Differences in Antipsychotic Treatment Discontinuation Among Veterans With Schizophrenia in the U.S. Department of Veterans Affairs
Abstract
Objective:
Effectiveness of antipsychotic drugs is inferred from relatively small randomized clinical trials conducted with carefully selected and monitored participants. This evidence is not necessarily generalizable to individuals treated in daily clinical practice. The authors compared the clinical effectiveness between all oral and long-acting injectable (LAI) antipsychotic medications used in the treatment of schizophrenia in the U.S. Department of Veterans Affairs (VA) health care system.
Methods:
This was an observational study utilizing VA pharmacy data from 37,368 outpatient veterans with schizophrenia. Outcome measures were all-cause antipsychotic discontinuation and psychiatric hospitalizations. Oral olanzapine was used as the reference group.
Results:
In multivariable analysis, clozapine (hazard ratio=0.43), aripiprazole long-acting injectable (LAI) (hazard ratio=0.71), paliperidone LAI (hazard ratio=0.76), antipsychotic polypharmacy (hazard ratio=0.77), and risperidone LAI (hazard ratio=0.91) were associated with reduced hazard of discontinuation compared with oral olanzapine. Oral first-generation antipsychotics (hazard ratio=1.16), oral risperidone (hazard ratio=1.15), oral aripiprazole (hazard ratio=1.14), oral ziprasidone (hazard ratio=1.13), and oral quetiapine (hazard ratio=1.11) were significantly associated with an increased risk of discontinuation compared with oral olanzapine. No treatment showed reduced risk of psychiatric hospitalization compared with oral olanzapine; quetiapine was associated with a 36% worse outcome in terms of hospitalizations compared with olanzapine.
Conclusions:
In a national sample of veterans with schizophrenia, those treated with clozapine, two of the LAI second-generation antipsychotics, and antipsychotic polypharmacy continued the same antipsychotic therapy for a longer period of time compared with the reference drug. This may reflect greater overall acceptability of these medications in clinical practice.
Randomized controlled trials are the gold-standard design used to test the efficacy of antipsychotics but only reflect effectiveness in patients who volunteer for such trials. Despite the obvious shortcomings, an alternative approach is to carefully analyze data from available health care databases, including medication and hospitalization data on large samples of individuals. This may offer insight into antipsychotic prescribing practices in clinical practice, enable comparison of the effectiveness among all medications used, and allow direct comparisons between oral and long-acting injectable (LAI) formulations of the same medications (1). The majority of extant observational studies of administrative databases were performed outside of the United States (2–5), used smaller and regional samples from the Veterans Administration system (6, 7) and Medicaid (8), and/or focused on the use of specific, rather than all, antipsychotics (6, 9). Hence, there is a need to study all-cause discontinuation of all oral and LAI antipsychotics, as well as psychiatric hospitalization, in a large nationwide study in the United States, including comparison of LAI formulations with the same oral medication, using a comprehensive health care database.
The U.S. Department of Veterans Affairs (VA) health care system maintains a large, comprehensive health care service utilization database on all veterans who receive their care within the VA system. The database includes information on diagnosis, demographic characteristics, prescriptions, and service utilization, including psychiatric hospitalizations. Thus, the database provides a unique opportunity to examine the relative effectiveness of oral and LAI antipsychotics in a real-world setting. In this study, we analyzed all-cause discontinuation of antipsychotic medications and psychiatric hospitalizations for 37,368 people with schizophrenia treated in the VA health care system. To our knowledge, this is the largest study comparing all-cause discontinuation of antipsychotics and psychiatric hospitalizations in the United States and the first to compare LAI formulations with the same oral medication in a nationwide, multiyear U.S. sample using nonprofit funding. Based on findings from previous studies (10), we hypothesized that clozapine and LAI antipsychotics would be associated with longer time to discontinuation and fewer psychiatric hospitalizations compared with oral olanzapine and that LAI formulations would be associated with longer time to discontinuation and fewer psychiatric hospitalizations than oral formulations of the same medication (11).
Methods
Data Source and Sample Selection
We used VA health care service utilization data from October 1, 2010, to September 30, 2015, which consisted of all health care records for inpatients and outpatients receiving health care services provided through VA hospital and outpatient clinic services. The records include demographic characteristics, ICD-9-CM diagnostic codes, all outpatient prescription medications dispensed from VA pharmacies, and all psychiatric hospitalizations. The study was approved by the institutional review board of the University of Maryland, Baltimore.
We selected all 101,434 VA health care system users who had at least one inpatient or outpatient record with an ICD-9-CM code for schizophrenia (295.x) during the study period. Individuals who had both a schizophrenia diagnosis (code 295.x) and one or more diagnoses for another serious mental illness (code 296.x, 297.x, 298.x) were included if schizophrenia accounted for the majority of all serious mental illness diagnoses listed on the individual’s inpatient and outpatient records (12). Of these 101,434 individuals, 82,463 (81%) received at least one prescription as an outpatient for an oral or LAI antipsychotic medication during the study period (medication episode). Only one 30-day prescription was recorded in many of these episodes. To ensure that all the antipsychotic medications had an adequate number of observations and an adequate exposure period, we focused on antipsychotics that had at least 150 episodes in our sample and on episodes that lasted at least 60 days. Hence, episodes for oral asenapine, oral iloperidone, LAI olanzapine, and oral thioridazine and all medication episodes for other antipsychotics that lasted <60 days were excluded. Because we required information about the total duration of each medication episode, we further limited the sample to incident-prescribing episodes (13, 14), defined as episodes with no fills for the same antipsychotic during the preceding 6 months. These exclusions resulted in a final sample of 62,056 incident antipsychotic episodes of at least 60 days’ duration among 37,368 individuals with schizophrenia (for details, see the online supplement).
Measures
Dependent measures.
Outcomes were assessed by examining time to all-cause discontinuation of an antipsychotic medication and time to psychiatric hospitalization. Discontinuing or changing medication, often after hospitalization, is a frequent occurrence and major problem in the treatment of schizophrenia. According to Lieberman et al. (15), all-cause discontinuation “integrates patients’ and clinicians’ judgments of efficacy, safety, and tolerability into a global measure of effectiveness that reflects their evaluation of therapeutic benefits in relation to undesirable effects.” To measure time to discontinuation, we defined discrete, continuous medication episodes for each antipsychotic dispensed to an individual, using prescription fill dates and days of medication supplied (16, 17). Discontinuation was indicated by the start date of a lapse of at least 45 days in prescription refills or LAI formulations during which patients ostensibly would have had no medication (for further details, see the online supplement). The second dependent variable, days to psychiatric hospitalization, was defined as the number of days elapsed between the medication start date and the first subsequent date of a psychiatric admission to a VA hospital or data censoring.
Data on medications prescribed during inpatient stays were unavailable to us and were considered less relevant for determining the duration of medication use than outpatient prescriptions.
Independent variables.
The independent variables were binary indicator variables for each antipsychotic medication, which included all second-generation oral and LAI antipsychotic medications available during the study period that met the inclusion criteria described above. All episodes of first-generation oral antipsychotics were analyzed as a group (i.e., one indicator for any oral first-generation medication incident medication episode). Antipsychotic polypharmacy was defined as follows: if an incident medication episode for one antipsychotic overlapped with an incident medication episode for another antipsychotic for ≥60 consecutive days, those episodes were classified as polypharmacy of the same length as the original episodes. Incident antipsychotic episodes that overlapped for less than 60 consecutive days were classified as monotherapy episodes for a particular type of antipsychotic so that all episodes of treatment not defined as polypharmacy were monotherapy. Because this might have resulted in overestimation of the duration of antipsychotic polypharmacy, all antipsychotic polypharmacy episodes were down-weighted by half in the Cox regression models to prevent undue weight to this group relative to the monotherapy groups. Among all episodes included in the polypharmacy group, the average percentage during which overlapping treatment occurred ranged from 63% to 87% (mean, 76%) of the episode. Incident antipsychotic episodes that overlapped for less than 60 consecutive days were classified as monotherapy episodes for a particular type of antipsychotic.
Covariates.
The following measures were used as covariates in the multivariate analyses (see the online supplement): demographic characteristics, service-connected disability ratings, geographic location, psychiatric comorbidity, medications and visits to outpatient mental health services, inpatient and outpatient medical care and comorbidities, psychiatric or medical emergency department visits, and fiscal year.
Statistical Analysis
We used the Cox proportional hazards regression model (18) to compare the effect of antipsychotic medications on two event-time outcomes: time to all-cause antipsychotic discontinuation and time to psychiatric hospitalization. Analysis results are reported as hazard ratios reflecting the risk of all-cause treatment discontinuation or psychiatric hospitalization compared with oral olanzapine. Oral olanzapine was used as the comparator in order to allow comparison to the study conducted by Tiihonen et al. (2) as closely as possible; data on people not receiving medication were not available to us. We selected oral olanzapine as the comparator because it demonstrated longer time to treatment discontinuation than risperidone and quetiapine in the Clinical Antipsychotic Trials of Intervention Effectiveness schizophrenia study (15), and it was used as the comparator in the prior comparative effectiveness study most similar to ours (2). The data set included a separate observation for each incident medication episode. We included all incident medication episodes from individuals in our analysis (some individuals contributed more than one incident medication episode to the analysis). Episodes that were ongoing at the end of the study period (September 30, 2015) were analyzed as right-censored. The pairs of overlapping episodes in the polypharmacy group were not truncated to only the days of consecutive overlap; each episode of a pair retained its original length of time, reflecting the entire time of exposure, which included a mixture of time on two or more drugs and on monotherapy.
Adjusted hazard ratios for discontinuation were estimated with 95% confidence intervals. Hazard ratios <1 indicated a lower likelihood of discontinuation or psychiatric hospitalization compared with the reference group (oral olanzapine), whereas hazard ratios >1 had the opposite interpretation. To account for clustering of discontinuation times from the same individual, robust “sandwich” standard errors were used (19). All models were adjusted for the demographic and clinical characteristics described above, because of the potential effect of illness severity on the choice of antipsychotic prescribed and the likelihood of discontinuation. To address multiplicity, p values were adjusted to control the Benjamini and Hochberg false discovery rate (FDR) to 5%. In separate Cox proportional hazards regression models, we also compared the risk of discontinuation and time to hospitalization for each LAI antipsychotic medication with its oral counterpart as the reference.
In sensitivity analyses, we lengthened the number of consecutive days of overlap required to indicate antipsychotic polypharmacy from 60 days to 90 and 120 days. In further sensitivity analyses, we excluded antipsychotic polypharmacy episodes that included either LAI antipsychotics or clozapine, because these agents may be associated with longer times to discontinuation or psychiatric hospitalization.
Results
Of the 37,368 individuals with schizophrenia included in the study, 91% were male, 41% were nonwhite, 61% had ever been married, 45% had at least a 50% service connection, and the mean age was 54.3 years (SD=12.6).
In analyses of large databases, it is not possible to ascertain the precise reasons for overlapping antipsychotic prescriptions. We found that the average length of the incident episodes classified as polypharmacy was 395 days in this study. While the incident episodes must have overlapped for at least 60 days to be included in this group, on average, the actual duration of overlap was almost 250 days (SD=256 days, median=146 days). The strengths and limitations of operationalizing polypharmacy in this manner are described below in the Discussion section.
Treatment Discontinuation
Among the 62,056 incident antipsychotic episodes that were included, the median duration of the antipsychotic medication episodes was 183 days (interquartile range=99–401). The median time to all-cause discontinuation, based on unadjusted Cox proportional hazards regression to account for right censoring, for each antipsychotic cohort is presented in Table 1.
| Time to Discontinuation (days) | ||||
|---|---|---|---|---|
| Medication | Number of Patients | Number of Episodes | Median | 95% CI |
| Total | 37,368a | 62,056 | 193 | 191, 196 |
| Clozapine | 666 | 678 | 716 | 619, 912 |
| Antipsychotic polypharmacy | 5,739 | 13,438 | 295 | 288, 304 |
| Aripiprazole LAI | 224 | 229 | 275 | 206, 424 |
| Paliperidone LAI | 2,606 | 2,745 | 254 | 234, 270 |
| Risperidone LAI | 1,362 | 1,431 | 204 | 187, 230 |
| Lurasidone | 1,004 | 1,013 | 201 | 185, 224 |
| Olanzapine | 5,155 | 5,515 | 194 | 189, 202 |
| Fluphenazine LAI | 483 | 506 | 194 | 160. 219 |
| Haloperidol LAI | 1,203 | 1,278 | 186 | 165, 199 |
| Quetiapine | 7,678 | 8,290 | 183 | 177, 187 |
| Paliperidone | 219 | 226 | 181 | 153, 224 |
| Aripiprazole | 5,951 | 6,361 | 178 | 172, 183 |
| Ziprasidone | 2,041 | 2,132 | 178 | 169, 189 |
| Oral first-generation antipsychotics | 6,342 | 7,085 | 173 | 170, 178 |
| Risperidone | 10,224 | 11,129 | 169 | 165, 172 |
TABLE 1. Unadjusted median time to discontinuation by antipsychotic medication in a national sample of patients in the U.S. Department of Veterans Affairs health care system
Adjusting for covariates, the Cox proportional hazards regression results yielded a hazard ratio of 0.43 (95% CI=0.38, 0.48) for clozapine, a hazard ratio of 0.71 (95% CI=0.58, 0.85) for aripiprazole LAI, a hazard ratio of 0.76 (95% CI=0.72, 0.81) for paliperidone LAI, and a hazard ratio of 0.91 (95% CI=0.85, 0.97) for risperidone LAI (all p values <0.05, after FDR adjustment) (Figure 1). We also found that antipsychotic polypharmacy was associated with a lower risk (hazard probability) of all-cause discontinuation compared with oral olanzapine (hazard ratio=0.77, 95% CI=0.74, 0.80, p<0.05).
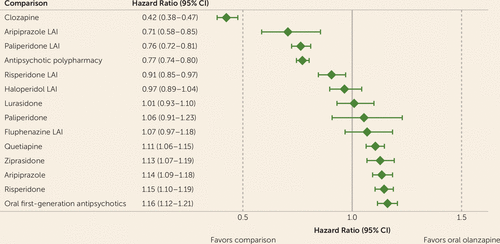
a LAI=long-acting injectable.
Figure 1 also shows that oral first-generation antipsychotics, oral risperidone, oral aripiprazole, oral ziprasidone, and oral quetiapine had a statistically significant higher hazard probability of discontinuation than olanzapine (all p values <0.05, after FDR adjustment). There were no significant differences in the hazard of discontinuation between haloperidol LAI, oral lurasidone, oral paliperidone, or fluphenazine LAI and oral olanzapine, but the 95% confidence intervals were wider for these comparisons, partly because of fewer observations (i.e., less frequent use) of these antipsychotics.
Similar results were obtained in sensitivity analyses, in which the definition of antipsychotic polypharmacy episodes was modified to require 90 and 120 consecutive days of overlap between episodes (for further details, see Figures 1 and 2 in the online supplement). The results were also unaffected by whether the antipsychotic polypharmacy group included episodes of LAI antipsychotics or episodes of clozapine or only included overlapping episodes not involving these agents.
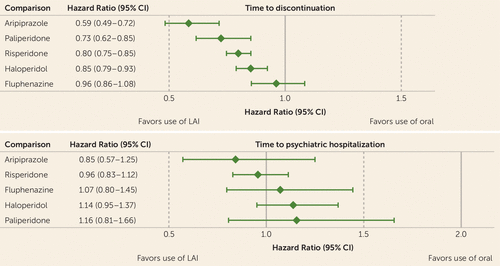
Figure 2 shows the comparison of time to discontinuation between each LAI antipsychotic and its oral version. Aripiprazole LAI (hazard ratio=0.59, 95% CI=0.49, 0.72), paliperidone LAI (hazard ratio=0.73, 95% CI=0.62, 0.85), risperidone LAI (hazard ratio=0.80, 95% CI=0.75, 0.85), and haloperidol LAI (hazard ratio=0.85, 95% CI=0.79, 0.93) were all associated with lower risks of discontinuation compared with their oral formulations (all p values <0.05, after FDR adjustment), whereas fluphenazine LAI was not (hazard ratio=0.96, 95% CI=0.85, 1.08).
Psychiatric Hospitalizations
Median time to psychiatric hospitalization, based on unadjusted Cox proportional hazards regression to account for right censoring, for each antipsychotic cohort is summarized in Table 2. Compared with oral olanzapine and adjusting for covariates, no drug was associated with lower risk for psychiatric hospitalization (Figure 3). Use of oral first-generation antipsychotics (hazard ratio=1.22, 95% CI=1.10, 1.36), paliperidone LAI (hazard ratio=1.27, 95% CI=1.12, 1.43), lurasidone (hazard ratio=1.33, 95% CI=1.09, 1.63), quetiapine (hazard ratio=1.36, 95% CI=1.22, 1.51), haloperidol LAI (hazard ratio=1.39, 95% CI=1.18, 1.62), fluphenazine LAI (hazard ratio=1.40, 95% CI=1.11, 1.76), and ziprasidone (hazard ratio=1.46, 95% CI=1.27, 1.68) was associated with increased risk of hospitalizations compared with oral olanzapine (all p values <0.05, after FDR adjustment). When we compared time to hospitalization between each LAI antipsychotic and its oral version, we found no statistically significant difference between any of the oral LAI comparisons: aripiprazole LAI compared with oral aripiprazole (p=0.41), fluphenazine LAI compared with oral fluphenazine (p=0.63), haloperidol LAI compared with oral haloperidol (p=0.15), paliperidone LAI compared with oral paliperidone (p=0.42), and risperidone LAI compared with oral risperidone (p=0.62) (Figure 2).
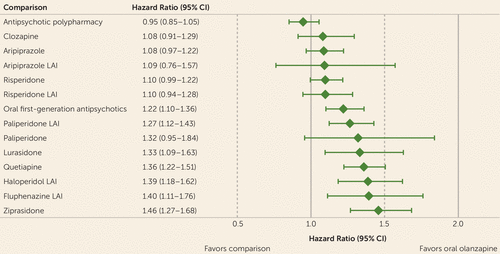
a LAI=long-acting injectable.
| Episodes With a Hospitalization Before Being Right Censored | ||||
|---|---|---|---|---|
| Medication | Number of Patients | Number of Episodes | N | % |
| Total | 35,072 | 54,803 | 7,257 | 13.24 |
| Clozapine | 653 | 659 | 177 | 26.86 |
| Antipsychotic polypharmacy | 4,171 | 9,221 | 1,364 | 14.79 |
| Aripiprazole LAI | 220 | 224 | 32 | 14.29 |
| Paliperidone LAI | 2,630 | 2,758 | 543 | 19.69 |
| Risperidone LAI | 1,323 | 1,367 | 225 | 16.46 |
| Lurasidone | 962 | 968 | 114 | 11.78 |
| Olanzapine | 4,699 | 5,000 | 609 | 12.18 |
| Fluphenazine LAI | 489 | 508 | 85 | 16.73 |
| Haloperidol LAI | 1,222 | 1,294 | 221 | 17.08 |
| Quetiapine | 7,220 | 7,722 | 1,000 | 12.95 |
| Paliperidone | 205 | 210 | 35 | 16.67 |
| Aripiprazole | 5,685 | 6,043 | 620 | 10.26 |
| Ziprasidone | 1,916 | 1,995 | 289 | 14.49 |
| Oral first-generation antipsychotics | 6,063 | 6,689 | 893 | 13.35 |
| Risperidone | 9,406 | 10,145 | 1,050 | 10.35 |
TABLE 2. Unadjusted median time to psychiatric hospitalization by antipsychotic medication in a national sample of patients in the U.S. Department of Veterans Affairs health care systema
Discussion
In support of our study hypothesis, we found that in a nationwide sample of veterans, clozapine and LAI second-generation antipsychotics were less likely to be discontinued, on average, than oral olanzapine and other oral antipsychotic medications. In addition, we observed that antipsychotic polypharmacy was associated with a longer time to discontinuation. The clozapine and LAI second-generation antipsychotic findings are consistent with the results from a similar, smaller VA study (7), as well as with the results from other database studies conducted in Sweden (2), Finland (20), and Canada (3). These results are partially similar to those of a study of Medicaid patients with treatment-resistant schizophrenia (21), which also found that clozapine was associated with increased time to discontinuation but differed in that the authors found a clear superiority of clozapine in reducing hospitalization. This difference may be related to the fact that their study focused only on patients defined as treatment nonresponders, whereas our study examined hospitalizations of all patients.
The issue of replication in medicine (22, 23) is particularly important in nonrandomized studies (24), and showing findings in this large U.S.-based study that are almost identical to those from recently published findings from Scandinavia and Finland greatly strengthens the validity and generalizability of the results. In contrast to our hypothesis, neither clozapine nor LAI antipsychotic medications were better than oral olanzapine in decreasing risk for hospitalizations, a result also very similar to findings reported by Tiihonen et al. (2) (see Figure S9 in the online supplement).
Our study extends the results from the Tiihonen et al. study (2) in that we included oral and LAI aripiprazole, whereas Tiihonen et al. did not include aripiprazole LAI, and we found that aripiprazole LAI was associated with a lower discontinuation rate than oral aripiprazole. In addition, the Tiihonen et al. study did not include oral lurasidone, which is a newer and more expensive oral medication and which did not outperform oral olanzapine in our study. Finally, although we used a different approach than Tiihonen et al. to define antipsychotic polypharmacy, our analyses were between individuals for incident users, whereas theirs were within individuals. Regardless, the results of the two studies are strikingly similar.
In our study, as in the Tiihonen et al. study, first-generation antipsychotics were more likely to be discontinued than second-generation antipsychotics. A possible explanation for this observation may be that prescribers and patients opt to discontinue first-generation antipsychotics because of their concerns about extrapyramidal side effects, although not all second-generation antipsychotics (e.g., risperidone, paliperidone) are devoid of these effects, and randomized controlled trials do not always demonstrate that first-generation antipsychotics are associated with greater rates of extrapyramidal side effects (25).
Clozapine was the treatment with the smallest risk for all-cause discontinuation. The smaller risk of discontinuation for clozapine may be related to the use of this agent in people who are more likely to be adherent to the monitoring requirements or the increased frequency of visits required to monitor the use of this agent. The smaller risk of clozapine discontinuation may also be related to the lack of alternative treatments for people who fail to demonstrate a robust response to clozapine. However, other database studies (26), as well as meta-analyses of randomized controlled trials, have also found that clozapine was the most efficacious treatment in schizophrenia (27–29). Prospective studies would have to be conducted to address this issue empirically.
We also found increased time to discontinuation among veterans receiving the LAI formulation compared with the oral formulation of the same medication. Meta-analyses have revealed similar findings in studies that used mirror-image (before-after) designs (11, 30, 31), while a meta-analysis of randomized controlled trials comparing the effectiveness of the same oral antipsychotic compared with the LAI antipsychotic showed no significant difference (32). This difference in effectiveness of LAI antipsychotic medications in observational studies compared with randomized controlled trials may be because many of the more severely ill, nonadherent patients, who might benefit most from LAIs, are often not included in randomized controlled trials. In addition, the increased time to LAI discontinuation may also reflect the reluctance of prescribers to discontinue an LAI once a patient has been started on such a medication. In light of the limited use of LAIs in the United States, our results add further evidence supporting and encouraging broader use of LAIs (33). The findings of differences between different medications regarding time to discontinuation were not present when the effect of these antipsychotic medications on time to hospitalization was examined, which revealed no medication to be better than oral olanzapine, including no difference between oral and LAI antipsychotics (Figure 2), but commonly used second-generation antipsychotics, such as quetiapine and ziprasidone, were found to be worse than olanzapine, consistent with the findings of Tiihonen et al. (2) and Taipale et al. (20) in Sweden and Finland, respectively. It is conceivable that discontinuation as a proxy for acceptability or effectiveness works best in blinded randomized trials. Hospitalization, perhaps more objective and less influenced by knowledge of treatment, seems to be more patient oriented and is more affected by factors other than antipsychotic medication.
Previous studies (34–37) and treatment guidelines (38) have suggested that the use of antipsychotic polypharmacy may not be recommended. However, the findings from our study, together with findings from similar studies conducted in Canada and Finland using population-based data (3, 5), showed a significantly longer time to discontinuation with antipsychotic polypharmacy compared with monotherapy. It may not be that polypharmacy is always better but that monotherapy with certain antipsychotics may be worse, as Tiihonen et al. found with polypharmacy compared with quetiapine (Figure 3). In contrast, a meta-analysis of randomized controlled trials of antipsychotic polypharmacy compared with monotherapy did not find a better treatment response for polypharmacy, defined as study-specific inefficacy and all-cause discontinuation (37). We note that our approach to operationalizing antipsychotic polypharmacy included periods of time during an episode of treatment when two antipsychotics overlapped and when such combination treatment had yet to begin or had ended. As opposed to analyzing only periods of overlapping treatment, we included the entire episode of each drug treatment that was part of the overlap. This approach reflects the possibility that polypharmacy as an augmentation strategy allows for an individual drug episode to continue longer than it would when used alone. Our findings are nevertheless similar to those of Tiihonen et al. (2) and Taipale et al. (20), and together with the conflicting data between randomized controlled trials and registry-based studies, they suggest that further research on polypharmacy for schizophrenia is warranted.
Strengths and Limitations
We analyzed a large and diverse sample of incident users of antipsychotic medications over 5 years and adjusted the analyses for many demographic and clinical covariates available in the VA health care service utilization data set. Compared with similar database studies by Tiihonen et al. (2) and Taipale et al. (20), this study is limited in that our between-subject design may not have adequately addressed residual confounding related to selection bias compared with those studies that used within-subject approaches; however, our results, which were very similar to those of Tiihonen et al. and Taipale et al., indicate that this is not a major limitation.
The results may be biased because of unmeasured confounding variables. In addition, the analyses performed on some of the newer antipsychotics (oral paliperidone and aripiprazole LAI) include a relatively small number of veterans, although each sample did include several hundred veterans. As a result of infrequent prescribing, we were not able to analyze rates of discontinuation of LAI olanzapine. Additionally, the results of this study are relevant to veterans with schizophrenia treated by the VA, who are more likely to be male, be older, have higher incomes, and be hospitalized and less likely to receive psycho-social treatment (39) compared with patients treated via Medicaid. Also, the VA does not limit the use of long-acting paliperidone and aripiprazole, which are more expensive compared with generic LAIs. An additional potential limitation is the assumption that time to all-cause discontinuation is an overall effectiveness measure; this study did not include clinical measures, such as symptoms and functioning.
Conclusions
Among veterans with schizophrenia, those who initiated antipsychotic treatment with clozapine, long-acting injectable second-generation medications, and antipsychotic polypharmacy experienced longer episodes of continuous therapy and lower rates of treatment discontinuation but not of psychiatric hospitalization compared with those who initiated oral olanzapine. This may be because clozapine, long-acting injectables, and polypharmacy are often used as a last resort, and once clinicians have put patients on one of these options, they may be reluctant to change the treatment, because they do not see any other option. No medications were associated with decreased risk of hospitalization, but quetiapine was associated with a 36% worse outcome compared with olanzapine. These results support earlier evidence from European data samples.
1. : Role of electronic health records in comparative effectiveness research . J Comp Eff Res 2013 ; 2 : 529 – 532 Crossref, Medline, Google Scholar
2. : Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29, 823 patients with schizophrenia . JAMA Psychiatry 2017 ; 74 : 686 – 693 Crossref, Medline, Google Scholar
3. : Comparative effectiveness and safety of antipsychotic drugs in schizophrenia treatment: a real-world observational study . Acta Psychiatr Scand 2016 ; 134 : 374 – 384 Crossref, Medline, Google Scholar
4. : Ambulatory use of olanzapine and risperidone: a population-based study on persistence and the use of concomitant therapy in the treatment of schizophrenia . Can J Psychiatry 2005 ; 50 : 901 – 908 Crossref, Medline, Google Scholar
5. : Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia . JAMA Psychiatry 2019 ; 76 : 499 – 507 Crossref, Medline, Google Scholar
6. : Treatment persistence: a comparison among patients with schizophrenia who were initiated on atypical antipsychotic agents . J Clin Pharm Ther 2006 ; 31 : 57 – 65 Crossref, Medline, Google Scholar
7. : Time to discontinuation of first- and second-generation antipsychotic medications in the treatment of schizophrenia . Schizophr Res 2011 ; 131 : 127 – 132 Crossref, Medline, Google Scholar
8. : Risk of discontinuation of atypical antipsychotic agents in the treatment of schizophrenia . Schizophr Res 2008 ; 98 : 8 – 15 Crossref, Medline, Google Scholar
9. : Antipsychotic polypharmacy and risk of death from natural causes in patients with schizophrenia: a population-based nested case-control study . J Clin Psychiatry 2010 ; 71 : 103 – 108 Crossref, Medline, Google Scholar
10. : Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis . Br J Psychiatry 2016 ; 209 : 385 – 392 Crossref, Medline, Google Scholar
11. : Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies . J Clin Psychiatry 2013 ; 74 : 957 – 965 Crossref, Medline, Google Scholar
12. :
13. : Good research practices for comparative effectiveness research: approaches to mitigate bias and confounding in the design of nonrandomized studies of treatment effects using secondary data sources: the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Retrospective Database Analysis Task Force Report, Part II . Value Health 2009 ; 12 : 1053 – 1061 Medline, Google Scholar
14. : Evaluating medication effects outside of clinical trials: new-user designs . Am J Epidemiol 2003 ; 158 : 915 – 920 Crossref, Medline, Google Scholar
15. : Effectiveness of antipsychotic drugs in patients with chronic schizophrenia . N Engl J Med 2005 ; 353 : 1209 – 1223 Crossref, Medline, Google Scholar
16. : Estimating medication persistency using administrative claims data . Am J Manag Care 2005 ; 11 : 449 – 457 Medline, Google Scholar
17. : Adherence to atypical antipsychotic treatment among newly treated patients: a population-based study in schizophrenia . J Clin Psychiatry 2007 ; 68 : 818 – 825 Crossref, Medline, Google Scholar
18. : Regression models and life‐tables . J R Stat Soc B 1972 ; 34 : 187 – 202 Crossref, Google Scholar
19. : Longitudinal data analysis using generalized linear models . Biometrika 1986 ; 73 : 13 – 22 Crossref, Google Scholar
20. : Comparative effectiveness of antipsychotic drugs for rehospitalization in schizophrenia: a nationwide study with 20-year follow-up . Schizophr Bull 2018 ; 44 : 1381 – 1387 Crossref, Medline, Google Scholar
21. : Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia . Am J Psychiatry 2016 ; 173 : 166 – 173 Link, Google Scholar
22. : 1,500 scientists lift the lid on reproducibility . Nature 2016 ; 533 : 452 – 454 Crossref, Medline, Google Scholar
23. ; Open Science Collaboration: Psychology: estimating the reproducibility of psychological science . Science 2015 ; 349 : aac4716 Crossref, Medline, Google Scholar
24. : Contradicted and initially stronger effects in highly cited clinical research . JAMA 2005 ; 294 : 218 – 228 Crossref, Medline, Google Scholar
25. : Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial . JAMA 2014 ; 311 : 1978 – 1987 Crossref, Medline, Google Scholar
26. : Time to discontinuation of antipsychotic drugs in a schizophrenia cohort: influence of current treatment strategies . Ther Adv Psychopharmacol 2014 ; 4 : 228 – 239 Crossref, Medline, Google Scholar
27. : A meta-analysis of the efficacy of second-generation antipsychotics . Arch Gen Psychiatry 2003 ; 60 : 553 – 564 Crossref, Medline, Google Scholar
28. : Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis . Lancet 2013 ; 382 : 951 – 962 Crossref, Medline, Google Scholar
29. : Association With hospitalization and all-cause discontinuation among patients with schizophrenia on clozapine vs other oral second-generation antipsychotics: a systematic review and meta-analysis of cohort studies . JAMA Psychiatry 2019 ; 76 : 1052 – 1062 Crossref, Medline, Google Scholar
30. : Depot antipsychotic drugs . Drugs 1994 ; 47 : 741 – 773 Crossref, Medline, Google Scholar
31. : Assessing the comparative effectiveness of long-acting injectable vs. oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry . J Clin Epidemiol 2013 ; 66 ( Suppl ): S37 – S41 Crossref, Medline, Google Scholar
32. : Does formulation matter? a systematic review and meta-analysis of oral versus long-acting antipsychotic studies . Schizophr Res 2017 ; 183 : 10 – 21 Crossref, Medline, Google Scholar
33. : Expanding therapy with long-acting antipsychotic medication in patients with schizophrenia . JAMA Psychiatry 2015 ; 72 : 745 – 746 Crossref, Medline, Google Scholar
34. : Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res 2007 ; 89 : 91 – 100 Crossref, Medline, Google Scholar
35. : Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials . Schizophr Bull 2009 ; 35 : 443 – 457 Crossref, Medline, Google Scholar
36. : Critical review of antipsychotic polypharmacy in the treatment of schizophrenia . Int J Neuropsychopharmacol 2014 ; 17 : 1083 – 1093 Crossref, Medline, Google Scholar
37. : Antipsychotic augmentation vs. monotherapy in schizophrenia: systematic review, meta-analysis and meta-regression analysis . World Psychiatry 2017 ; 16 : 77 – 89 Crossref, Medline, Google Scholar
38. : American Psychiatric Association Steering Committee on Practice Guidelines: Practice guideline for the treatment of patients with schizophrenia, second edition . Am J Psychiatry 2004 ; 161 : 1 – 56 Link, Google Scholar
39. : Benchmarking treatment of schizophrenia: a comparison of service delivery by the national government and by state and local providers . J Nerv Ment Dis 2000 ; 188 : 209 – 216 Crossref, Medline, Google Scholar



