pendleton MG: Use of neuropsychological tests to predict patients' everyday functioning. J Consult Clin Psychol 1981; 49:807–821Crossref, Medline, Google Scholar
38 Mortimer JA, Ebbitt B, Jun AP: Predictors of cognitive and functional progression in patients with probable Alzheimer's disease. Neurology 1992; 42:1689–1696Crossref, Medline, Google Scholar
39 Green CR, Mohs RC, Schmeidler J, Lawlor B, Ryan T, Davis KL: Functional decline in Alzheimer's disease: a longitudinal study. J Am Geriatr Soc 1993; 41:654–661Crossref, Medline, Google Scholar
40 Green MF: What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry 1996; 153:321–330Google Scholar
41 Wyatt RJ: Neuroleptics and the natural course of schizophrenia. Schizophr Bull 1991; 17:325–351Crossref, Medline, Google Scholar
42 Olney JW, Farber NB: Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 1995; 52:998–1007Crossref, Medline, Google Scholar
43 Davidson M, Keefe RSE: Cognitive impairment as a target for pharmacological treatment in schizophrenia. Schizophr Res 1995; 17:123–129Crossref, Medline, Google Scholar
44 Harvey PD, Keefe RSE: Cognitive impairment and the implications of atypical neuroleptic treatment. CNS Spectrums 1997; 2:41–55Google Scholar



