Sex-Specific Distributed White Matter Microarchitectural Alterations in Preadolescent Youths With Anxiety Disorders: A Mega-Analytic Study
Abstract
Objective:
Anxiety disorders are among the most common psychiatric disorders in youths and emerge during childhood. This is also a period of rapid white matter (WM) development, which is critical for efficient neuronal communication. Previous work in preadolescent children with anxiety disorders demonstrated anxiety disorder–related reductions in WM microstructural integrity (fractional anisotropy [FA]) in the uncinate fasciculus (UF), the major WM tract facilitating prefrontal cortical–limbic structural connectivity. Importantly, this association was found only in boys with anxiety disorders. To confirm this finding and more comprehensively understand WM changes in childhood anxiety, this mega-analytic study characterizes WM alterations related to anxiety disorders and sex in the largest sample of preadolescent children to date.
Methods:
Diffusion tensor imaging data from published studies of preadolescent children with anxiety disorders and healthy volunteers (ages 8–12) (N=198) were combined with a new data set (N=97) for a total sample of 165 children with anxiety disorders and 132 healthy volunteers. Children with anxiety disorders met DSM-5 criteria for current generalized, separation, and/or social anxiety disorder. Analyses of tractography and voxel-wise data assessed between-group differences (anxiety disorder vs. healthy volunteer), effects of sex, and their interaction.
Results:
Tract-based and voxel-wise analyses confirmed a significant reduction in UF FA in boys but not girls with anxiety disorders. Results also demonstrated other significant widespread anxiety disorder–related WM alterations specifically in boys, including in multiple commissural, association, projection, and brainstem regions.
Conclusions:
In addition to confirming male-specific anxiety disorder–related reductions in UF FA, the results demonstrate that anxiety disorders in boys and not girls are associated with broadly distributed WM alterations across the brain. These findings support further studies focused on understanding the extent to which WM alterations in boys with anxiety disorders are involved in pathophysiological processes that mediate anxiety disorders. The findings also suggest the possibility that WM microarchitecture could serve as a novel treatment target for childhood anxiety disorders.
Anxiety disorders emerge in childhood and early adolescence and are among the most common psychiatric illnesses in this age group (1, 2). Youths with anxiety disorders, such as generalized, separation, and social anxiety disorder, suffer from significant impairment and face increased risk for additional stress-related psychopathology (3). A more in-depth understanding of the pathophysiological processes underlying childhood anxiety disorders will provide rationales for novel treatment targets in children with anxiety disorders. Our work and that of others has identified alterations in white matter (WM) pathways in children and adults with anxiety disorders (4–9), which is of interest because WM plays an important role in mediating optimal neuronal communication. It is important to recognize that WM is modifiable, as preclinical and clinical studies demonstrate that WM and myelin dynamically change with development and in response to environmental factors, including behavioral and pharmacological interventions (10–14).
Prefrontal-limbic dysfunction is thought to underlie the pathophysiological processes associated with anxiety disorders (15). In a previous study (4), we found anxiety disorder–related alterations in the WM tract that connects prefrontal cortical regulatory regions to limbic regions (i.e., the uncinate fasciculus [UF]) in youths with anxiety disorders. Of particular interest is that these UF alterations appeared to occur selectively in boys and not girls. In further support of this finding, our nonhuman primate studies in anxious young monkeys showed a similar sexually dimorphic effect (16). The absence of apparent UF alterations in girls does not obviate the possibility of prefrontal cortical–limbic involvement in pathological anxiety. The UF fractional anisotropy (FA) effects could be present in girls but less robust than in boys and, therefore, not detectable in the sample size analyzed in our previous study. Also, it is possible that boys and girls differ in the prefrontal cortical–limbic mechanisms that underlie the expression of pathological anxiety. In addition to the sexually dimorphic anxiety disorder–related effects in UF WM, lower FA was observed in other brain regions in both boys and girls with anxiety disorders, including regions such as the corpus callosum, inferior fronto-occipital fasciculus, and internal capsule (4). Only one study has examined WM microstructural integrity in adolescents with anxiety disorders (6). While sexually dimorphic effects were not reported, that study in adolescents with generalized anxiety disorder also found FA reductions in the UF as well as in the inferior fronto-occipital fasciculus, inferior longitudinal fasciculus, and corona radiata.
Given the small number of youths in whom the relation between WM integrity and anxiety disorders has been examined, we performed a mega-analysis across our studies to maximize the sample size and enhance confidence in the findings. A particular focus was to further explore the extent to which anxiety disorder–related WM alterations are sexually dimorphic. Understanding sex-related differences in the pathophysiology of anxiety disorders may be helpful in explaining the increased risk faced by girls, as with puberty, girls are twice as likely as boys to develop anxiety disorders (17). The present analysis includes data from the two largest cross-sectional diffusion tensor imaging studies of pediatric anxiety disorders (4, 18) in addition to a newly collected sample, for a total of 163 children with anxiety disorders and 132 healthy volunteers (total N=295). Medication-free children with current diagnoses of generalized, separation, and/or social anxiety disorders were studied. We utilized tractography and voxel-wise approaches to assess anxiety disorder–related alterations in WM microstructure.
Methods
In this study, we combined data from three samples collected across a 9-year period. Samples 1 (4), 2 (18), and 3 were collected during the periods of July 2013–July 2015, December 2017–February 2020, and June 2019–March 2022, respectively.
Participants
Recruitment and clinical assessment.
Samples 1, 2, and 3 included 98, 100, and 97 participants, respectively, for a total of 295 preadolescent children included in the present study (163 with anxiety disorders, 132 healthy volunteers). Of the 295 participants, data from 103 children with anxiety disorders and 95 healthy volunteers (samples 1 and 2) have been published previously (4, 18). For samples 1 and 3, participants were enrolled at two sites: University of Wisconsin–Madison (UW) and the National Institute of Mental Health (NIMH). Sample 2 was acquired entirely at UW. Informed assent and consent were obtained from all participants and their parents, in accordance with the institutional review boards of UW and NIMH. Individuals were compensated for their time and effort.
For all samples, children were enrolled between the ages of 8 and 12 and underwent clinical, behavioral, developmental, and neuroimaging assessments. Diagnostic assessment included the Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL) interview (19), administered by a child psychologist or psychiatrist or by a trained study team member under the supervision of a child psychologist or psychiatrist. All cases were reviewed by two authors (N.H.K. and D.S.P.) prior to inclusion in the study. Based on K-SADS-PL evaluation, participants were categorized as either healthy volunteers or as having an anxiety disorder. Children in the anxiety disorder group met DSM-5 criteria for current generalized, separation, and/or social anxiety disorder; healthy volunteers did not meet criteria for any past or current DSM-5 disorder other than past adjustment disorder. Our study focused on generalized, social, and separation anxiety disorders because preadolescent anxiety most commonly presents with admixtures of symptoms of these three disorders, and they are highly comorbid with one another (20, 21). A similar approach has been taken for large-scale treatment studies related to childhood anxiety disorders (22, 23), as well as many neuroimaging studies focused on childhood anxiety disorders (24). Children’s anxiety symptoms were rated by both child and parent using the Screen for Child Anxiety and Related Emotional Disorders (25). Across the three samples, participants also provided self-report measures of depression, using either the Child Depression Inventory (N=193) (26) or the Mood and Feelings Questionnaire (N=93) (27), and of externalizing symptoms, using the Conners’ Parent Rating Scale–Revised (28). The Childhood Opportunity Index—a census-derived composite metric of neighborhood-level education, health and environment, and socioeconomic conditions that relates to several health outcomes (29, 30)—was used in a subanalysis in the 230 participants for whom data from this index were available, to account for potential differences in children’s environment (see the online supplement).
The majority of participants had never been treated for anxiety or any other psychiatric disorder, and all had been treatment free for a minimum of 6 months at the time of the study. Major exclusion criteria included psychotropic medication use, severe psychopathology in need of immediate treatment, and comorbid diagnoses of major depressive disorder, obsessive-compulsive disorder, posttraumatic stress disorder, autism spectrum disorder, bipolar disorder, or schizophrenia. Children in the anxiety disorder group with comorbid diagnoses of attention deficit hyperactivity disorder (ADHD) and/or oppositional defiant disorder were eligible to be included, provided that the ADHD or oppositional defiant disorder symptoms were less severe than anxiety disorder symptoms.
MRI Data Acquisition and Processing
Diffusion tensor imaging (DTI) acquisition.
At both sites (UW and NIMH), brain images were collected on a 3.0-T GE MR750 scanner (GE Healthcare; Waukesha, Wisc.). Diffusion-weighted MRI scans were obtained using a two-dimensional echo planar imaging diffusion-weighted spin-echo sequence with 48 optimal non-collinear directions and eight non-diffusion weighted images (see the online supplement).
DTI processing, harmonization, and analysis.
Image processing steps were identical to those outlined in our previous publications (4, 18) (see the online supplement). Briefly, the FMRIB Software Library (FSL) tools for rigid registration were used to correct diffusion-weighted images for distortions resulting from head motion and eddy currents (31). Images were normalized across all participants to create a study-specific template that was then warped via rigid, affine, and diffeomorphic (i.e., nonlinear) transformations to an MNI-152 standard space structural template. Individual tensor maps were generated in MNI space. Deterministic tractography was performed in TrackVis (32) to delineate seven bilateral tracts of interest across the brain, including the UF, corpus callosum, cingulum, internal capsule, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, and stria terminalis/fornix, which were selected based on reports suggesting their involvement in the pathophysiology of anxiety disorders and related disorders in both youth and adult cohorts (6, 8, 33–38). An FA threshold of 0.1 was used for fiber tracking, based on our previous work and given the developmental nature of our work (4, 16, 18). For each image, a weighted means approach was used to calculate each diffusion metric (FA, mean diffusivity, radial diffusivity, axial diffusivity) per tract, which down-weights the contribution of voxels with relatively low numbers of fibers in each tract (see the online supplement). Right and left hemisphere metrics were averaged for bilateral tracts given no a priori hypotheses related to laterality. Prior to analysis, tract-based and voxel-wise DTI data were harmonized to account for differences in site (UW vs. NIMH), sample (1 vs. 2 vs. 3), and head coil (8- vs. 32-channel), using the neuroCombat program in R (39, 40) and the neuroHarmonize program in Python (41, 42), respectively (see the online supplement). Whole-brain WM voxel-wise analyses were performed using the randomize program in FSL to assess anxiety disorder–related WM effects across the brain. Input data for both analyses consisted of normalized FA images in standard MNI space.
Sex Hormone Collection and Analyses
Details on sex hormone (testosterone and estradiol) collection and analyses are provided in the Supplemental Methods section in the online supplement.
Statistical Analysis
Tract-based DTI analyses.
Multiple regression was used to assess the relationship between harmonized tract DTI metrics and group (healthy volunteer vs. anxiety disorder). FA was considered the primary metric of interest, and mean diffusivity, radial diffusivity, and axial diffusivity were secondary measures meant to inform the interpretation of the FA results. The following model estimated the main effects of group and sex, as well as their interaction, in relation to a given tract DTI metric, while covarying for age:

For the tract-based DTI analyses, we used a Bonferroni-adjusted p value when determining statistical significance to correct for multiple comparisons (seven WM tracts; corrected p<0.05/7=0.00714). Multiple regression modeling was performed using the stats and lmSupport packages in RStudio, version 2022.02.1.
Voxel-wise DTI analyses.
In the voxel-wise analysis of harmonized DTI parameter maps, general linear models using permutation methods were implemented with the FSL randomize tool (43) to estimate anxiety disorder–related differences in FA—and in mean diffusivity, radial diffusivity, and axial diffusivity as secondary measures—across whole-brain WM, as well as the moderating role of sex while controlling for age. Voxel-wise analyses were restricted to a liberal WM mask (see the online supplement). Using threshold-free cluster enhancement (TFCE), results were assessed at a family-wise error (FWE)–corrected threshold of p<0.05.
Results
Demographic and Clinical Characteristics
The combined sample consisted of 295 participants, of whom 163 had anxiety disorders and 132 were healthy volunteers (Table 1). Sex was reported by the parent at the time of study enrollment; 94 were male and 201 were female. Participants self-reported their race and ethnicity; 221 (74.9%) were White, 33 (11.2%) were multiracial, 23 (7.8%) were Black, nine (3.1%) were Asian, six (2.0%) were unknown, two (0.7%) were Native American, and one (0.3%) was other. Racial and ethnic distributions did not differ significantly between children with anxiety disorders and healthy volunteers (race: χ2=2.33, df=6, p=0.89; ethnicity: χ2=2.20, df=2, p=0.33). In the subset of participants for whom Childhood Opportunity Index data were available (N=230), the mean rating was 0.035 standard deviations above the national mean, and ratings did not differ significantly by sex or group or show a group-by-sex interaction (all p values >0.1). The mean age across the combined sample was 10.56 years, and age did not differ significantly between children with anxiety disorders and healthy volunteers (t=0.44, df=293, p=0.66). Physical development, as assessed by Tanner stage, did not differ significantly by group or sex and did not show a group-by-sex interaction (all p values >0.1). Additionally, sex distributions did not differ significantly between children with anxiety disorders and healthy volunteers (χ2=0.55, df=1, p=0.46).
| Characteristic or Measure | Full Sample | Girls | Boys | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Healthy Volunteer Group (N=132) | Anxiety Disorder Group (N=163) | Healthy Volunteer Group (N=87) | Anxiety Disorder Group (N=114) | Healthy Volunteer Group (N=45) | Anxiety Disorder Group (N=49) | |||||||
| | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Age (years) | 10.53 | 1.19 | 10.59 | 1.23 | 10.58 | 1.10 | 10.62 | 1.16 | 10.43 | 1.36 | 10.51 | 1.40 |
| IQ (WASI) | 116.71 | 13.02 | 113.85 | 14.57 | 115.06 | 13.98 | 113.35 | 14.95 | 119.82 | 10.40 | 114.96 | 13.78 |
| Tanner stage | 1.76 | 0.87 | 1.97 | 1.00 | 1.80 | 0.89 | 2.05 | 1.02 | 1.69 | 0.83 | 1.78 | 0.92 |
| COI (national z-scores) | 0.03 | 0.02 | 0.04 | 0.02 | 0.03 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.04 | 0.02 |
| | N | % | N | % | N | % | N | % | N | % | N | % |
| Race | | | | | | | | | | | | |
| Asian | 4 | 3.0 | 5 | 3.1 | 3 | 3.4 | 3 | 2.6 | 1 | 2.2 | 2 | 4.1 |
| Black | 13 | 9.8 | 10 | 6.1 | 9 | 10.3 | 7 | 6.1 | 4 | 8.9 | 3 | 6.1 |
| Multiracial | 14 | 10.6 | 19 | 11.7 | 10 | 11.5 | 17 | 14.9 | 4 | 8.9 | 2 | 4.1 |
| Native American | 1 | 0.8 | 1 | 0.6 | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 | 1 | 2.0 |
| Other | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 |
| Unknown | 3 | 2.3 | 3 | 1.8 | 3 | 3.4 | 2 | 1.8 | 0 | 0.0 | 1 | 2.0 |
| White | 97 | 73.5 | 124 | 76.1 | 61 | 70.1 | 84 | 73.7 | 36 | 80.0 | 40 | 81.6 |
| Ethnicity | | | | | | | | | | | | |
| Hispanic or Latino | 8 | 6.1 | 17 | 10.4 | 8 | 9.2 | 12 | 10.5 | 0 | 0.0 | 5 | 10.2 |
| Not Hispanic or Latino | 121 | 91.2 | 144 | 88.3 | 77 | 88.5 | 100 | 87.7 | 44 | 97.8 | 44 | 89.8 |
| Unknown | 3 | 2.3 | 2 | 1.2 | 2 | 2.3 | 2 | 1.8 | 1 | 2.2 | 0 | 0.0 |
| | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Parent-rated SCARED (anxiety)b | 4.35 | 5.11 | 29.59 | 11.31 | 4.18 | 4.41 | 30.06 | 10.80 | 4.67 | 6.30 | 28.52 | 12.44 |
| Child-rated SCARED (anxiety)b,c,d | 8.92 | 7.50 | 29.91 | 13.74 | 8.63 | 7.15 | 32.43 | 13.30 | 9.49 | 8.18 | 24.20 | 13.11 |
| CDI (N=193) (depression)b,c | 34.02 | 14.47 | 44.50 | 17.19 | 36.19 | 12.63 | 48.17 | 14.60 | 27.52 | 17.73 | 33.32 | 19.80 |
| MFQ (N=93) (depression)b | 0.26 | 0.51 | 5.79 | 4.51 | 0.43 | 0.65 | 6.09 | 4.90 | 0.14 | 0.36 | 5.38 | 3.94 |
| CPRS-R (ADHD)b,c | 44.40 | 3.77 | 58.99 | 11.95 | 45.32 | 3.88 | 59.95 | 12.87 | 42.51 | 2.73 | 56.79 | 9.29 |
TABLE 1. Demographic and clinical characteristics of children with anxiety disorders and healthy volunteersa
Children with anxiety disorders exhibited significantly higher symptom scores on all clinical measures compared with healthy volunteers, including on anxiety, depression, and externalizing symptoms (all p values <0.001) (see Table 1). Additionally, compared with boys, girls had significantly higher scores on child-rated anxiety, child-rated depression, and externalizing symptoms (all p values <0.01). Finally, a significant group-by-sex interaction was observed for child-rated anxiety (p<0.001), with post hoc tests indicating that girls with anxiety disorders had significantly higher scores compared with boys with anxiety disorders.
Anxiety-DTI Relations
Tractography-based results.
In our combined sample, we examined anxiety disorder–related differences in the WM microstructure of seven major WM tracts (UF, corpus callosum, cingulum, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, stria terminalis/fornix, and internal capsule), as described in the Methods section. As expected, FA increased with age in six of the seven tracts (p<0.05, corrected). A main effect of sex was found only in the cingulum (see Table S1 in the online supplement).
In relation to anxiety disorder–related associations, there was a main effect of group for UF FA (t=−2.86, df=290, p=0.005), such that children with anxiety disorders exhibited significantly reduced FA compared with healthy volunteers (Figures 1 and 2). Significant group-by-sex interactions were observed for UF FA (t=3.08, df=290, p=0.002) and inferior fronto-occipital fasciculus FA (t=2.98, df=290, p=0.003), such that the anxiety disorder–related reductions in FA occurred predominantly in boys (see Figures 1 and 2). Separate analyses of the boys and girls revealed significant anxiety disorder–related FA reductions in the boys and not the girls. When comparing boys with anxiety disorders to healthy volunteer boys, in addition to anxiety disorder–related FA reductions in the UF (t=−3.60, df=91, p<0.001) and inferior fronto-occipital fasciculus (t=−3.06, df=91, p=0.003), we observed significantly lower FA in the corpus callosum for boys with anxiety disorders (t=−2.91, df=91, p=0.005) (see Figures 1 and 2). Inclusion of additional covariates, including sex hormones (testosterone, estradiol), Childhood Opportunity Index rating, depression, and externalizing symptoms, did not affect the anxiety disorder–FA associations (see Table S1 in the online supplement). Together, the results suggest a sex-specific and widespread pattern of reductions in WM microstructural integrity in children with anxiety disorders relative to healthy volunteers.
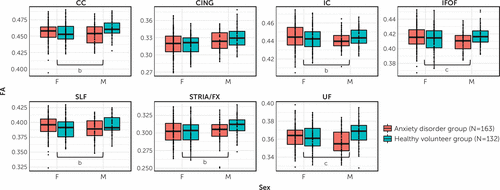
FIGURE 1. Group-by-sex interactions in relation to fractional anisotropy in seven bilateral white matter tractsa
aEach panel represents the group-by-sex interaction in relation to fractional anisotropy (harmonized values) in a given tract. CC=corpus callosum; CING=cingulum; FA=fractional anisotropy; IC=internal capsule; IFOF=inferior fronto-occipital fasciculus; SLF=superior longitudinal fasciculus; STRIA/FX=stria terminalis/fornix; UF=uncinate fasciculus.
bGroup-by-sex interaction significant at the uncorrected threshold (p<0.05, uncorrected).
cGroup-by-sex interaction significant at the Bonferroni-corrected threshold (p<0.05, corrected).
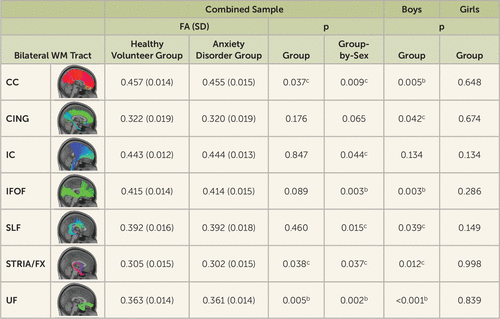
FIGURE 2. Tract-level analysis of group differences and group-by-sex interactions in the fractional anisotropy of seven bilateral white matter tractsa
aAll analyses reflect harmonized data and include age as a covariate. CC=corpus callosum; CING=cingulum; FA=fractional anisotropy; IC=internal capsule; IFOF=inferior fronto-occipital fasciculus; SLF=superior longitudinal fasciculus; STRIA/FX=stria terminalis/fornix; UF=uncinate fasciculus; WM=white matter.
bStatistically significant at the Bonferroni-corrected threshold (p<0.05, corrected).
cStatistically significant at the uncorrected threshold (p<0.05, uncorrected).
Tract-based analyses of mean diffusivity, radial diffusivity, and axial diffusivity revealed no significant anxiety disorder–related effects or group-by-sex interactions (see Table S2 and Figure S2 in the online supplement). At the uncorrected level, significant effects of group were found for radial diffusivity in boys, such that for several tracts, boys with anxiety disorders exhibited increased radial diffusivity relative to healthy volunteer boys (see Table S2 in the online supplement).
Voxel-wise results.
Voxel-wise analyses revealed a main effect of group, demonstrating significant anxiety disorder–related FA reductions in clusters that overlapped with portions of the UF, corpus callosum, cingulum, internal capsule, inferior fronto-occipital fasciculus, corona radiata, external capsule, fornix, cerebral peduncles, inferior longitudinal fasciculus, and sagittal striatum (TFCE p<0.05, FWE corrected) (Figure 3A; see also Table S3 in the online supplement). Significant group-by-sex interactions were evident for FA in clusters that overlapped with portions of the UF, corpus callosum, internal capsule, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, cingulum, external capsule, corona radiata, superior fronto-occipital fasciculus, anterior commissure, anterior thalamic radiation, cerebral peduncles, corticospinal tract, medial lemniscus, cerebellar peduncles, sagittal striatum, and inferior longitudinal fasciculus (TFCE p<0.05, FWE corrected) (Figure 3B; see also Table S3 in the online supplement). Separate analyses of the boys and the girls revealed no anxiety disorder–related effects in girls, whereas boys had significant FA reductions in WM regions across the brain (TFCE p<0.05, FWE corrected) (Figure 3C; see also Table S3 in the online supplement).
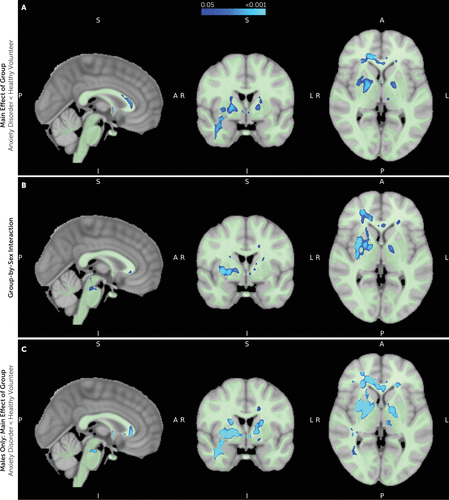
FIGURE 3. Voxel-wise analysis of group differences and group-by-sex interactions across whole-brain white matter fractional anisotropya
aAll analyses reflect harmonized data and include age as a covariate. All three panels show sagittal, coronal, and transverse views at MNI coordinates 92, 126, 74). Results shown are using threshold-free cluster enhancement (TFCE) and are corrected for multiple comparisons using the family-wise error rate (p<0.05, FWE corrected). Panel A shows voxels in which fractional anisotropy (FA) is significantly reduced in children with anxiety disorders compared with healthy volunteers across the combined sample. Panel B shows voxels in which there is a significant group-by-sex interaction in relation to FA. Panel C shows voxels, among the males alone, in which FA is significantly reduced in boys with anxiety disorders compared with healthy volunteer boys. In analyses of females alone, there were no significant differences in FA between those with anxiety disorders and healthy volunteers. In all panels, the binary white matter mask used in the voxel-wise analyses is underlaid in light green.
Voxel-wise analyses of radial diffusivity, mean diffusivity, and axial diffusivity were also performed, and results are presented in the online supplement.
Discussion
In this mega-analytic study comparing preadolescent children with anxiety disorders to healthy volunteers, we combined data from two published samples from our laboratories (4, 18) with data from a new sample of 97 children. Our aim was to increase confidence in the previous findings by maximizing the sample size for exploring the relationships among WM microstructural alterations, anxiety disorders, and sex in preadolescent children. We found 1) reduced UF FA in boys with anxiety disorders relative to healthy volunteer boys, consistent with our previous work (4); 2) beyond the UF, more broadly distributed WM reductions in boys with anxiety disorders; and 3) no evidence for anxiety disorder–related WM changes in preadolescent girls.
Findings from the tract-based and voxel-wise analyses demonstrated significantly decreased UF FA in boys with anxiety disorders and not girls with anxiety disorders. Anxiety disorder–related FA reductions were also observed in other WM tracts in boys, including association connections (UF, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, external capsule, sagittal striatum, inferior longitudinal fasciculus), commissural connections (corpus callosum), projection connections (corona radiata, internal capsule), and brainstem connections (corticospinal tract, cerebral peduncles, medial lemniscus). Among youths with anxiety disorders, radial diffusivity analyses also revealed findings in boys and not girls in many of the regions in which the FA alterations were detected in boys (see Figure S4 in the online supplement). Studies have demonstrated that DTI metrics can reflect different aspects of WM microstructure (44). FA values broadly reflect microstructural integrity (e.g., myelination, cellular density, and/or axonal coherence) (45, 46), whereas radial diffusivity, which is incorporated into FA, has been more consistently associated with changes in myelination (47, 48). Taken together, the FA and radial diffusivity findings suggest that the WM changes observed in boys with anxiety disorders could be due to alterations in myelination. These broadly distributed WM findings in boys with anxiety disorders generally overlap with and connect components of the neural circuitry that underlie anxiety (49, 50). For example, the UF is the primary WM tract linking prefrontal cortical and limbic regions, which are involved in the regulation and expression of anxiety, and the inferior fronto-occipital fasciculus facilitates prefrontal interactions with occipital and parietal regions thought to subserve functions relevant to emotion recognition and affective processing (51, 52). Considering the essential role of myelination in supporting efficient neuronal communication and neural network synchrony, it is possible that the reductions in WM microstructural integrity in these pathways, observed in boys with anxiety disorders, contribute to aberrant communication between and among key neural networks implicated in the pathophysiology of anxiety disorders.
DTI studies of individuals with anxiety disorders have generally reported findings linking anxiety to reduced FA across various WM regions throughout the brain. The majority of this work has been performed in adult populations (7–9, 34, 53–59). Importantly, very few studies have characterized WM alterations in youths with anxiety disorders (4, 6, 18). A single study has been performed in adolescents (6), and two in preadolescents (4, 18), both conducted in our laboratory. Studies examining WM alterations in relation to anxiety disorder–related measures in normative populations also generally find negative associations between WM metrics and anxiety across preadolescents, adolescents, and adults (33, 38, 60–68). We note that a minority of studies also report positive WM-anxiety correlations, including two in participants with anxiety disorders and three in normative samples (34, 35, 59, 69, 70).
Few studies in the WM-anxiety literature have examined the interactions between sex, anxiety, and WM microstructure. Our previous studies in preadolescents with anxiety disorders revealed a sexually dimorphic WM-anxiety relationship, such that boys with anxiety disorders, and not girls, demonstrated reduced UF FA (4, 18). However, with regard to DTI studies of adolescents and adults with anxiety disorders, it is unclear whether any of these studies explicitly tested anxiety disorder-by-sex interactions in relation to WM metrics. Some studies in normative populations have tested the moderating effect of sex on WM-anxiety associations (38, 62, 64, 66, 67, 69, 70). The two largest studies in adults reported significant negative correlations between corticolimbic pathway FA and trait anxiety only in females, and not males (66, 67). These findings contrast with the male-specific anxiety disorder–related FA decreases in preadolescents detailed in the present study.
While the findings in this study demonstrate effects exclusively in boys and, given reductions in UF FA, provide a potential mechanism by which prefrontal-limbic interactions could be altered in relation to anxiety disorders, this does not preclude the possibility that girls with anxiety disorders also have prefrontal-limbic alterations. The sexually dimorphic nature of the finding here in preadolescent children is striking. While boys with anxiety disorders exhibit significant and widespread reductions in microstructural integrity relative to healthy volunteer boys, we find no evidence of anxiety disorder–related WM effects in girls in this cross-sectional analysis. We note that in a previous report from our laboratory (18), we examined a large sample of girls longitudinally and found a within-participant negative association between anxiety symptom severity and whole-brain FA. The lack of a between-participant anxiety-WM effect in girls in the context of a significant longitudinal finding may suggest that anxiety disorder–related WM alterations in girls are more subtle than those in boys.
While the potential mechanisms underlying the sexually dimorphic findings in the present study are unclear, sex differences in WM maturation and oligodendrocyte biology have been reported. Some studies suggest that patterns of WM growth differ between males and females, although these are generally reported to be small effects (71, 72). One study demonstrated a more protracted trajectory of WM growth in males and also a greater magnitude of FA in males (71). Additionally, previous research has indicated sex-specific pubertal effects on WM microstructure, as well as differential relationships between sex hormones (i.e., testosterone, estradiol) and WM (73–75). Therefore, based on these findings, it would not be surprising to observe differences in WM parameters between boys and girls with anxiety disorders. However, in our sample, we did not find significant effects of age, pubertal status, or sex hormones on the observed group differences. It is still possible that a period of delayed WM growth could have occurred in boys with anxiety disorders prior to the age at which we studied them, and this could potentially contribute to the observed differences in FA magnitude between boys with anxiety disorders and healthy volunteer boys. A few preclinical studies have examined sex differences in oligodendrocyte and oligodendrocyte progenitor cell (OPC) biology (76–78). Findings include the demonstration that female OPCs exhibit more robust cell proliferation and migration capacities in response to injury, in addition to having higher levels of intracellular ATP (76, 77). There is also some evidence to suggest that progesterone may protect female oligodendrocytes from cellular stress to a greater degree than for male oligodendrocytes (78). In addition, male OPCs have been shown to have heightened sensitivity to cytotoxic stress and increased likelihood of cell death (77). Because of the data supporting greater vulnerability of male OPCs, it is possible that elevated levels of early-life adversity/stress—which are known to increase the risk for psychopathology and negatively affect WM microstructure—may differentially impact male and female OPCs.
Some limitations must be considered in the interpretation of the study results. Although this study was performed in a relatively large sample of children, the majority of the study participants were non-Hispanic White children. Studying more individuals from other racial and ethnic groups in future studies is critical to more fully understand the relations between WM and anxiety across diverse populations. This study focused on the preadolescent period (8–12 years old), and interpretation of the results is therefore limited to this age range. Also, the subgroup of anxious boys was relatively small. While we assessed estradiol and testosterone in relation to WM-anxiety associations, it may be important to include other hormonal markers, including dehydroepiandrosterone, in future studies. In relation to interpreting the data from this study, it is important to emphasize that DTI metrics are indirect measures that reflect water diffusion patterns within brain tissue and therefore may reflect not only WM properties but also other factors that influence water diffusion (e.g., other glial cells, microtubules) (79). Additionally, while DTI metrics can reflect myelin content, they can also reflect other microstructural properties, including fiber density and cellular permeability (45). Another important consideration is that tensor-based metrics are limited in their ability to resolve complex microstructural features, such as crossing and bending fibers, as well as partial volume effects. Finally, other evidence suggests that girls may have more subtle anxiety disorder–related WM relations, which, in a cross-sectional design, might be elucidated with other methods, such as neurite orientation dispersion and density imaging (NODDI) and quantitative relaxometry.
In summary, in the largest cross-sectional DTI study of childhood anxiety to date, the results demonstrate that childhood anxiety disorders are associated with broadly distributed alterations in WM microarchitecture across the brain, and, importantly, this cross-sectional relationship is evident only in boys. Anxiety disorders most commonly emerge during preadolescence and early adolescence, periods during which there is rapid WM development (1, 10, 80). While not addressed in this study, it is possible that the WM microstructural alterations observed here are related to the underlying pathophysiology of childhood anxiety disorders. Because evidence demonstrates that WM can dynamically change in relation to various environmental, behavioral, and pharmacological factors (12–14, 81), WM microarchitecture could be a potentially modifiable target for treating childhood anxiety disorders.
1. : Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:593–602Crossref, Medline, Google Scholar
2. : Prevalence, persistence, and sociodemographic correlates of DSM-IV disorders in the National Comorbidity Survey Replication Adolescent Supplement. Arch Gen Psychiatry 2012; 69:372–380Crossref, Medline, Google Scholar
3. : What do childhood anxiety disorders predict? J Child Psychol Psychiatry 2007; 48:1174–1183Crossref, Medline, Google Scholar
4. : Altered uncinate fasciculus microstructure in childhood anxiety disorders in boys but not girls. Am J Psychiatry 2019; 176:208–216Link, Google Scholar
5. : Reduced structural connectivity of a major frontolimbic pathway in generalized anxiety disorder. Arch Gen Psychiatry 2012; 69:925–934Crossref, Medline, Google Scholar
6. : White matter abnormalities in adolescents with generalized anxiety disorder: a diffusion tensor imaging study. BMC Psychiatry 2014; 14:41Crossref, Medline, Google Scholar
7. : Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol Psychiatry 2009; 66:691–694Crossref, Medline, Google Scholar
8. : White matter alterations in social anxiety disorder. J Psychiatr Res 2011; 45:1366–1372Crossref, Medline, Google Scholar
9. : Evidence of frontotemporal structural hypoconnectivity in social anxiety disorder: a quantitative fiber tractography study. Hum Brain Mapp 2013; 34:437–446Crossref, Medline, Google Scholar
10. : Longitudinal development of human brain wiring continues from childhood into adulthood. J Neurosci 2011; 31:10937–10947Crossref, Medline, Google Scholar
11. : Spatiotemporal dynamics of nonhuman primate white matter development during the first year of life. Neuroimage 2021; 231:117825Crossref, Medline, Google Scholar
12. : Rapid and widespread white matter plasticity during an intensive reading intervention. Nat Commun 2018; 9:2260Crossref, Medline, Google Scholar
13. : Experience-dependent plasticity in white matter microstructure: reasoning training alters structural connectivity. Front Neuroanat 2012; 6:32Crossref, Medline, Google Scholar
14. : Clemastine enhances myelination in the prefrontal cortex and rescues behavioral changes in socially isolated mice. J Neurosci 2016; 36:957–962Crossref, Medline, Google Scholar
15. : Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci 2007; 10:1116–1124Crossref, Medline, Google Scholar
16. : The relationship between the uncinate fasciculus and anxious temperament is evolutionarily conserved and sexually dimorphic. Biol Psychiat 2019; 86:890–898Crossref, Medline, Google Scholar
17. : Longitudinal patterns of anxiety from childhood to adulthood: the Great Smoky Mountains Study. J Am Acad Child Adolesc Psychiatry 2014; 53:21–33Crossref, Medline, Google Scholar
18. : A dynamic relation between whole-brain white matter microstructural integrity and anxiety symptoms in preadolescent females with pathological anxiety. Transl Psychiat 2022; 12:57Crossref, Medline, Google Scholar
19. : Schedule for Affective Disorders and Schizophrenia for School-Age Children (K-SADS-PL) for the assessment of preschool children: a preliminary psychometric study. J Psychiatr Res 2009; 43:680–686Crossref, Medline, Google Scholar
20. : Clinical characteristics of anxiety disordered youth. J Anxiety Disord 2010; 24:360–365Crossref, Medline, Google Scholar
21. : Patterns and predictors of comorbidity of DSM-IV anxiety disorders in a clinical sample of children and adolescents. J Anxiety Disord 2013; 27:306–311Crossref, Medline, Google Scholar
22. : Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med 2001; 344:1279–1285 Crossref, Medline, Google Scholar
23. : Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 2008; 359:2753–2766Crossref, Medline, Google Scholar
24. : Neural substrates of childhood anxiety disorders: a review of neuroimaging findings. Child Adolesc Psychiatr Clin N Am 2012; 21:501–525Crossref, Medline, Google Scholar
25. : Psychometric properties of the Screen for Child Anxiety Related Emotional Disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry 1999; 38:1230–1236Crossref, Medline, Google Scholar
26. : The Children’s Depression Inventory: a systematic evaluation of psychometric properties. J Consult Clin Psych 1984; 52:955–967Crossref, Medline, Google Scholar
27. : Scales to assess child and adolescent depression: checklists, screens, and nets. J Am Acad Child Adolesc Psychiatry 1988; 27:726–737Crossref, Medline, Google Scholar
28. : The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998; 26:257–268Crossref, Medline, Google Scholar
29. : The Child Opportunity Index: improving collaboration between community development and public health. Heal Aff (Millwood) 2014; 33:1948–1957Crossref, Medline, Google Scholar
30. : Racial and ethnic inequities in children’s neighborhoods: evidence from the new Child Opportunity Index 2.0. Heal Aff (Millwood) 2020; 39:1693–1701Crossref, Medline, Google Scholar
31. : FSL. Neuroimage 2012; 62:782–790Crossref, Medline, Google Scholar
32. TrackVis. 2017. http://trackvis.org/ Google Scholar
33. : Cingulum-callosal white-matter microstructure associated with emotional dysregulation in children: a diffusion tensor imaging study. Neuroimage Clin 2020; 27:102266Crossref, Medline, Google Scholar
34. : White matter integrity alterations in first episode, treatment-naive generalized anxiety disorder. J Affect Disord 2013; 148:196–201Crossref, Medline, Google Scholar
35. : Individual differences in trait anxiety are associated with white matter tract integrity in fornix and uncinate fasciculus: preliminary evidence from a DTI based tractography study. Behav Brain Res 2013; 238:188–192Crossref, Medline, Google Scholar
36. : Fronto-occipital fasciculus, corpus callosum and superior longitudinal fasciculus tract alterations of first-episode, medication-naïve and late-onset panic disorder patients. J Affect Disord 2013; 146:378–382Crossref, Medline, Google Scholar
37. : White matter alterations in the internal capsule and psychomotor impairment in melancholic depression. Plos One 2018; 13:e0195672Crossref, Medline, Google Scholar
38. : Linking an anxiety-related personality trait to brain white matter microstructure: diffusion tensor imaging and harm avoidance. Arch Gen Psychiat 2011; 68:369–377Crossref, Medline, Google Scholar
39. : ComBat harmonization in R. 2021. https://github.com/Jfortin1/neuroCombat_Rpackage Google Scholar
40. : Harmonization of multi-site diffusion tensor imaging data. Neuroimage 2017; 161:149–170Crossref, Medline, Google Scholar
41. : neuroHarmonize. 2019. https://github.com/rpomponio/neuroHarmonize Google Scholar
42. : Harmonization of large MRI datasets for the analysis of brain imaging patterns throughout the lifespan. NeuroImage 2020; 208:116450Crossref, Medline, Google Scholar
43. : Permutation inference for the general linear model. Neuroimage 2014; 92:381–397Crossref, Medline, Google Scholar
44. : Diffusion tensor imaging of the brain. Neurotherapeutics 2007; 4:316–329Crossref, Medline, Google Scholar
45. : The relationship between axon density, myelination, and fractional anisotropy in the human corpus callosum. Cereb Cortex 2020; 30:2042–2056Crossref, Medline, Google Scholar
46. : Fiber density index correlates with reduced fractional anisotropy in white matter of patients with glioblastoma. AJNR Am J Neuroradiol 2005; 26:2183–2186Medline, Google Scholar
47. : Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage 2005; 26:132–140Crossref, Medline, Google Scholar
48. : Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. Neuroimage 2011; 55:1454–1460Crossref, Medline, Google Scholar
49. : Intergenerational neural mediators of early-life anxious temperament. Proc Natl Acad Sci U S A 2015; 112:9118–9122Crossref, Medline, Google Scholar
50. : The overlapping neurobiology of induced and pathological anxiety: a meta-analysis of functional neural activation. Am J Psychiatry 2021; 178:156–164Link, Google Scholar
51. : Damage to association fiber tracts impairs recognition of the facial expression of emotion. J Neurosci 2009; 29:15089–15099Crossref, Medline, Google Scholar
52. : Structural correlates of facial emotion recognition deficits in Parkinson’s disease patients. Neuropsychologia 2012; 50:2121–2128Crossref, Medline, Google Scholar
53. : Pilot multimodal twin imaging study of generalized anxiety disorder. Depress Anxiety 2012; 29:202–209Crossref, Medline, Google Scholar
54. : Reduced white matter integrity and its correlation with clinical symptom in first-episode, treatment-naive generalized anxiety disorder. Behav Brain Res 2016; 314:159–164Crossref, Medline, Google Scholar
55. : Diffusion tensor imaging studies on Chinese patients with social anxiety disorder. Biomed Res Int 2014; 2014:860658Crossref, Medline, Google Scholar
56. : Abnormal white matter integrity in Chinese young adults with first-episode medication-free anxious depression: a possible neurological biomarker of subtype major depressive disorder. Neuropsych Dis Treat 2018; 14:2017–2026Crossref, Medline, Google Scholar
57. : White matter architecture in major depression with anxious distress symptoms. Prog Neuropsychopharmacol Biol Psychiatry 2019; 94:109664Crossref, Medline, Google Scholar
58. : White-matter abnormalities in the right posterior hemisphere in generalized anxiety disorder: a diffusion imaging study. Psychol Med 2012; 42:427–434Crossref, Medline, Google Scholar
59. : Different white matter abnormalities between the first-episode, treatment-naive patients with posttraumatic stress disorder and generalized anxiety disorder without comorbid conditions. J Affect Disord 2011; 133:294–299Crossref, Medline, Google Scholar
60. : Anxious/depressed symptoms are related to microstructural maturation of white matter in typically developing youths. Dev Psychopathol 2017; 29:751–758Crossref, Medline, Google Scholar
61. : Tracking brain development and dimensional psychiatric symptoms in children: a longitudinal population-based neuroimaging study. Am J Psychiatry 2018; 175:54–62Link, Google Scholar
62. : Development of white matter microstructure and intrinsic functional connectivity between the amygdala and ventromedial prefrontal cortex: associations with anxiety and depression. Biol Psychiatry 2017; 82:511–521Crossref, Medline, Google Scholar
63. : Cerebral white matter changes in young healthy individuals with high trait anxiety: a tract-based spatial statistics study. Front Neurol 2018; 9:704Crossref, Medline, Google Scholar
64. : Sex-related differences in stress reactivity and cingulum white matter. Neuroscience 2021; 459:118–128Crossref, Medline, Google Scholar
65. : The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. J Neurosci 2009; 29:11614–11618Crossref, Medline, Google Scholar
66. : Neurogenetic plasticity and sex influence the link between corticolimbic structural connectivity and trait anxiety. Sci Rep 2017; 7:10959Crossref, Medline, Google Scholar
67. : The inverse relationship between the microstructural variability of amygdala-prefrontal pathways and trait anxiety is moderated by sex. Front Syst Neurosci 2016; 10:93Crossref, Medline, Google Scholar
68. : Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J Neurosci 2015; 35:6020–6027Crossref, Medline, Google Scholar
69. : Individual differences in trait anxiety are associated with white matter tract integrity in the left temporal lobe in healthy males but not females. Neuroscience 2012; 217:77–83Crossref, Medline, Google Scholar
70. : Age-related reduced prefrontal-amygdala structural connectivity is associated with lower trait anxiety. Neuropsychology 2014; 28:631–642Crossref, Medline, Google Scholar
71. : Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 2007; 36:1065–1073Crossref, Medline, Google Scholar
72. : Sex differences in the growth of white matter during adolescence. Neuroimage 2009; 45:1055–1066Crossref, Medline, Google Scholar
73. : Sex differences in the effects of gonadal hormones on white matter microstructure development in adolescence. Dev Cogn Neurosci 2020; 42:100773Crossref, Medline, Google Scholar
74. : White matter development in adolescence: a DTI study. Cereb Cortex 2010; 20:2122–2131Crossref, Medline, Google Scholar
75. : The impact of sex, puberty, and hormones on white matter microstructure in adolescents. Cereb Cortex 2012; 22:1979–1992Crossref, Medline, Google Scholar
76. : Sexual dimorphism in the white matter of rodents. J Neurol Sci 2009; 286:76–80Crossref, Medline, Google Scholar
77. : Sex-specific differences in transcriptomic profiles and cellular characteristics of oligodendrocyte precursor cells. Stem Cell Res 2020; 46:101866Crossref, Medline, Google Scholar
78. : Sexual dimorphism of oligodendrocytes is mediated by differential regulation of signaling pathways. J Neurosci Res 2009; 87:3306–3319Crossref, Medline, Google Scholar
79. : The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed 2002; 15:435–455Crossref, Medline, Google Scholar
80. : Global and regional white matter development in early childhood. Neuroimage 2019; 196:49–58Crossref, Medline, Google Scholar
81. : Training induces changes in white-matter architecture. Nat Neurosci 2009; 12:1370–1371Crossref, Medline, Google Scholar



