Polygenic Scores and Onset of Major Mood or Psychotic Disorders Among Offspring of Affected Parents
Abstract
Objective:
Family history is an established risk factor for mental illness. The authors sought to investigate whether polygenic scores (PGSs) can complement family history to improve identification of risk for major mood and psychotic disorders.
Methods:
Eight cohorts were combined to create a sample of 1,884 participants ages 2–36 years, including 1,339 offspring of parents with mood or psychotic disorders, who were prospectively assessed with diagnostic interviews over an average of 5.1 years. PGSs were constructed for depression, bipolar disorder, anxiety, attention deficit hyperactivity disorder (ADHD), schizophrenia, neuroticism, subjective well-being, p factor, and height (as a negative control). Cox regression was used to test associations between PGSs, family history of major mental illness, and onsets of major mood and psychotic disorders.
Results:
There were 435 onsets of major mood and psychotic disorders across follow-up. PGSs for neuroticism (hazard ratio=1.23, 95% CI=1.12–1.36), schizophrenia (hazard ratio=1.15, 95% CI=1.04–1.26), depression (hazard ratio=1.11, 95% CI=1.01–1.22), ADHD (hazard ratio=1.10, 95% CI=1.00–1.21), subjective well-being (hazard ratio=0.90, 95% CI=0.82–0.99), and p factor (hazard ratio=1.14, 95% CI=1.04–1.26) were associated with onsets. After controlling for family history, neuroticism PGS remained significantly positively associated (hazard ratio=1.19, 95% CI=1.08–1.31) and subjective well-being PGS remained significantly negatively associated (hazard ratio=0.89, 95% CI=0.81–0.98) with onsets.
Conclusions:
Neuroticism and subjective well-being PGSs capture risk of major mood and psychotic disorders that is independent of family history, whereas PGSs for psychiatric illness provide limited predictive power when family history is known. Neuroticism and subjective well-being PGSs may complement family history in the early identification of persons at elevated risk.
Major mood and psychotic disorders, including major depressive disorder, bipolar disorder, and schizophrenia spectrum disorder, have a pervasive impact on individuals across the life course (1). As a result, there have been calls to prioritize early identification and prevention of these disorders (2). The best available predictor of major mental illness is having a biological relative who is affected (3). However, family history information is not sufficient to accurately identify which individuals are most likely to become ill. This is partly because most people with a positive family history will not be affected (4). Additionally, family history may not be known in full because of stigma associated with mental illness, and the demographic trend toward smaller families limits our ability to obtain a complete picture of family history. It has been shown that genetic and environmental risk factors for major mental disorders are largely shared across diagnostic categories (5). Therefore, it is desirable to identify early, stable predictors of illness across diagnostic categories. In the present study, we sought to test whether polygenic scores improved identification of risk for mood and psychotic disorders over family history information alone.
Polygenic scores (PGSs) can be used to summarize genetic predisposition to a disease or trait based on common variants across the genome (6). PGSs are calculated by summing associated alleles from a discovery genome-wide association study (GWAS), weighted by their effect sizes (7). Using this method, we can calculate PGSs indexing genetic liability to a range of phenotypes. It is also possible to combine genetic predisposition to multiple forms of psychopathology into a single score indexing general genetic liability to psychopathology (a “p factor”) (8). The utility of PGSs in prospectively identifying risk of major mood and psychotic disorders among youths with a family history of these disorders has not yet been examined.
There is a clear relationship between PGSs for psychiatric disorders and psychopathology among youths (9–11). Among individuals from families densely affected by mental illness, higher PGSs for schizophrenia and bipolar disorder are associated with the presence of these disorders (12). PGSs for schizophrenia have been shown to improve the prediction of psychosis among adults in a clinical high-risk sample (13). However, it is not known whether PGSs can meaningfully complement family history of major mood and psychotic disorders to identify risk of these disorders.
In this study, we examined the relationship between PGSs, family history of major mood and psychotic disorders, and onset of these disorders in young people. We defined family history of mental illness as the presence of a major mood or psychotic disorder in a biological parent. We selected PGSs for major depressive disorder (14), bipolar disorder (15), schizophrenia (16), anxiety disorders (17), and attention deficit hyperactivity disorder (ADHD) (18) and constructed a p factor PGS that encompassed the combined genetic liability to these disorders (19). We included PGSs for neuroticism and subjective well-being, because these dimensional phenotypes have been associated with multiple forms of mental illness (20, 21). We hypothesized that PGSs would improve prediction of major mood and psychotic disorders over family history information alone. We tested this hypothesis in a unique sample enriched for familial risk of mood and psychotic disorders, composed of eight cohorts from six countries.
Methods
Sample Description
Participants ranged in age from 2 to 31 years (mean=13.66 years, SD=5.37) at first assessment and were enrolled in one of eight longitudinal cohorts: the Families Overcoming Risks and Building Opportunities for Well-Being study (22), the Maritime Bipolar Family Study (23), the U.S. Bipolar High-Risk Study (24), the Pittsburgh Bipolar Offspring Study (25), the Early Prediction of Adolescent Depression study (26), the Bipolar and Schizophrenia Young Offspring Study (27), the Dutch Bipolar and Schizophrenia Offspring Study (28), and the Sydney Bipolar Kids and Sibs Study (29). The sample included offspring of parents with major mood or psychotic disorders, defined as bipolar disorder, recurrent or chronic major depressive disorder, or schizophrenia spectrum disorder, in addition to offspring of control parents. Offspring of affected parents were recruited through mental health services. Offspring of control parents were recruited through advertisements and community organizations. Participants were assessed at regular intervals, and the median follow-up duration was 3 years (range, 0–18 years). Study protocols were approved by each cohort’s local institutional research ethics board, and all participants or their parents or guardians provided informed consent.
Assessment of Family History
The parents of participants were assessed for mental disorders using semistructured diagnostic interviews. Offspring of parents with bipolar disorder, recurrent or chronic major depressive disorder, or schizophrenia spectrum disorder were considered to have a positive family history of major mood or psychotic disorders. Additional information outlining assessment of parent psychopathology is provided in Supplemental Methods in the online supplement.
Assessment of Psychopathology
Participants were assessed for mental disorders at regular intervals using diagnostic interviews (30–32). Assessors were blind to parent psychopathology and to the specific hypotheses of the study. Diagnoses were confirmed in consensus meetings with clinicians who were blind to parent psychopathology. We defined an onset of major mood or psychotic disorder as a prospectively assessed diagnosis of major depressive disorder, bipolar disorder, or schizophrenia spectrum disorder. Additional cohort-specific information outlining recruitment and assessment of psychopathology is provided in Supplemental Methods in the online supplement.
Polygenic Scores
Genotyping was carried out separately for each cohort. Genetic quality control was performed following the same procedure for each cohort (see Supplemental Methods in the online supplement). Genetic data from all cohorts were merged after imputation. We excluded individuals with self-reported non-European ancestry. PGSs were constructed with PRSice-2 (7), using GWAS summary statistics for major depressive disorder (14), bipolar disorder (15), schizophrenia (16), anxiety disorders (17), ADHD (18), subjective well-being (21), and neuroticism (20). As a negative control, we also calculated a PGS for height (33), a trait with a large genetic contribution, to establish specificity of the effects. We pruned genotypes using clumping to obtain an independent set of single-nucleotide polymorphisms (SNPs) in approximate linkage equilibrium with an r2 <0.1 within any 500-kb window. We weighted the contribution of each allele by the effect size of its association with each phenotype in the discovery GWAS. To avoid overlap between discovery and target samples, we used summary statistics omitting cohorts that had potential overlap with the target sample. Additionally, to construct a general psychopathology (p factor) PGS, we first used genomic structural equation modeling (19) to conduct a multivariate GWAS using univariate summary statistics from GWASs of major depressive disorder, bipolar disorder, schizophrenia, anxiety disorders, and ADHD. Next, we used summary statistics from the resulting multivariate GWAS to calculate the p factor PGS. To minimize the number of tests conducted, we used PGSs at p-value thresholds that maximally capture the phenotypic variance in the discovery GWAS samples in analyses: 0.001 for height (33), 0.05 for major depressive disorder and schizophrenia (14, 16), 0.10 for bipolar disorder (15), 0.50 for ADHD and neuroticism (18, 20), and 1.00 for anxiety (17), subjective well-being (21), and p factor (19). The independent discovery GWAS sample size for each phenotype and the number of SNPs included in each PGS are listed in Tables S1 and S2 in the online supplement.
Statistical Analysis
We tested the associations between PGSs, family history of major mood and psychotic disorders, and onset of any of these disorders using Cox proportional hazards regression (coxme package [34]) and Kaplan-Meier estimation (survminer [35] and survival [36] packages). We used chronological age as survival time in all models. We accounted for the nonindependence of observations from related individuals by including the family identifier as a random effect in Cox proportional hazards models. We verified the proportional hazards assumption using the Schoenfeld residuals test (see Supplemental Results and Figure S6 in the online supplement). Diagnostic information from all follow-ups was included in analyses. We first tested the effect of each PGS on onsets of major mood and psychotic disorders. We estimated the proportion of variance in time until diagnosis of major mood or psychotic disorder explained by the fixed effects in each model using likelihood-ratio-based pseudo R2 (see Table S4 in the online supplement). Next, we tested the independent effect of each PGS on onsets of mood and psychotic disorders when accounting for familial high-risk status as a dichotomous variable. We then tested interactions between PGSs and family history where an independent effect of PGS was observed. In addition to survival time, all models accounted for sex, follow-up duration, and genetic population structure indexed with 10 genetic principal components. To ensure that results were consistent across cohorts and not driven by a subset of participants, we tested the robustness of the main findings using cohort-wise leave-one-out analyses. Associations were quantified as hazard ratios. All PGSs were standardized so that hazard ratios represent the effect of an increase of one standard deviation in the PGSs. Analyses were conducted in RStudio (R, version 4.0.1).
Results
Demographic and Clinical Characteristics
After genetic quality control, the final sample included 1,884 participants from 925 families, ages 6–36 years at most recent follow-up. Table 1 summarizes the participants’ characteristics. The age distribution at most recent assessment across cohorts is shown in Figure 1. Nearly three-quarters (N=1,339, 71.1%) of participants had a biological parent with a major mood or psychotic disorder. Of the participants, 435 developed major depressive disorder, bipolar disorder, or a psychotic disorder by the end of follow-up. As expected, family history of major mood and psychotic disorders was strongly positively associated with risk of onset of these disorders (hazard ratio=2.82, 95% CI=2.15–3.70, p<0.001; see Figure S1 in the online supplement). We also found the expected relationships between PGSs and family history of major mood or psychotic disorders; higher PGSs for bipolar disorder, p factor, neuroticism, major depression, schizophrenia, ADHD, and anxiety were significantly positively associated with family history of illness (see Figure S2 in the online supplement). The relationships between PGSs are shown in Figure S3 in the online supplement.
| Cohort | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | BASYS (N=96) | FORBOW (N=325) | EPAD (N=285) | USAB (N=274) | DBSOS (N=97) | BK&S (N=229) | BIOS (N=414) | MBFS (N=164) | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age at first assessment (years) | 11.1 | 3.4 | 10.8 | 4.0 | 12.2 | 2.1 | 15.6 | 3.2 | 13.2 | 2.3 | 20.2 | 5.2 | 10.3 | 4.7 | 19.7 | 4.1 |
| Age at last assessment (years) | 14.1 | 3.5 | 15.1 | 4.2 | 14.6 | 2.1 | 17.7 | 3.2 | 17.2 | 2.6 | 22.3 | 5.2 | 22.9 | 5.9 | 23.1 | 4.4 |
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Female | 51 | 53.1 | 173 | 53.2 | 168 | 58.9 | 133 | 48.5 | 49 | 50.5 | 119 | 52.0 | 215 | 51.9 | 96 | 58.5 |
| Parent diagnosis | ||||||||||||||||
| Major depression | 0 | 0.0 | 143 | 44.0 | 285 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 21 | 12.8 |
| Bipolar disorder | 28 | 29.1 | 66 | 20.3 | 0 | 0.0 | 198 | 72.3 | 49 | 50.5 | 145 | 63.3 | 256 | 61.8 | 73 | 44.5 |
| Psychotic disorder | 16 | 16.7 | 23 | 7.1 | 0 | 0.0 | 0 | 0.0 | 20 | 20.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Control subjects | 52 | 54.2 | 93 | 28.6 | 0 | 0.0 | 76 | 27.7 | 28 | 28.9 | 84 | 36.3 | 158 | 38.2 | 70 | 42.7 |
| Offspring diagnosis | ||||||||||||||||
| Major depression | 5 | 5.2 | 56 | 17.2 | 29 | 10.2 | 40 | 14.6 | 16 | 16.5 | 51 | 22.3 | 139 | 33.6 | 30 | 18.3 |
| Bipolar disorder | 0 | 0.0 | 7 | 2.2 | 4 | 1.4 | 26 | 9.5 | 6 | 6.2 | 2 | 0.9 | 28 | 6.8 | 26 | 15.9 |
| Psychotic disorder | 0 | 0.0 | 7 | 2.2 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 4 | 1.0 | 3 | 1.8 |
TABLE 1. Demographic and clinical characteristics of the study population, stratified by cohorta
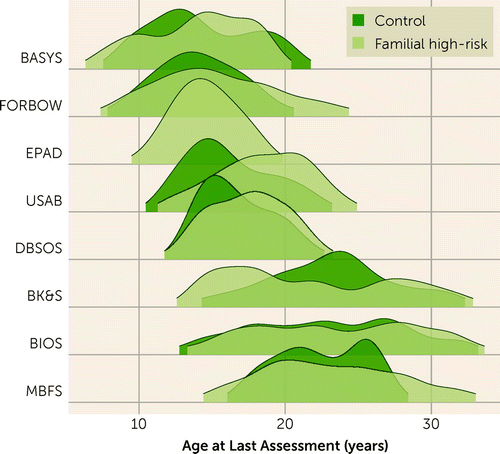
FIGURE 1. Age at most recent assessment, stratified by cohorta
aBASYS=Bipolar and Schizophrenia Young Offspring Study (27); FORBOW=Families Overcoming Risks and Building Opportunities for Well-Being study (22); EPAD=Early Prediction of Adolescent Depression study (39); USAB=U.S. Bipolar High-Risk Study (24); DBSOS=Dutch Bipolar and Schizophrenia Offspring Study (40); BK&S=Sydney Bipolar Kids and Sibs Study (29); BIOS=Pittsburgh Bipolar Offspring Study (25); MBFS=Maritime Bipolar Family Study (23).
Relationship Between PGSs and Onsets of Major Mental Disorders
Higher PGSs for multiple phenotypes were associated with onset of mood and psychotic disorders (Figure 2). After accounting for age, follow-up duration, sex, and genetic principal components, PGSs for neuroticism (hazard ratio=1.23, 95% CI=1.12–1.36, p<0.001), schizophrenia (hazard ratio=1.15, 95% CI=1.04–1.26, p=0.007), depression (hazard ratio=1.11, 95% CI=1.01–1.22, p=0.038), ADHD (hazard ratio=1.10, 95% CI=1.00–1.21, p=0.044), and p factor (hazard ratio=1.14, 95% CI=1.04–1.26, p=0.006) were positively associated with onset of major mood and psychotic disorders. PGS for subjective well-being was negatively associated with illness onset (hazard ratio=0.90, 95% CI=0.82–0.99, p=0.040). As expected, PGS for height was not associated with disorder onset (hazard ratio=0.96, 95% CI=0.87–1.06, p=0.470). This pattern of associations was confirmed, with all effect sizes within one standard error of the original finding, in sensitivity analyses restricted to individuals followed up until age 15 or older and adjusting for study (see Supplemental Results in the online supplement).
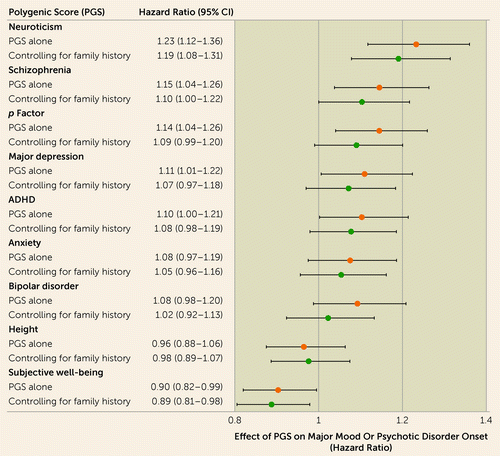
FIGURE 2. Relationships between polygenic scores and onsets of major mood and psychotic disordersa
a ADHD=attention deficit hyperactivity disorder. Error bars indicate 95% confidence intervals.
Unique Contribution of PGSs to Onsets of Major Mental Illness
PGSs for neuroticism and subjective well-being were significantly associated with onsets of major mood and psychotic disorders, independent of family history of these disorders (Figure 2). After accounting for family history, neuroticism PGS remained positively associated with onsets of major mood and psychotic disorders (hazard ratio=1.19, 95% CI=1.08–1.31, p<0.001), and subjective well-being PGS remained negatively associated with onsets of these disorders (hazard ratio=0.89, 95% CI=0.81–0.98, p=0.017). The independent effect of neuroticism PGS was consistent across all cohort-wise leave-one-out analyses (hazard ratio range, 1.16–1.22; see Figure S4 in the online supplement). The independent effect of the subjective well-being PGS was directionally consistent across cohorts (hazard ratio range, 0.82–0.92) and was statistically significant in six of the eight cohort-wise leave-one-out analyses (see Figure S5 in the online supplement). The effect of neuroticism PGS on disorder onset was stronger in the absence of family history of mood and psychotic disorders, reflected in a statistically significant interaction between family high-risk status and neuroticism PGS (hazard ratio=0.75, 95% CI=0.58–0.98, p=0.035). Offspring of control subjects with low neuroticism PGS had the lowest probability of diagnosis, followed by offspring of control subjects with high neuroticism PGS, then offspring of affected parents with low neuroticism PGS, and finally offspring of affected parents with high neuroticism PGS, who had the highest probability of diagnosis (Kaplan-Meier χ2=39.40, p<0.001) (Figure 3). There was no interaction between subjective well-being PGS and family history of major mood and psychotic disorders (hazard ratio=1.09, 95% CI=0.85–1.41, p=0.480). This pattern of associations was confirmed, with all effect sizes within one standard error of the original finding, in sensitivity analyses restricted to individuals followed up until age 15 or older and adjusting for study (see Supplemental Results in the online supplement).
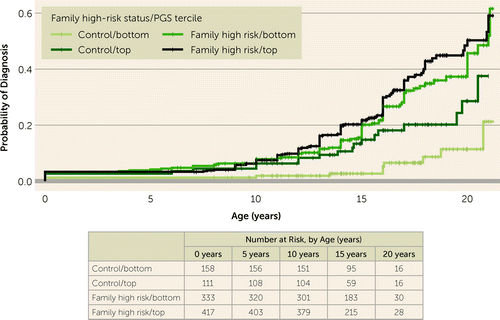
FIGURE 3. Kaplan-Meier plot showing the relationship between neuroticism PGS, family history of major mood and psychotic disorders, and onsets of these disorders, with the sample divided into terciles based on PGSa
aCurves shown are 1) no family history of major mental illness and bottom neuroticism PGS tercile (control/bottom); 2) no family history of major mental illness and top neuroticism PGS tercile (control/top); 3) family history of major mental illness and bottom neuroticism PGS tercile (family high risk/bottom); and 4) family history of major mental illness and top neuroticism PGS tercile (family high risk/top). PGS=polygenic score.
Discussion
This study identified associations between polygenic scores, family history of major mood and psychotic disorders, and onset of these disorders in youths. We found that PGSs for neuroticism and subjective well-being uniquely contributed to identification of risk of major mental illness, over and above the well-established effect of family history. In contrast, individuals with higher genetic predisposition to schizophrenia, major depressive disorder, ADHD, and a genetic “p factor” were, as expected, more likely to develop major mood or psychotic disorders, but these PGSs did not improve identification of risk beyond family history alone. These findings suggest that PGSs have the potential to improve risk identification in cases where family history information is not available or not known in full.
This study was motivated by a need to improve identification of risk for major mental disorders. It has been shown that PGSs derived from GWASs of adult psychiatric phenotypes are associated with psychopathology earlier in life (11). We confirmed that genetic liabilities to neuroticism, depression, and subjective well-being indexed from GWASs of adult participants are associated with psychopathology among youths. Our results also aligned with those from past studies showing that offspring of parents with mood and psychotic disorders and individuals with these disorders have elevated PGSs for psychopathology compared to control subjects (10, 12). Our findings expand on these studies to show that PGSs have the potential to improve upon current strategies for identification of risk for major mental disorders. While PGSs for several disorders were associated with onset of mood or psychotic disorders, their effects largely overlapped with the known effect of family history. These findings suggest that PGSs for psychiatric illness may provide limited predictive power when family history information is available. In contrast, the effects of neuroticism and subjective well-being PGSs were unique and independent of family history. This may be due in part to the transdiagnostic nature of these phenotypes. Transdiagnostic indicators of risk may be particularly valuable in the developmental context, where early manifestations of psychopathology typically begin in childhood or adolescence but often change in form over time. Neuroticism and subjective well-being PGSs may capture some of this higher-level transdiagnostic liability to major mood and psychotic disorders that is independent of family history. The effect of neuroticism PGS on disorder onset was stronger in the absence of family history of mood and psychotic disorders. Thus, neuroticism PGS may help stratify degrees of risk, especially among offspring of unaffected parents. To our knowledge, this is the first time that an interaction between family history and a polygenic score has been reported in a prospective study of risk for mental disorders, and the replicability of this result should be tested in independent samples. Interestingly, the association between subjective well-being PGS and onset of major mood and psychotic disorders strengthened when we accounted for family history of these disorders. This suggests that the subjective well-being PGS may be useful in identifying individuals at higher risk of major mood and psychotic disorders, particularly those with a positive family history of these disorders.
Our findings have implications for future research. The finding that genetic liability to neuroticism is associated with increased risk of major mood and psychotic disorders independent of family history suggests that neuroticism PGS may be a useful tool for improved risk identification in early intervention studies. While the effects of PGSs on psychopathology are modest, clinical implementation of PGSs may be most useful in populations with a higher prior probability of disease, such as among offspring of affected parents (6). Approximately one of every three offspring of a parent with a mood or psychotic disorder will become ill by adulthood (3). However, family history information is inherently limited (4) and is often not known in full, so it is necessary to identify additional predictive factors. Genetic factors are set from conception and thus provide a stable tool that could be used in conjunction with other factors, such as family history information, and more dynamic risk factors such as environmental exposures or clinical features, to allow for early identification of individuals at the highest risk. It has been suggested that earlier interventions produce the greatest benefit (37). The identification of stable, transdiagnostic indicators of risk opens the door for targeted early interventions that may eventually reduce the individual and societal burden of these disorders.
Our study benefited from the inclusion of offspring of parents with major mood and psychotic disorders. Over 70% of the participants in our study have a biological parent with major depressive disorder, bipolar disorder, or a schizophrenia spectrum disorder, resulting in a concentration of familial risk and thus a higher rate of psychopathology than in the general population. The study also benefited from our use of eight longitudinal cohorts, each with thorough diagnostic assessments repeated over multiple follow-up years and across ages, with high retention rates. This allowed us to follow participants through the period of highest risk of major mental illness onset and to examine the association between PGSs and psychopathology over time. However, the results should be interpreted in the context of the study’s limitations. One limitation is the heterogeneity of parent diagnoses across cohorts. We opted to include all available cohorts and to select a transdiagnostic outcome (onset of any major mood or psychotic disorder) because it has been shown that familial transmission of major mental illness is largely transdiagnostic (3, 5). We have performed leave-one-out analyses to probe the robustness to cohort effects and confirm that the main findings are not driven by any single cohort. Our study is also limited by the binary definition of family history. More nuanced family history information may be more predictive, but this information was not consistently collected across all contributing samples. Even with the combined sample, our study is also limited by statistical power. The limited statistical power increases the likelihood of false negative results, particularly if a true weak relationship exists between predictor and outcome. This may be the case for PGSs that were not significantly associated with illness onset when accounting for family history. Thus, these results await confirmation in larger studies. Another important limitation is the ethnically homogeneous nature of the sample. We included only individuals of European descent, because current PGS methods rely on having matched genetic ancestry between the target sample and the GWAS from which reference effect sizes were derived. Currently, most large-scale GWASs are based on White individuals of European ancestry. As PGSs move from the research setting to the clinic, this limitation may lead to exacerbation of the systemic health disadvantages already experienced by racially marginalized populations (38). It is our hope that our analyses will be extended to more inclusive samples as large-scale GWASs of racially diverse populations become available.
In summary, we found that genetic predisposition to neuroticism and subjective well-being uniquely contribute to risk of major mood and psychotic disorders beyond the effect of family history of these disorders. Future studies could probe the ability of neuroticism and subjective well-being PGSs to predict onsets of major mood and psychotic disorders in diverse independent samples. The results may inform targeted early interventions to prevent onset of major mood and psychotic disorders among high-risk children and adolescents.
1. : Longitudinal assessment of mental health disorders and comorbidities across 4 decades among participants in the Dunedin Birth Cohort Study. JAMA Netw Open 2020; 3:e203221Crossref, Medline, Google Scholar
2. : Why we need a transdiagnostic staging approach to emerging psychopathology, early diagnosis, and treatment. JAMA Psychiatry 2016; 73:191Crossref, Medline, Google Scholar
3. : Risk of mental illness in offspring of parents with schizophrenia, bipolar disorder, and major depressive disorder: a meta-analysis of family high-risk studies. Schizophr Bull 2014; 40:28–38Crossref, Medline, Google Scholar
4. : Modelling the effects of penetrance and family size on rates of sporadic and familial disease. Hum Hered 2011; 71:281–288Crossref, Medline, Google Scholar
5. : Etiology in psychiatry: embracing the reality of poly-gene-environmental causation of mental illness. World Psychiatry 2017; 16:121–129Crossref, Medline, Google Scholar
6. : Polygenic risk scores: from research tools to clinical instruments. Genome Med 2020; 12:44Crossref, Medline, Google Scholar
7. : PRSice-2: polygenic risk score software for biobank-scale data. GigaScience 2019; 8:giz082Crossref, Medline, Google Scholar
8. : A polygenic p factor for major psychiatric disorders. Transl Psychiatry 2018; 8:205Crossref, Medline, Google Scholar
9. : Polygenic risk for depression, anxiety, and neuroticism are associated with the severity and rate of change in depressive symptoms across adolescence. J Child Psychol Psychiatry 2021; 62:1462–1474Crossref, Medline, Google Scholar
10. : Polygenic risk: predicting depression outcomes in clinical and epidemiological cohorts of youths. Am J Psychiatry 2019; 176:615–625Link, Google Scholar
11. : Genetic associations between childhood psychopathology and adult depression and associated traits in 42 998 individuals: a meta-analysis. JAMA Psychiatry 2020; 77:715–728Crossref, Medline, Google Scholar
12. : Polygenic risk scores distinguish patients from non-affected adult relatives and from normal controls in schizophrenia and bipolar disorder multi-affected kindreds. Am J Med Genet B Neuropsychiatr Genet 2018; 177:329–336Crossref, Medline, Google Scholar
13. : Polygenic risk score contribution to psychosis prediction in a target population of persons at clinical high risk. Am J Psychiatry 2020; 177:155–163Link, Google Scholar
14. : Genome-wide association analyses identify 44 risk variants and refine the genetic architecture of major depression. Nat Genet 2018; 50:668–681Crossref, Medline, Google Scholar
15. : Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet 2021; 53:817–829Crossref, Medline, Google Scholar
16. : Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet 2018; 50:381–389Crossref, Medline, Google Scholar
17. : Genetic variants associated with anxiety and stress-related disorders: a genome-wide association study and mouse-model study. JAMA Psychiatry 2019; 76:924–932Crossref, Medline, Google Scholar
18. : Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet 2019; 51:63–75Crossref, Medline, Google Scholar
19. : Genomic structural equation modelling provides insights into the multivariate genetic architecture of complex traits. Nat Hum Behav 2019; 3:513–525Crossref, Medline, Google Scholar
20. : Meta-analysis of genome-wide association studies for neuroticism in 449,484 individuals identifies novel genetic loci and pathways. Nat Genet 2018; 50:920–927Crossref, Medline, Google Scholar
21. : Genetic variants associated with subjective well-being, depressive symptoms, and neuroticism identified through genome-wide analyses. Nat Genet 2016; 48:624–633Crossref, Medline, Google Scholar
22. : A familial risk enriched cohort as a platform for testing early interventions to prevent severe mental illness. BMC Psychiatry 2014; 14:344Crossref, Medline, Google Scholar
23. : Rare susceptibility variants for bipolar disorder suggest a role for G protein-coupled receptors. Mol Psychiatry 2018; 23:2050–2056Crossref, Medline, Google Scholar
24. : A high-risk study of bipolar disorder: childhood clinical phenotypes as precursors of major mood disorders. Arch Gen Psychiatry 2011; 68:1012–1020Crossref, Medline, Google Scholar
25. : Lifetime psychiatric disorders in school-aged offspring of parents with bipolar disorder: the Pittsburgh Bipolar Offspring Study. Arch Gen Psychiatry 2009; 66:287–296Crossref, Medline, Google Scholar
26. : Mental health resilience in the adolescent offspring of parents with depression: a prospective longitudinal study. Lancet Psychiatry 2016; 3:49–57Crossref, Medline, Google Scholar
27. : Neuropsychological characteristics of child and adolescent offspring of patients with schizophrenia or bipolar disorder. Schizophr Res 2017; 183:110–115Crossref, Medline, Google Scholar
28. : Brain structure, IQ, and psychopathology in young offspring of patients with schizophrenia or bipolar disorder. Eur Psychiatry 2020; 63:e5Crossref, Medline, Google Scholar
29. : Longitudinal changes in structural connectivity in young people at high genetic risk for bipolar disorder. Am J Psychiatry 2022; 179:350–361Link, Google Scholar
30. : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
31. : Structured Clinical Interview for DSM-5–Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Washington, DC, American Psychiatric Association, 2015 Google Scholar
32. : A test-retest reliability study of child-reported psychiatric symptoms and diagnoses using the Child and Adolescent Psychiatric Assessment (CAPA-C). Psychol Med 1995; 25:755–762Crossref, Medline, Google Scholar
33. : Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum Mol Genet 2018; 27:3641–3649Crossref, Medline, Google Scholar
34. : coxme: Mixed effects Cox models. 2020. https://cran.r-project.org/package=coxme Google Scholar
35. : survminer: Drawing survival curves using “ggplot2.” 2021. https://cran.r-project.org/package=survminer Google Scholar
36. : survival: A package for survival analysis in R. 2020. https://cran.r-project.org/package=survival Google Scholar
37. : The developmental origins of health. Health Econ 2012; 21:24–29 Crossref, Medline, Google Scholar
38. : Clinical use of current polygenic risk scores may exacerbate health disparities. Nat Genet 2019; 51:584–591Crossref, Medline, Google Scholar
39. : Offspring of parents with recurrent depression: which features of parent depression index risk for offspring psychopathology? J Affect Disord 2012; 136:44–53Crossref, Medline, Google Scholar
40. : Prevalence of psychopathology in children of a bipolar parent. J Am Acad Child Adolesc Psychiatry 2001; 40:1094–1102Crossref, Medline, Google Scholar



