Cannabidiol: Not a Cure-All, but a Candidate for Coping With Cue-Induced Craving
The Size of It
In May of this year, headlines and tweets began to have their way with a preprint of the article published in this issue by Hurd and colleagues (1). Their gist: “CBD effective in treating heroin, opioid addiction.” The article itself was not guilty of any such claims; it was a thoughtful, scholarly write-up of a well-conducted experiment (which focused on self-reported craving in a laboratory setting, with no attempt to measure opioid use). Even so, I was expecting to find that it was overblown. I’m already on record as having said that ad lib smoking of whole-plant cannabis is no remedy for opioid withdrawal (2). My finding with cannabis didn’t rule out a benefit for pure cannabidiol (CBD) in the very different context of preventing opioid craving in already tapered people with opioid use disorder—but I was, I admit, ready to rain on CBD’s parade.
My skepticism regarding the finding presented in the Hurd et al. article (1) centered on the absence of information about an effect size for the observed benefit. I got out my calipers (or the digital equivalent: an old, freely downloadable Mac application called GraphClick) and turned the authors’ Figure 3 into means and standard deviations, from which I calculated Cohen’s d effect sizes (Figure 1). That’s what changed my mind. By almost any standard, the effects of CBD were large, and the 95% confidence intervals around them were narrow. A very conservative summary estimate would be that CBD, compared with placebo, blocked cue-induced opioid craving with a Cohen’s d of 2.0. A defensible estimate would be 3.0, but let’s take it as 2.0, given the “winner’s curse” of regression to the mean. The narrowness of the confidence intervals strongly suggests that there’s something here, something of that general magnitude (3).
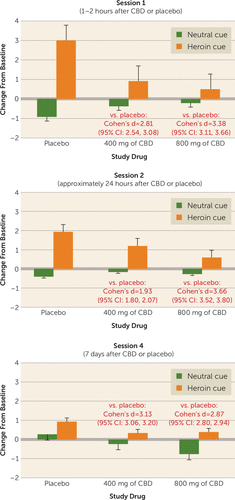
FIGURE 1. Change from baseline scores on the visual analogue scale for craving in a study of cannabidiol (CBD) for the reduction of craving and anxiety in heroin use disordera
a Annotated reproduction of Figure 3 from Hurd et al. (1). Red text shows Cohen’s d effect sizes and 95% confidence intervals for each CBD condition compared with the corresponding placebo condition.
Practicing clinicians might want to see that “something” in terms of a practical measure of effect size, such as number needed to treat. The number needed to treat addresses the question, “How many patients need to receive the new treatment in order for one patient to have a favorable outcome that would not have occurred otherwise?” (“Otherwise” can mean “with placebo,” or “with treatment as usual,” or with any other comparison condition.) Thus, the lower the number needed to treat, the better. Converting a Cohen’s d to a number needed to treat relies on a host of assumptions, such as the rate of favorable outcomes in the comparison condition (4). But even if you set that rate as high as 80% (using the slider for “control event rate” at the online calculator https://rpsychologist.com/d3/cohend/), a Cohen’s d of 2.0 yields a number needed to treat of just 5.06. Should you be impressed? That’s up to you, but you can start by recalling that varenicline (5) and bupropion (6) are approved by the Food and Drug Administration (FDA) for smoking cessation with numbers needed to treat for 6-month abstinence on the order of 7.0 (varenicline) or 8.0 (bupropion), or that naltrexone (7) and acamprosate (8) are FDA-approved to treat alcohol use disorder with numbers needed to treat for relapse prevention on the order of 7.0 (naltrexone) and 9.0–13.3 (acamprosate).
Already I detect a light breeze from the wagging of fingers: yes, the number needed to treat that I calculated for CBD is from a laboratory experiment, while the higher (i.e., worse) numbers needed to treat for other medications are from the larger, noisier, longer-duration world of clinical trials. Let’s discuss that.
The Place of It
If you don’t read much human research on opioid use disorder—and maybe even if you do—you might not realize how large a proportion of our knowledge derives from studying people who are currently maintained on methadone or buprenorphine treatment—the upper circle in Figure 2. There are good reasons why we rely so heavily on that population. First, there’s a clinical need for it: even though agonist maintenance treatment for opioid use disorder is one of the clearest success stories in addiction medicine (9–11), typical outcomes still leave room for betterment, both in terms of sporadic opioid craving and sporadically ongoing use (12, 13). Second, the first-line status of agonist maintenance treatment for opioid use disorder complicates the ethics of a study in which treatment-seeking people are randomly assigned to anything that doesn’t include it, given that the stakes are life and death. Third, investigators’ resources are limited, and when we offer agonist maintenance as part of our study, we have faster enrollment, better retention, and the statistical noise reduction that comes from synchronizing study enrollment with the start of a treatment episode rather than enrolling people wherever they happen to be in the course of their opioid use disorder.
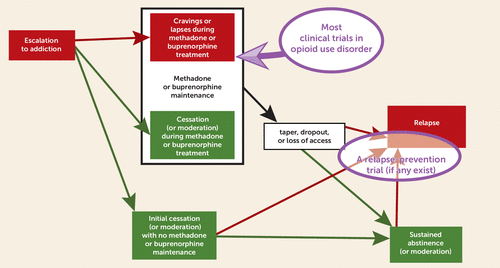
FIGURE 2. Partial schematic diagram of the possible courses of opioid use disorder and its treatmenta
a Red boxes and arrows show unfavorable outcomes; green boxes and arrows show favorable outcomes. The upper purple circle shows the setting of most clinical trials in opioid use disorder (and perhaps most human laboratory research in opioid use disorder): agonist maintenance, where sporadic cravings and lapses persist for a large enough proportion of patients to justify reliance on them for study. The lower purple circle shows the setting of the Hurd et al. (1) experiment: people who are abstinent and who are not taking medications, who are at greater risk of relapse than patients on agonist maintenance treatment. The boxes are not to scale, and, for readability, many arrows (such as all the possible paths out of relapse) are omitted.
What this means, however, is that the results of most human research on opioid use disorder cannot confidently be extrapolated to people with opioid use disorder who are not on agonist maintenance treatment. My own group, running clinical trials of adjunctive medications during agonist maintenance, has taken pains to separate “relapse prevention” from reduction of ongoing use, but we’ve had to discuss our results in terms of “lapse prevention” instead (14). That’s because opioid use that persists during agonist maintenance (in patients who keep coming in for treatment) is usually sporadic and is rarely accompanied by the return to prior symptom severity that the term “relapse” implies. To study relapse prevention, we clinical trialists probably need to venture into the not-so-expedient world of people with opioid use disorder who are currently abstinent and not taking medications (the lower circle in Figure 2), and we probably need to keep them enrolled considerably longer than the few months to which we are accustomed. I can’t think of even one clinical trial in opioid use disorder that looks like that—at least, not for a medication or other biomedical treatment. Therefore, I don’t know what to recommend for people with histories of opioid use disorder who don’t want to embark on methadone or buprenorphine maintenance treatment but who would like some biomedically based protection from bursts of craving. (Before you say “naltrexone,” see reference 15.)
So, rather than wave off the Hurd et al. study as a mere laboratory demonstration, I’m inclined to credit it for being a comparatively rare example of a study that has something to offer those people, the inhabitants of the lower circle in Figure 2. The Hurd et al. sample consisted of 42 people with histories of opioid use disorder, with the main exclusion criterion being a positive urine toxicology test for any opioids, licit or not. This was not, I acknowledge, a broad cross-section: most participants had been abstinent less than a month, and a few had been abstinent for 2 or 3 months, but none for any longer than that. Inclusion of people with longer durations of abstinence might have made for a more stringent test of CBD’s effects on craving, and it certainly would make the results more generalizable. Still, this was basically the population from which one would recruit to study relapse prevention in people who are not taking medication and who are recently abstinent, and the experimental results generalize to them.
The Long and Short of It
As I said, I know of no instance in which the population tested here (people in the lower circle in Figure 2) were enrolled in a long-term randomized trial of a medication or other biomedical intervention to prevent opioid use disorder relapse. Clinical trialists like me are waiting for a treatment with a strong enough signal to justify the time, expense, and effort. Clinicians, bioethicists, and patient advocates would probably scrutinize the treatment in terms of its risk-benefit ratio and its accessibility.
From all of these angles, CBD is starting to look good, but that does not mean that a relapse-prevention trial using CBD would be easy. Hurd et al. found that their participants, who received once-daily CBD for (only) 3 consecutive days, showed no sign of a general reduction in tonic at-home craving for opioids, as assessed by questionnaire.
As Hurd et al. aptly noted, this is to be expected when you give a treatment for cue-induced craving and then measure all craving: tonic background craving is dissociable from induced craving (16). In a short clinical trial, a medication that prevents only one type of craving or lapse can leave a fingerprint if you know where to look—our group has seen it in the real-time reports we obtain through ecological momentary assessment. For example, in people receiving buprenorphine maintenance treatment for opioid use disorder, we hypothesized that the addition of daily clonidine maintenance would prevent craving and lapses induced by stress but not by other precipitants such as cue exposure, because alpha-adrenergic agonists had acted with that kind of specificity in a laboratory-animal model of relapse (17). We found strong support for our hypothesis because we were able to assess stress, cue exposure, and craving in real time throughout the trial via smartphone-based ecological momentary assessment (14). As it happened, we didn’t need to disaggregate lapses into “stress-induced” and “cue-induced” subsets to show an overall benefit of clonidine, but that won’t always be the case; sometimes lumping all lapses together can bury important information (18), and a clinical trial using CBD should be built to withstand that risk.
But I’m not confident that our ecological momentary assessment approach would be sustainable for a trial of sufficient duration to test relapse prevention in a broad swath of people who are abstinent and not taking medication. The main outcome measure might need to be actual relapse rates, perhaps with an after-the-fact assessment of the cause of each recorded relapse, although that is not an ideal way to determine the cause of a relapse (19). A relapse-prevention trial could also incorporate short intervals of ecological momentary assessment to assess whether CBD decouples cue exposure from craving in daily life, irrespective of whether the craving leads to drug use during those intervals. Craving is a worthwhile treatment target on its own because it is distressing and distracting for people who are trying not to relapse (20). And, as in our lofexidine trial (14), ecological momentary assessment documentation of a behaviorally specific action for CBD in daily life would bolster the believability and clinical relevance of even a small reduction in actual relapse rates in the same trial. CBD could then be viewed as one part of a multipronged approach to relapse prevention in opioid use disorder. The results of the Hurd et al. study don’t make CBD a sure thing in that regard, but, if the safety and tolerability of CBD remain as good as they currently appear (21), it’s a more attractive candidate than it was before.
1 : Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry 2019; 176:911–922Link, Google Scholar
2 : No evidence for reduction of opioid-withdrawal symptoms by cannabis smoking during a methadone dose taper. Am J Addict 2015; 24:323–328Crossref, Medline, Google Scholar
3 : The new statistics: why and how. Psychol Sci 2014; 25:7–29Crossref, Medline, Google Scholar
4 : How to obtain NNT from Cohen’s d: comparison of two methods. PLoS One 2011; 6:
5 : Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA 2014; 312:155–161Crossref, Medline, Google Scholar
6 : A placebo-controlled trial of bupropion combined with nicotine patch for smoking cessation in schizophrenia. Biol Psychiatry 2008; 63:1092–1096Crossref, Medline, Google Scholar
7 : Naltrexone for the treatment of alcoholism: a meta-analysis of randomized controlled trials. Int J Neuropsychopharmacol 2005; 8:267–280Crossref, Medline, Google Scholar
8 : Acamprosate: a review of its use in the maintenance of abstinence in patients with alcohol dependence. CNS Drugs 2005; 19:445–464Crossref, Medline, Google Scholar
9 : Science-based actions can help address the opioid crisis. Trends Pharmacol Sci 2018; 39:911–916Crossref, Medline, Google Scholar
10 : Maintenance medication for opiate addiction: the foundation of recovery. J Addict Dis 2012; 31:207–225Crossref, Medline, Google Scholar
11 : Changes in quality of life following buprenorphine treatment: relationship with treatment retention and illicit opioid use. J Psychoactive Drugs 2015; 47:149–157Crossref, Medline, Google Scholar
12 : Correlates of long-term opioid abstinence after randomization to methadone versus buprenorphine/naloxone in a multi-site trial. J Neuroimmune Pharmacol 2018; 13:488–497Crossref, Medline, Google Scholar
13 : Diminished illicit drug use as a consequence of long-term methadone maintenance. J Addict Dis 1993; 12:45–57Crossref, Medline, Google Scholar
14 : Clonidine maintenance prolongs opioid abstinence and decouples stress from craving in daily life: a randomized controlled trial with ecological momentary assessment. Am J Psychiatry 2015; 172:760–767Link, Google Scholar
15 : Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol 2007; 15:134–143Crossref, Medline, Google Scholar
16 : Dissociable effect of acute varenicline on tonic versus cue-provoked craving in non-treatment-motivated heavy smokers. Drug Alcohol Depend 2013; 130:135–141Crossref, Medline, Google Scholar
17 : Repeated lofexidine treatment attenuates stress-induced, but not drug cues–induced reinstatement of a heroin-cocaine mixture (speedball) seeking in rats. Neuropsychopharmacology 2001; 25:320–331Crossref, Medline, Google Scholar
18 : Negative affect and smoking lapses: a prospective analysis. J Consult Clin Psychol 2004; 72:192–201Crossref, Medline, Google Scholar
19 : Remember that? A comparison of real-time versus retrospective recall of smoking lapses. J Consult Clin Psychol 1997; 65:292–300Crossref, Medline, Google Scholar
20 : The clinical significance of drug craving. Ann N Y Acad Sci 2012; 1248:1–17Crossref, Medline, Google Scholar
21 : Safety and pharmacokinetics of oral cannabidiol when administered concomitantly with intravenous fentanyl in humans. J Addict Med 2015; 9:204–210Crossref, Medline, Google Scholar



