Treatment-Specific Associations Between Brain Activation and Symptom Reduction in OCD Following CBT: A Randomized fMRI Trial
Abstract
Objective:
The authors sought to examine whether brain activity is associated with treatment response to cognitive-behavioral therapy (CBT) in adolescents and adults with obsessive-compulsive disorder (OCD), and whether any associations are treatment specific relative to an active control psychotherapy (stress management therapy; SMT).
Methods:
Eighty-seven patients with OCD (age range 12–45 years; 57 female, 39 medicated) were randomly assigned to receive 12 weeks of CBT or SMT. Prior to treatment, functional MRI scans were conducted in patients performing an incentive flanker task, which probes brain activation to both cognitive control and reward processing. Voxelwise linear mixed-effects models examined whether baseline brain activation was differentially associated with change in scores on the Yale-Brown Obsessive Compulsive Scale (standard or Children’s version) over the course of CBT or SMT treatment.
Results:
Within the CBT group, a better treatment response was significantly associated with greater pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control and within the ventromedial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. In contrast, reduced pretreatment activation within a largely overlapping set of regions was significantly associated with a better treatment response to SMT.
Conclusions:
The study findings demonstrate that associations between brain activation and treatment response were treatment specific to CBT relative to a control psychotherapy and that these associations were stable from adolescence to mature adulthood. Such treatment-specific associations are important for the development of biomarkers to personalize treatment in OCD.
Obsessive-compulsive disorder (OCD) affects 1%−3% of children and adolescents and 2%−3% of adults, and is characterized by recurrent and intrusive obsessive thoughts that patients attempt to neutralize with behavioral and/or mental compulsions (1). Psychological treatment for OCD consists primarily of cognitive-behavioral therapy (CBT) incorporating in vivo exposure and response prevention (1). During exposure and response prevention, patients interact with symptom-provoking stimuli while resisting compulsions, thereby learning that compulsive rituals are not necessary to prevent feared outcomes (2). Meta-analyses show large effect sizes for symptom severity reductions following CBT, even when compared with active control psychotherapies (1, 3). However, approximately 30%−50% of patients with OCD do not respond adequately to treatment, and reliable predictors of response to treatment have yet to be established (3).
The neural mechanisms underlying OCD remain poorly understood, but cingulo-opercular and orbito-striato-thalamic networks are commonly implicated in patients across the lifespan (4). OCD patients show impaired performance as well as hypoactivation within cingulo-opercular regions during cognitive control, identifying possible mechanisms of impaired control over obsessions and compulsions (4, 5). The orbito-striato-thalamic network appears hyperconnected at rest and hyperactive during symptom provocation and habit-driven responding in the disorder, whereas orbito-striatal regions are hypoactive during reward processing and decision making, suggesting an imbalance of habit and goal-directed functions within these regions in OCD (6–8). Other work has linked cingulo-opercular and orbito-striatal activation during cognitive control and reward processing with treatment response to CBT (9, 10). Greater cingulo-opercular functioning during cognitive control may indicate a greater ability to engage this network during self-regulation to implement response-prevention strategies (10). Orbito-striatal and connected limbic regions are key for maintaining motivation as well as for learning new associations for environmental stimuli and behavioral actions, as required during exposure and response prevention (11, 12).
Previous studies have examined whether individual differences in pretreatment brain activation or structure are associated with treatment response to CBT in OCD, implicating cingulo-opercular, orbito-striatal, and amygdalar regions (13–15). One study (10) reported that adult patients with more pretreatment activation during cognitive control within cingulo-opercular, dorsolateral prefrontal, posterior cingulate, striatal, and temporal regions had a better response to CBT. However, the existing literature has limitations. First, published neuroimaging studies have not included control psychotherapy groups and have not been able to separate findings associated with symptom change due to CBT from nonspecific symptom reduction (10, 16). Findings of treatment-specific associations are critical in developing a mechanistic understanding of CBT, as well as in developing individually tailored treatment algorithms (14, 15). Second, neuroimaging studies of treatment response in patients with OCD have focused primarily on adults. Since early intervention may well have advantages in disorders like OCD that are often early in onset, understanding whether treatment-specific predictors of recovery generalize beyond mature adulthood is important (1, 9).
Therefore, in this study, we explored the associations between task activation and treatment response to CBT compared with a control psychotherapy—stress management therapy (SMT)—in adolescent and adult patients with OCD. Effective engagement with CBT likely places greater demands than control therapies on self-regulatory processes, which allow patients to control emotions, cognitions, and behaviors in the face of symptom triggers (10). It also requires the capacity for maintaining the motivation to work toward long-term goals (e.g., symptom recovery) in the face of challenging exposures (9, 12). Consequently, we anticipated that more pretreatment activation within cingulo-opercular regions during cognitive control and orbito-striatal regions during reward processing would be associated with larger symptom reductions in the CBT group, and that these associations would be treatment specific relative to SMT. Secondary analyses tested whether associations remained in both adolescent and adult subgroups.
Methods
Participants
Eighty-seven patients were included in the analysis. Of these, 42 (19 adolescents, 23 adults; 20 medicated; 28 females) were assigned to CBT and 45 (20 adolescents, 25 adults; 19 medicated; 29 females) were assigned to SMT. Linear mixed-effects models allow for the inclusion of patients with missing data points, and all patients for whom usable scans and assessment data were available were included. The patients’ demographic and clinical characteristics are summarized in Table 1. Patients were recruited from outpatient programs at the University of Michigan Health System, from social media advertisements, and via referrals from clinicians. We focused on two critical age periods: adolescence (13–17 years) and adulthood (25–45 years). Patients were required to have an early age at symptom onset (≤15 years) and moderate or greater levels of symptoms at baseline, as indicated by a score ≥16 on either the standard or the Children’s version of the Yale-Brown Obsessive Compulsive Scale (Y-BOCS) (17, 18). Developed to measure OCD across the lifespan, the Y-BOCS instruments use nearly identical wording and are comparable in terms of validity and reliability in adolescents and adults (17, 18). Full details on inclusion and exclusion criteria, characterization of participants, and a CONSORT chart (Figure S1) are provided in the online supplement.
| SMT Group | CBT Group | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adolescents (N=20) | Adults (N=25) | Full Group (N=45) | Adolescents (N=19) | Adults (N=23) | Full Group (N=42) | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |
| Female | 13 | 65 | 16 | 64 | 29 | 64 | 14 | 74 | 14 | 61 | 28 | 67 |
| Antidepressant medication | 8 | 40 | 11 | 44 | 19 | 42 | 10 | 53 | 10 | 44 | 20 | 48 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 15.35 | 1.77 | 31.84 | 5.55 | 24.51 | 9.32 | 15.53 | 1.63 | 31.42 | 5.80 | 24.23 | 9.13 |
| Number of interference errors | 27.45 | 12.51 | 27.20 | 13.99 | 27.31 | 13.21 | 30.37 | 11.62 | 30.74 | 14.01 | 30.57 | 12.83 |
| Reaction time (ms) | ||||||||||||
| Interference | 17.18 | 10.02 | 19.94 | 11.25 | 18.72 | 10.69 | 15.42 | 9.63 | 22.46 | 12.86 | 19.28 | 11.92 |
| Incentive | –13.28 | 31.11 | –22.30 | 36.33 | –18.29 | 34.04 | –15.70 | 31.01 | –9.80 | 28.97 | –12.47 | 29.69 |
| Y-BOCS score | ||||||||||||
| Week 1 | 28.05 | 5.23 | 26.88 | 4.04 | 27.40 | 4.59 | 26.74 | 5.64 | 23.74 | 4.92 | 25.10 | 5.41 |
| Week 6 | 25.33 | 7.02 | 23.00 | 5.00 | 24.13 | 6.11 | 21.28 | 6.36 | 17.98 | 6.18 | 19.41 | 6.39 |
| Week 12 | 21.13 | 9.61 | 21.45 | 6.84 | 21.30 | 8.16 | 14.50 | 10.48 | 11.73 | 5.15 | 12.96 | 7.96 |
| QIDS score | ||||||||||||
| Week 1 | 7.26 | 4.13 | 7.65 | 4.23 | 7.48 | 4.14 | 8.12 | 5.45 | 6.70 | 3.69 | 7.30 | 4.51 |
| Week 6 | 6.53 | 3.60 | 6.58 | 3.76 | 6.55 | 3.63 | 8.67 | 5.10 | 5.25 | 2.82 | 6.71 | 4.26 |
| Week 12 | 6.11 | 4.60 | 6.62 | 4.67 | 6.38 | 4.58 | 6.73 | 4.88 | 4.53 | 2.59 | 5.50 | 3.86 |
| HAM-A score | ||||||||||||
| Week 1 | 10.10 | 6.95 | 12.76 | 7.06 | 11.58 | 7.06 | 14.32 | 11.88 | 9.17 | 6.99 | 11.50 | 9.74 |
| Week 6 | 8.85 | 7.42 | 11.57 | 6.34 | 10.24 | 6.94 | 12.00 | 9.40 | 10.05 | 8.38 | 10.89 | 8.76 |
| Week 12 | 7.11 | 5.82 | 10.76 | 6.22 | 9.03 | 6.24 | 10.88 | 12.76 | 7.90 | 6.74 | 9.22 | 9.84 |
| CGI severity score | ||||||||||||
| Week 1 | 4.60 | 0.75 | 4.60 | 0.65 | 4.60 | 0.69 | 4.58 | 0.77 | 4.39 | 0.72 | 4.48 | 0.74 |
| Week 6 | 4.40 | 0.82 | 4.33 | 0.73 | 4.37 | 0.77 | 4.25 | 1.07 | 3.86 | 0.85 | 4.03 | 0.96 |
| Week 12 | 3.68 | 1.11 | 4.05 | 0.97 | 3.87 | 1.04 | 3.25 | 1.39 | 2.80 | 0.83 | 3.00 | 1.12 |
TABLE 1. Characteristics and behavioral performance of patients in an fMRI study of CBT and SMT for obsessive-compulsive disordera
Written informed consent and assent were obtained from all patients and/or their legal guardians, according to procedures reviewed and approved by the Institutional Review Board of the University of Michigan. Data for included patients were collected between March 2015 and October 2018 as part of a larger clinical trial (ClinicalTrials.gov identifier: NCT02437773). The present report is a planned interim analysis focused on the examination of pretreatment neural associations with subsequent treatment response.
Study Design
Patients were randomly assigned to receive either 12 weeks of individual CBT incorporating exposure and response prevention or 12 weeks of SMT (19, 20). Assignment was stratified by medication, gender, and age, using block randomization. SMT has been used in non-imaging studies of CBT and has been shown to produce small to moderate reductions in OCD symptoms (2, 21, 22). SMT was included to control for potential nonspecific effects of time and weekly meetings with a therapist on symptom change. Y-BOCS assessment of OCD severity occurred at the beginning, middle, and end of treatment (weeks 1, 6, and 12) by an independent rater blind to treatment assignment. Patients were also assessed for anxiety and depression symptoms (see the online supplement) and underwent scanning at the Functional MRI Laboratory at the University of Michigan <6 weeks before treatment (CBT group, mean=1.52 weeks, SD=0.7; SMT group, mean=1.76 weeks, SD=1.19). Patients were assigned to treatment group after the scan. Details of treatment protocols and MRI acquisition are provided in the online supplement.
Incentive flanker task.
In the incentive flanker task, patients pressed one of two buttons to identify a target letter (S, K, H, and C) surrounded by four flankers, which either mapped to the same button response (low interference) or the opposite response (high interference) as the target. Target and flanker stimuli were preceded by cues (1.5–10 seconds) indicating how much money patients stood to lose (for an error) or gain (for a correct response) in the upcoming trial (0¢ in 50% of the trials, 10¢ in 25% of the trials, and 25¢ in 25% of the trials). Patients’ responses lead to a feedback signal—white (correct) or red (incorrect) asterisks in place of the target/flanker stimuli. In total, participants completed four runs, each consisting of 48 trials (scan duration, ∼25 minutes). Prior to the fMRI session, patients practiced the incentive flanker task to achieve an error rate of ∼15%, titrated using a subject-specific response deadline (6). Previous studies indicate that rewards and punishments on the incentive flanker task decrease reaction times in both OCD patients and healthy subjects, in line with a motivational effect (23). Performance measures include interference reaction time, incentivized reaction time, and interference errors (see the online supplement). Patients received approximately $10–$20, based on amounts earned and lost for each incentive trial (see Figure S2 in the online supplement).
Statistical Analysis
Clinical and behavioral data.
Linear mixed-effects models were used to test for differences in treatment response between the CBT and SMT groups, as well as for relationships between task performance, treatment group, and treatment outcome (see the online supplement).
fMRI data.
Standard preprocessing steps were performed in SPM12 (http://www.fil.ion.ucl.ac.uk/spm). First-level contrasts examined brain activation during cognitive control (“interference inhibition”—correct high- versus correct low-interference trials), interference errors (incorrect versus correct high-interference trials), and reward processing (rewarded correct trials versus nonrewarded correct trials, regardless of interference level). At the second level, voxelwise linear mixed-effects analyses were performed in the nlme package (24) for R (http://www.r-project.org). These models examined voxel-activation-by-week-by-treatment-group interactions on Y-BOCS scores collected at treatment weeks 1, 6, and 12 while controlling for age group and medication status. A random intercept term for patient was included to account for the nonindependence of observations. T-scores for the interaction of interest were used to create whole-brain t-maps, which were then thresholded at a voxel-level threshold of p<0.001 and a family-wise error cluster-level-corrected threshold of p<0.05. The MarsBar toolbox (http://marsbar.sourceforge.net/) was used to extract mean blood-oxygen-level-dependent signal for each patient in significant clusters. To determine which treatment group(s) drove significant interactions, extracted values were plotted and subjected to follow-up analyses examining mean cluster-activation-by-week interactions on Y-BOCS scores performed within each level of treatment group (i.e., CBT, SMT). Voxelwise follow-up analyses were also performed within CBT and SMT subgroups. Further details on fMRI preprocessing and analyses are provided in the online supplement.
Results
Clinical Outcomes
Both the CBT and SMT groups showed a significant decrease in symptoms over time (CBT group: B=−6.13, t=−12.89, p<0.001, 95% CI=−7.07, −5.18; SMT group: B=−2.94, t=−5.64, p<0.001, 95% CI=−3.9, −1.9). There was a significant treatment-group-by-week interaction, such that CBT resulted in a steeper reduction in Y-BOCS scores over time compared with SMT (B=−3.21, t=−4.52, p=0.001, 95% CI=−4.61, −1.81) (Table 1 and Figure 1).
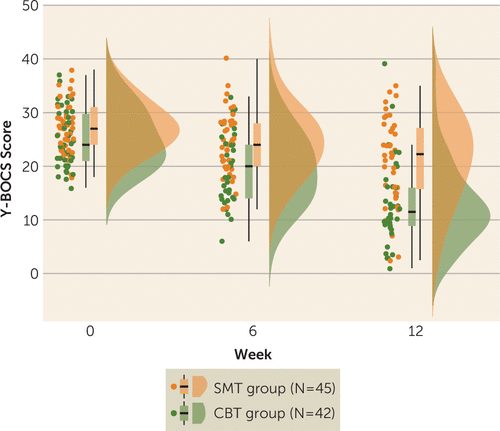
FIGURE 1. Raincloud plot of the change in OCD symptoms over the course of CBT or SMT treatment in patients with OCDa
a CBT=cognitive-behavioral therapy; OCD=obsessive-compulsive disorder; SMT=stress management therapy; Y-BOCS=Yale-Brown Obsessive Compulsive Scale (standard or Children’s version). Patients who underwent CBT (B=−6.13, t=−12.89, p<0.001, 95% CI=−7.07, −5.18) and those who underwent SMT (B =−2.94, t=−5.64, p<0.001, 95% CI=−3.9, −1.9) showed a significant decrease in symptoms over the course of treatment. There was a significant treatment-group-by-week interaction. Patients who underwent CBT showed a steeper reduction over time in scores on the Y-BOCS compared with patients who underwent SMT (B=−3.21, t=−4.52, p=0.001, 95% CI=−4.61, −1.81).
Behavioral Data
There were no significant task-performance-by-treatment-group-by-week interactions on Y-BOCS scores (all p values >0.05; see the online supplement).
fMRI Results
Brain activation maps are provided in Figures S3–S5 in the online supplement. During cognitive control, activation within the left premotor cortex and right temporal lobe showed a significant voxel-activation-by-treatment-group-by-week interaction. At a relaxed cluster-forming threshold of p<0.0025, a similar finding was observed within a hypothesized cingulo-opercular region of interest—the rostral anterior cingulate cortex (rACC). Follow-up analyses revealed that while more pretreatment activation within the right temporal lobe (CBT group: B=−3.96, p=0.003; SMT group: B=5.09, p<0.001), the rACC (CBT group: B=−2.68, p=0.004; SMT group: B=2.94, p=0.01), and the left premotor cortex (CBT group: B=−2.91, p=0.002; SMT group: B=3.61, p=0.008) was associated with a better treatment response in patients undergoing CBT, less activation was associated with a better treatment response in the same regions in patients undergoing SMT (Table 2 and Figure 2; see also Figure S6 in the online supplement).
| Contrast | MNI Coordinates (x, y, z) | Max-T | No. of Voxels | Brodmann Areas |
|---|---|---|---|---|
| Cognitive control | ||||
| Left and right rACCb | –9, 50, 29 | –4.09 | 70 | 9,10,32 |
| Left premotor cortex | –9, 5, 68 | –5.28 | 43 | 6 |
| Right temporal lobe | 60, –19, –4 | –4.68 | 52 | 21,22 |
| Reward processing | ||||
| Left and right vmPFC/OFC/amygdala/IFG/DLPFC | –6, 65, –10 | –6.21 | 1,502 | 10, 11, 8, 47, 6, 32, 25, 9, 45, |
| Left temporal lobe | –57, –13, –16 | –6.07 | 283 | 21, 22 |
| Left inferior parietal lobe | –42, –70, 41 | –4.12 | 108 | 39, 40, 19 |
| Right premotor cortex/posterior insula | 51, –1, 5 | 4.82 | 100 | 6 |
| Right inferior parietal lobe | 48, –34, 32 | 5.15 | 75 | 40 |
TABLE 2. Brain regions that were significant in the linear mixed-effects model of the interactive effect of voxel activation, treatment group, and week on Y-BOCS scoresa
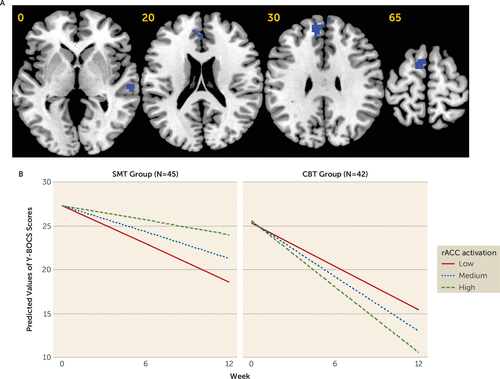
FIGURE 2. Brain regions significant in the linear mixed-effects model for the cognitive control contrast before initiation of CBT or SMT in patients with OCDa
a CBT=cognitive-behavioral therapy; OCD=obsessive-compulsive disorder; rACC=rostral anterior cingulate; SMT=stress management therapy; Y-BOCS=Yale-Brown Obsessive Compulsive Scale (standard or Children’s version). Panel A presents axial slices showing brain regions that were significant in the linear mixed-effects model of the interactive effect of voxel activation during cognitive control, treatment group, and week on Y-BOCS scores. All regions are presented at an uncorrected cluster-forming threshold of p<0.001 and a family-wise error corrected cluster threshold of p<0.05, except for the cluster in the rACC, which is presented using an uncorrected cluster-forming threshold of p<0.0025 and a family-wise error corrected cluster threshold of p<0.05. Blue indicates regions where more pretreatment activation was associated with greater symptom reduction over time in patients undergoing CBT, but a smaller reduction in symptoms over time in patients undergoing SMT. Panel B presents graphs showing predicted model estimates for the CBT and SMT groups. The y-axis represents the predicted Y-BOCS score based on model estimates, and separate lines indicate level of rACC activation (low=one standard deviation below the mean; medium=mean; high=one standard deviation above the mean). Graphs for other regions are provided in the online supplement.
During reward processing, a significant voxel-activation-by-treatment-group-by-week interaction was found within a large cluster incorporating the left and right ventromedial prefrontal cortex, orbitofrontal cortex, amygdala, inferior frontal gyrus, and dorsolateral prefrontal cortex. More pretreatment activation within these regions was associated with a better treatment response within the CBT group (B=−3.91, p<0.001) but a worse treatment response in the SMT group (B=5.62, p<0.001). A similar pattern of findings was found in the left temporal lobe (CBT group: B=−3.15, p<0.001; SMT group: B=4.37, p<0.001) and the parietal lobe (CBT group: B=−1.22, p=0.045; SMT group: B=2.53, p<0.001). Also significant in the interaction analysis were the right posterior insula (CBT group: B=5.35, p<0.001; SMT group: B=−2.53, p=0.03) and the parietal lobe (CBT group: B=5.26, p<0.001; SMT group: B=−2.75, p=0.015), in which relatively less activation was associated with a better response to CBT but more activation was associated with a better response to SMT (Table 2 and Figure 3; see also Figure S7 in the online supplement).
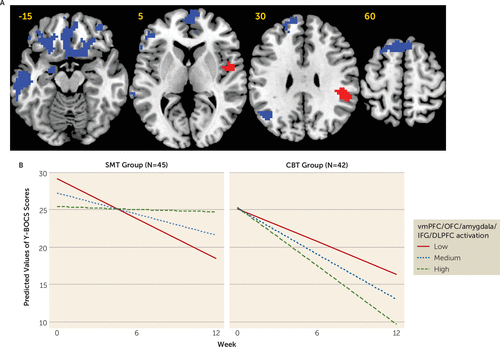
FIGURE 3. Brain regions significant in the linear mixed-effects model for the reward processing contrast before initiation of CBT or SMT in patients with OCDa
a CBT=cognitive-behavioral therapy; DLPFC=dorsolateral prefrontal cortex; IFG=inferior frontal gyrus; OCD=obsessive-compulsive disorder; OFC=orbitofrontal cortex; SMT=stress management therapy; vmPFC=ventromedial prefrontal cortex; Y-BOCS=Yale-Brown Obsessive Compulsive Scale (standard or Children’s version). Panel A presents axial slices showing brain regions that were significant in the linear mixed-effects model of the interactive effect of voxel activation during reward processing, treatment group, and week on Y-BOCS scores. All regions are presented at an uncorrected cluster-forming threshold of p<0.001 and a family-wise error corrected cluster threshold of p<0.05. Blue indicates regions where more pretreatment activation was associated with greater symptom reduction over time in patients undergoing CBT, but a smaller reduction in symptoms over time in patients undergoing SMT. Red indicates regions where more pretreatment activation was associated with a smaller reduction in symptoms over time in patients undergoing CBT, but a greater reduction in symptoms over time in patients undergoing SMT. Panel B presents graphs showing predicted model estimates for the CBT and SMT groups. The y-axis represents the predicted Y-BOCS score based on model estimates, and separate lines indicate level of vmPFC/OFC/amygdala/IFG/DLPFC activation (low=one standard deviation below the mean; medium=mean; high=one standard deviation above the mean). Graphs for other regions are provided in the online supplement.
Voxelwise findings at each level of treatment group (CBT, SMT) are presented in Figures S8–S11 in the online supplement.
Effects of Age
Both the adult and the adolescent subgroups showed similar symptom reductions during CBT (adults: B=−6.12, p<0.001; adolescents: B=−6.13, p<0.001) and SMT (adults: B=−2.74, p<0.001; adolescents: B=−3.21, p<0.001), as well as a group-by-time interaction, indicating greater efficacy of CBT relative to SMT (adults: B=−3.43, p<0.001; adolescents: B=−2.93, p=0.01). Adding an additional interaction term for treatment group by week by age group to the model decreased model fit, as determined by the Bayesian information criterion (without interaction with age group, 1,543.81; including interaction with age group, 1,552.15). Moreover, this interaction was nonsignificant (B=−0.57, t=−0.39, p=0.69, 95% CI=−3.4, 2.27). Adult and adolescent patients did not show any behavioral differences (all p values >0.05), aside from adolescents showing smaller interference reaction times (t=2, df=85, p=0.046). Clusters found to be significant in the primary voxel-activation-by-treatment-group-by-week interaction were extracted and subjected to robustness checks for the effects of age. Adolescents and adults did not differ on activation within any of the extracted clusters (all p values >0.05). As shown in Table S3 in the online supplement, results remained significant for both adolescents and adults when analyses were repeated within each subgroup using extracted region-of-interest data. Exploratory analyses performed on extracted data including age group in an additional interaction term were nonsignificant (all p values >0.05) (see Figures S12–S14 in the online supplement).
Discussion
In this study, we sought to examine whether pretreatment brain activation during cognitive control and reward processing is associated with treatment response to CBT in patients with OCD, and if so, whether the associations are treatment specific relative to an active control therapy (SMT) and whether they vary with age. Patients in both treatment groups showed significant reductions in OCD symptoms after treatment, but symptom reduction following CBT was steeper than symptom reduction following SMT. In the CBT group, a pattern of greater pretreatment activation during cognitive control and reward processing was associated with a better treatment response, while relatively less activation in these regions prior to treatment was associated with better outcomes after treatment in the SMT group. Follow-up analyses in adolescent and adult subgroups indicated that findings were conserved across age.
Greater symptom reduction after CBT treatment was associated with more baseline brain activation within the right temporal lobe and, at a relaxed cluster-forming threshold, within a hypothesized cingulo-opercular region, the rACC, that has been shown in meta-analyses to be reduced in gray matter volume and hypoactive during cognitive control in patients with OCD (4, 5). These findings replicate a recent study of treatment response to CBT in adults with OCD (10) and extend previous work by showing treatment specificity relative to SMT. Interestingly, in the CBT subgroup analysis, greater activation within anterior insular, dorsolateral prefrontal, and posterior cingulate regions was also associated with treatment response, providing further independent replication of this previous study, although these findings did not survive correction for multiple comparisons in the interaction analysis, leaving their treatment specificity unclear (see the online supplement). Meta-analytic studies have shown that reduced structure and function of the rACC is commonly implicated across multiple psychiatric disorders (5, 25, 26), and greater volume or activation within the rACC is arguably the most common predictor of a better treatment response to CBT (27, 28). The rACC is proposed to play important roles in flexible top-down control of emotions and self-regulation in response to potential symptom triggers, cognitive-affective functions which are critically involved in CBT (29). These findings, together with a converging research literature, suggest that patients with relatively preserved functioning within brain regions supporting cognitive control may be better candidates for CBT (10).
During reward processing before treatment initiation, greater activation in the left and right ventromedial prefrontal cortex/orbitofrontal cortex, lateral prefrontal, and amygdalar regions predicted better response to CBT. Recent work has suggested parallels between reward processing and fear extinction, as experiencing the presentation of a conditioned stimulus without the aversive unconditioned stimulus is a better than expected and therefore quasi-rewarding outcome, and studies in humans and animals have demonstrated that functioning within mesolimbic dopaminergic circuitry during extinction learning mirrors that seen during reward-related tasks (11). Fear extinction learning is likely a key mechanism of exposure and response prevention for OCD, and therefore patients with relatively robust orbito-striato-limbic brain activation may be better able to learn updated and less negative associations for their symptom triggers while undergoing exposure and response prevention (2, 11). More broadly, robust functioning within reward-processing regions may protect against impairments in motivation (e.g., anhedonia) and positive affect, which have been shown to have a negative impact on response to treatments, including CBT, across multiple disorders (12, 30). Therefore, relatively greater pretreatment activation in patients who respond strongly to CBT may also indicate a greater capacity for emotional resilience and the motivation to engage in challenging aspects of CBT therapy (12).
Interestingly, interaction analyses showed that while more brain activation within cognitive-control and reward-processing regions was associated with a better treatment response to CBT, a better treatment response to SMT was associated with less activation in an overlapping set of brain regions. While these findings in the SMT group were unexpected, they are consistent with studies comparing CBT with pharmacotherapy treatments, which have reported increased ventromedial prefrontal cortex/orbitofrontal cortex gray matter and resting-state metabolism to be associated with a better response to CBT, and the opposite for pharmacotherapy (13, 15). One possibility is that CBT is most effective in patients who already possess the degree of cognitive control and reward responsiveness required for engaging with and learning from exposure and response prevention (10, 11). SMT, on the other hand, may improve OCD symptoms indirectly by teaching patients how to relax and employ problem-solving techniques to reduce negative emotions in the face of common (i.e., non-OCD-specific) life stressors; thus, SMT may bring about therapeutic change via improved self-regulation and feelings of self-efficacy (20). Consequently, SMT may be better able to meet the needs of patients who have the most room for improvement in these domains. However, given that these findings were unanticipated, further work is needed to properly delineate the mechanisms driving these findings in the SMT group.
Findings of treatment-specific associations suggest that rather than being a general correlate of symptom reduction, greater activation during cognitive control and reward processing is likely linked in a more specific way to CBT response in patients with OCD. We have demonstrated in recent meta-analytic work that patients with OCD show impaired performance and reduced activation within the rACC during cognitive control (4, 31). Moreover, hypoactivation during reward processing has been reported in patients with OCD in prefrontal and orbito-striato-limbic regions similar to those associated with treatment response in the present study (8, 32). Findings indicate that treatment response to CBT depends on circuitry known to be dysfunctional in OCD, suggesting that engagement with CBT might be augmented by pharmacological or nonpharmacological treatments that target these underlying networks (25), and that it may be possible to identify good candidates for CBT on the basis of relatively preserved functioning in these brain regions during cognitive control and reward processing (14).
In the present study, analyses in age-defined subgroups indicated that the same pattern of treatment-specific associations was present in both adolescent and adult patients. The findings provide initial evidence for a preservation of neural predictors of CBT response across the lifespan. However, all patients in our study had early-onset forms of the disorder. OCD has a bimodal onset distribution, with peaks at around age 10 and age 20, and early- and late-onset forms of the disorder have been linked to distinct clinical, neuropsychological, and neurobiological correlates, as well as distinct treatment outcome trajectories (33). Future research should examine whether there are differences in brain activation during cognitive control and reward processing between early- and late-onset forms of the disorder, and whether there are distinct neural predictors of treatment response in these patient subgroups. No differences in activation were found between adolescent and adult patients during cognitive control and reward processing, unlike in some studies in healthy subjects (34). However, interpreting this as evidence of altered development in the disorder would be problematic, as findings in healthy subjects have been mixed and no normative data on the development of activation during the incentive flanker task have been published (4, 34).
Limitations of this study include the fact that, although outcome assessments were made blinded to treatment group, it was not possible to maintain blinded status for patients. This issue is common to previous clinical trials comparing CBT with SMT (2, 19). In addition, we excluded patients with common comorbidities, and the findings may not generalize to patients with these comorbid conditions. Furthermore, negative findings may be due to relatively lower power for the error processing contrast because of the smaller number of trials. Moreover, the sample size of the study was moderate, and while the present findings provide initial evidence for the potential of using task-based fMRI in distinguishing good and poor responders to CBT and SMT, before translation to the clinic these findings must be shown to be robust, replicable, and generalizable to other patient subgroups, as well as to other treatment and imaging sites. Although similar findings were found for adolescents and adults, the inclusion of two age groups added heterogeneity to the sample. While the study findings reveal treatment-specific outcome predictors in patients with OCD undergoing CBT or SMT, they do not speak to whether these predictors are conserved across different disorders that are treated with similar therapies and in which similar cingulo-opercular and orbito-striatal regions have been implicated (25, 30).
Conclusions
The aim of this study was to examine whether treatment response to CBT was associated with treatment-specific brain activation during cognitive control and reward processing examined prior to therapy in adolescent and adult OCD. The findings show that greater activation within two networks commonly implicated in the disorder, the cingulo-opercular network during cognitive control and the orbito-striato-thalamic network during reward processing, was associated with better treatment response to CBT but worse treatment response to SMT. The present study advances the field by demonstrating that associations between brain activation and treatment response were treatment specific to CBT relative to a control psychotherapy and, moreover, that these associations were stable from adolescence to mature adulthood.
1 : Obsessive-compulsive disorder: advances in diagnosis and treatment. JAMA 2017; 317:1358–1367Crossref, Medline, Google Scholar
2 : What matters more? Common or specific factors in cognitive behavioral therapy for OCD: therapeutic alliance and expectations as predictors of treatment outcome. Behav Res Ther 2018; 105:43–51Crossref, Medline, Google Scholar
3 : Pharmacological and psychotherapeutic interventions for management of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry 2016; 3:730–739Crossref, Medline, Google Scholar
4 : Error processing and inhibitory control in obsessive-compulsive disorder: a meta-analysis using statistical parametric maps. Biol Psychiatry 2019; 85:713–725Crossref, Medline, Google Scholar
5 : Identification of a common neurobiological substrate for mental illness. JAMA Psychiatry 2015; 72:305–315Crossref, Medline, Google Scholar
6 : Emotional processing in obsessive-compulsive disorder: a systematic review and meta-analysis of 25 functional neuroimaging studies. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:563–571Crossref, Medline, Google Scholar
7 : Functional neuroimaging of avoidance habits in obsessive-compulsive disorder. Am J Psychiatry 2015; 172:284–293Link, Google Scholar
8 : Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch Gen Psychiatry 2006; 63:1225–1236Crossref, Medline, Google Scholar
9 : Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci 2010; 10:107–118Crossref, Medline, Google Scholar
10 : Task-based fMRI predicts response and remission to exposure therapy in obsessive-compulsive disorder. Proc Natl Acad Sci USA 2019; 116:20346–20353Crossref, Medline, Google Scholar
11 : A dopaminergic basis for fear extinction. Trends Cogn Sci 2019; 23:274–277Crossref, Medline, Google Scholar
12 : What good are positive emotions for treatment? Trait positive emotionality predicts response to cognitive behavioral therapy for anxiety. Behav Res Ther 2017; 93:6–12Crossref, Medline, Google Scholar
13 : FDG-PET predictors of response to behavioral therapy and pharmacotherapy in obsessive compulsive disorder. Psychiatry Res 1998; 84:1–6Crossref, Medline, Google Scholar
14 : Multivariate resting-state functional connectivity predicts response to cognitive behavioral therapy in obsessive-compulsive disorder. Proc Natl Acad Sci USA 2018; 115:2222–2227Crossref, Medline, Google Scholar
15 : Differential prefrontal gray matter correlates of treatment response to fluoxetine or cognitive-behavioral therapy in obsessive-compulsive disorder. Eur Neuropsychopharmacol 2013; 23:569–580Crossref, Medline, Google Scholar
16 : Basolateral amygdala-ventromedial prefrontal cortex connectivity predicts cognitive behavioural therapy outcome in adults with obsessive-compulsive disorder. J Psychiatry Neurosci 2017; 42:378–385Crossref, Medline, Google Scholar
17 : Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry 1997; 36:844–852Crossref, Medline, Google Scholar
18 : The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
19 : A randomized, controlled trial of cognitive-behavioral therapy for augmenting pharmacotherapy in obsessive-compulsive disorder. Am J Psychiatry 2008; 165:621–630Link, Google Scholar
20 : Controlled trial of exposure and response prevention in obsessive-compulsive disorder. Br J Psychiatry 1997; 171:135–139Crossref, Medline, Google Scholar
21 : Treatment of obsessions: a randomized controlled trial. Behav Res Ther 2010; 48:295–303Crossref, Medline, Google Scholar
22 : Cognitive behavior therapy for comorbid obsessive-compulsive disorder in high-functioning autism spectrum disorders: a randomized controlled trial. Depress Anxiety 2013; 30:697–708Crossref, Medline, Google Scholar
23 : Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol Psychiatry 2011; 69:583–591Crossref, Medline, Google Scholar
24 Pinheiro J, Bates D, DebRoy S, et al: nlme: linear and nonlinear mixed effects models, R package version 3.1-148. 2020. https://CRAN.R-project.org/package=nlmeGoogle Scholar
25 : The neural crossroads of psychiatric illness: an emerging target for brain stimulation. Trends Cogn Sci 2016; 20:107–120Crossref, Medline, Google Scholar
26 : Identification of common neural circuit disruptions in cognitive control across psychiatric disorders. Am J Psychiatry 2017; 174:676–685Link, Google Scholar
27 : Resting state amygdala-prefrontal connectivity predicts symptom change after cognitive behavioral therapy in generalized social anxiety disorder. Biol Mood Anxiety Disord 2014; 4:14Crossref, Medline, Google Scholar
28 : Rostral anterior cingulate cortex morphology predicts treatment response to internet-based cognitive behavioral therapy for depression. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:255–262Crossref, Medline, Google Scholar
29 : Brain systems underlying anxiety disorders: a view from neuroimaging, in Anxiety Disorders: Theory, Research, and Clinical Perspectives. Edited by Simpson HB, Neria Y, Lewis-Fernández R, . Cambridge, UK, Cambridge University Press, 2010, pp 192–203Crossref, Google Scholar
30 : Anhedonia and reward-circuit connectivity distinguish nonresponders from responders to dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Biol Psychiatry 2014; 76:176–185Crossref, Medline, Google Scholar
31 : Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol Psychiatry 2017; 82:83–102Crossref, Medline, Google Scholar
32 : Increased default mode network connectivity in obsessive-compulsive disorder during reward processing. Front Psychiatry 2018; 9:254Crossref, Medline, Google Scholar
33 : Early- and late-onset obsessive-compulsive disorder in adult patients: an exploratory clinical and therapeutic study. J Psychiatr Res 2003; 37:127–133Crossref, Medline, Google Scholar
34 : A systematic review of fMRI reward paradigms used in studies of adolescents vs adults: the impact of task design and implications for understanding neurodevelopment. Neurosci Biobehav Rev 2013; 37:976–991Crossref, Medline, Google Scholar



