Complementary Features of Attention Bias Modification Therapy and Cognitive-Behavioral Therapy in Pediatric Anxiety Disorders
Abstract
Objective:
In the treatment of anxiety disorders, attention bias modification therapy (ABMT) and cognitive-behavioral therapy (CBT) may have complementary effects by targeting different aspects of perturbed threat responses and behaviors. ABMT may target rapid, implicit threat reactions, whereas CBT may target slowly deployed threat responses. The authors used amygdala-based connectivity during a threat-attention task and a randomized controlled trial design to evaluate potential complementary features of these treatments in pediatric anxiety disorders.
Method:
Prior to treatment, youths (8–17 years old) with anxiety disorders (N=54), as well as healthy comparison youths (N=51), performed a threat-attention task during functional MRI acquisition. Task-related amygdala-based functional connectivity was assessed. Patients with and without imaging data (N=85) were then randomly assigned to receive CBT paired with either active or placebo ABMT. Clinical response was evaluated, and pretreatment amygdala-based connectivity profiles were compared among patients with varying levels of clinical response.
Results:
Compared with the CBT plus placebo ABMT group, the CBT plus active ABMT group exhibited less severe anxiety after treatment. The patient and healthy comparison groups differed in amygdala-insula connectivity during the threat-attention task. Patients whose connectivity profiles were most different from those of the healthy comparison group exhibited the poorest response to treatment, particularly those who received CBT plus placebo ABMT.
Conclusions:
The study provides evidence of enhanced clinical effects for patients receiving active ABMT. Moreover, ABMT appears to be most effective for patients with abnormal amygdala-insula connectivity. ABMT may target specific threat processes associated with dysfunctional amygdala-insula connectivity that are not targeted by CBT alone. This may explain the observation of enhanced clinical response to CBT plus active ABMT.
In the treatment of anxiety disorders, attention bias modification therapy (ABMT) and cognitive-behavioral therapy (CBT) may target different aspects of dysfunctional threat processing. In this study, we compared clinical response to active and placebo forms of ABMT in patients receiving CBT. We also differentiated patients with varying clinical response to these treatments by assessing amygdala connectivity engaged during threat-related attention shifts. To do so, we used the dot-probe task and functional MRI (fMRI) in these patients and a group of matched healthy volunteers.
Threats influence attention more strongly in anxious than nonanxious individuals (1). Such effects manifest on paradigms such as the dot-probe task (e.g., 2, 3), which briefly presents task-irrelevant threats. This suggests that anxiety disorders involve implicit biases in attention, and ABMT was developed to alter these attention biases (4–7). ABMT adapts the dot-probe task to use implicit training to correct these biases by varying the location of task-relevant targets and task-irrelevant threats (Figure 1). In CBT, by contrast, patients learn how to change their attention and behavior through explicit instruction and practice, without receiving the repetitive, implicit training contained in ABMT. Therefore, ABMT could augment clinical response by altering components of implicit biases not fully alleviated by CBT. This may explain why some patients fail to benefit fully from CBT (8).
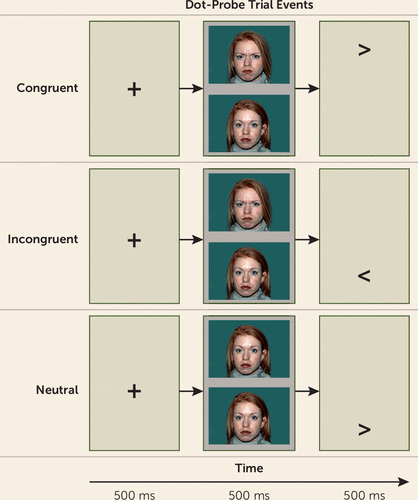
FIGURE 1. The Dot-Probe Task
The different techniques used in CBT and ABMT are reflected in a recently proposed “two-system” model contrasting neural processes engaged by threats (9). The model labels one set of processes “defensive reactions,” which involve rapid, stereotyped behaviors triggered by threats, and contrasts these with a second set, “defensive responses,” which involve more flexible, slowly deployed behaviors. From the perspective of this model, ABMT could target defensive reactions in ways that CBT does less directly. By comprehensively changing both sets of processes, CBT with ABMT may produce a greater clinical response than CBT alone or CBT with inactive ABMT. In the present study, we evaluated this possibility through a randomized controlled trial.
Combining a randomized controlled trial with an fMRI assessment of attention biases could identify factors associated with varying levels of clinical response. Such research is particularly needed in youths, where few therapies have been shown to enhance CBT response and few factors have been shown to differentiate youths with poor response to CBT (e.g., 10–13). To characterize patients who manifest varying levels of treatment response, we used fMRI to assess amygdala-based functional connectivity during a dot-probe task. We acquired these data prior to randomly assigning patients receiving CBT to receive, in addition, either an active or a placebo form of ABMT.
Imaging work using the dot-probe task to study anxious individuals has found consistent relationships between anxiety and altered function in circuitry connecting the amygdala to various cortical regions, particularly the insula and the prefrontal cortex (14–16). In the present study, we therefore hypothesized that patients and healthy comparison subjects differ by level of amygdala-insula and amygdala-prefrontal cortex connectivity during the dot-probe task. We also expected that dysfunctional connectivity between the amygdala and the insula and prefrontal cortex would differentiate patients with particular treatment outcomes, specifically to ABMT.
Method
Participants
As in previous National Institute of Mental Health (NIMH) studies, treatment-seeking patients were recruited and matched to healthy comparison subjects in available subject pools (17). All participants had an IQ >70, were medication free, and were assessed by structured interviews (18). Participants had diagnoses of generalized anxiety disorder, social anxiety disorder, and/or separation anxiety disorder. Current major depressive disorder, obsessive-compulsive disorder, and posttraumatic stress disorder were exclusionary, as were a lifetime history of psychosis, bipolar disorder, or extreme trauma. Study procedures were approved by the NIMH institutional review board. Parents and youths provided written consent or assent.
A total of 85 patients were randomly assigned to receive either active ABMT or a placebo version of ABMT, using published methods (19). The protocol under which this study was performed has multiple components, including one with open fluoxetine treatment; for the trial reported here, however, no patient received fluoxetine or any other medication. All personnel working with the patients were blind to ABMT group assignment. Data were collected from the summer of 2012 until the fall of 2015.
Of 85 patients who underwent randomized assignment, two declined participation after randomization, four completed only baseline assessments, and seven could not tolerate CBT; 72 patients completed at least one ABMT session. Of the 58 patients who underwent scanning, MRI data were usable for 54, and of these, 40 had posttreatment clinical assessments (for more information, see the Supplemental Methods section of the data supplement that accompanies the online edition of this article).
Fifty-one healthy youths, group-matched with the patients on age, sex, and IQ (23 of them were female, and the mean age was 12.86 years [SD=1.94]), completed the same preassessment protocol. The patient and healthy comparison groups did not differ significantly in attention bias on the dot-probe task, although reaction time was generally slower in the patient group. Mean reaction time was not related to baseline symptom severity or treatment response (Table 1).
| Characteristic or Measure | Anxiety Group (N=54) | Healthy Comparison Group (N=51) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Female | 32 | 59.3 | 23 | 45.1 |
| Mean | SD | Mean | SD | |
| Age (years) | 12.08 | 2.80 | 12.86 | 1.94 |
| IQ | 112.78 | 15.55 | 113.18 | 11.58 |
| Baseline SCARED total scorea | 29.40 | 9.59 | 5.44 | 4.68 |
TABLE 1. Demographic and Clinical Characteristics of Youths With Anxiety Disorders and Healthy Comparison Subjects, by Diagnostic Group, for fMRI Analysis
Attention Bias Assessment
Participants completed an event-related dot-probe MRI task (see Figure 1) at baseline (19), with a subgroup undergoing repeat fMRI after treatment. In the dot-probe task, fixation crosses preceded face-pair presentations exhibiting either angry-neutral or neutral-neutral expressions, followed by an arrow probe; participants were instructed to respond to the direction of the probe. The task had three conditions: 1) congruent trials, which presented probes behind the angry face; 2) incongruent trials, which presented probes behind the neutral face; and 3) neutral trials, which lacked angry faces and provided a nonthreat condition. The results highlight the incongruent-congruent contrast, considered a measure of “attention bias” reflecting differential brain function or behavior when attention is allocated away or toward the angry face. The fMRI task was presented across two runs to provide 80 trials of each task condition, interspersed with 80 “null” fixation-only trials. (For more information, see the Supplemental Methods section of the online data supplement.)
Treatments
The flow of participants in the study is illustrated in Figure 2. All patients received up to 12 weekly CBT sessions (see the data supplement) (12, 20); makeup sessions were not available, so patients who missed one or more CBT appointments had fewer than 12 sessions. Patients were randomly assigned to receive active or placebo ABMT, delivered from the fifth through the 12th CBT session. For ABMT, participants received a modified dot-probe task: active ABMT always presented probes in the opposite location of the angry face (incongruent trials); placebo ABMT presented probes with equal probability behind angry or neutral faces (19). Two 5-minute ABMT sessions occurred during each visit, one before and one after the CBT session.
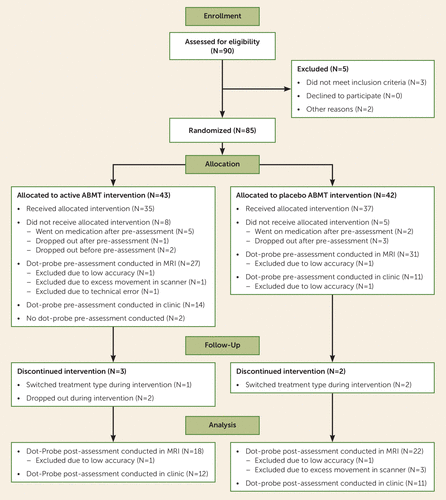
FIGURE 2. Flow Diagram of Patients in a Study of Pediatric Anxietya
a The diagram includes only the patient group, not the healthy comparison group (N=51).
Clinical Treatment Data Analysis
Primary outcome tests followed conventions in the Research Unit on Pediatric Psychopharmacology (RUPP) Anxiety Study (21). To provide the primary continuous clinician-derived index, treating therapists rated the 50-item Pediatric Anxiety Rating Scale (PARS) (22) at pretreatment, midtreatment, and posttreatment assessments. The effects of treatment were examined using the intent-to-treat principle, which included all patients who underwent randomized treatment assignment for whom baseline data and data on any outcome measure were available (N=68). PARS rating data were subjected to a linear mixed model with time (midtreatment, posttreatment assessment) as a within-group variable and ABMT group (active, placebo) as a between-group variable. Data were assumed to have an autoregressive (AR1) covariance structure. Pretreatment ratings were entered as a covariate. Determination of efficacy was based on the planned contrast that tested ABMT group differences on posttreatment PARS ratings. For the analysis of treatment response as a categorical outcome, determination of efficacy was based on a comparison of clinical response rates on the Clinical Global Impressions improvement (CGI-I) scale, as employed in the RUPP Anxiety Study. Patients who had improvement, defined as having a CGI-I score ≤3, were compared with those with no improvement with the chi-square test. In secondary analyses, models were conducted to test the effects of age and sex on treatment response.
Imaging Data Acquisition and Analysis
fMRI acquisition parameters.
Neuroimaging data were acquired with a 3-T GE scanner (Waukesha, Wisc.) with an eight-channel head coil with 2.5×2.5×2.5 mm resolution and T2* weighting (TR=2,300 ms, TE=25 ms, flip angle=50°, FOV=24 cm, matrix=96×96, 41 contiguous 3-mm interleaved axial slices). Coregistration and normalization used a high-resolution three-dimensional magnetization-prepared rapid gradient echo scan (NEX=1, TE/TI=min/725 ms, FOV=22 cm, matrix=256×192, bandwidth=31.25 Hz per 256 voxels).
fMRI preprocessing.
Processing in AFNI (Analysis of Functional Neuroimages) included slice timing correction, coregistration, and normalization and nonlinear registering of echoplanar data to anatomical scans. Data were smoothed (5 mm full width at half maximum) and scaled to 2.5-mm isotropic voxels. For motion correction, repetition time (TR) pairs with a Euclidean norm motion derivative >1 mm were censored prior to individual-level analyses. To be included in the analyses, no more than 20% of TRs across conditions could be censored.
Individual-level general linear models included regressors for correct trials across task conditions, incorrect trials, and for baseline drift and motion (i.e., rotational movement of roll, pitch, yaw, and motion displacement in the x, y, and z axes). Functional connectivity used generalized psychophysiological interaction (gPPI) to model connectivity between each anatomically defined amygdala in the AFNI Talairach Daemon atlas and other brain regions across each task condition. Separate individual-level general linear models were created for the right and left amygdala seeds. PPI terms for congruent, incongruent, and neutral conditions were the product of detrended and demeaned seed and trial condition regressors. Individual PPI general linear models used the same regressors for task-related changes in activation, in addition to the time series for the seed and the three PPI terms. With gPPI, individual differences in activation are controlled to better isolate task-specific differences in connectivity (23).
fMRI data analysis.
All analyses relied on an event-related design and focused on task-related amygdala-based connectivity. This focus reflected the consistency of previous findings (14–16) and the greater stability of amygdala-based connectivity than activation on the dot-probe task (24). Thus, the results presented in the main text emphasize omnibus statistical models testing for differences in amygdala-based gPPI functional connectivity across task condition. Other analyses appear in the Supplemental Results section of the online data supplement.
The results are presented in three sections examining how amygdala connectivity at baseline 1) differed between patients and healthy comparison subjects, 2) related to overall treatment response in patients, controlling for ABMT effects, and 3) related to ABMT-specific treatment effects. In the main text, connectivity findings are highlighted where consistent associations emerged across these three sets of analyses; this convergence occurred only for right amygdala connectivity. Other notable results appear in the data supplement, including between-group comparisons for amygdala activation, associations of age and sex with brain function, treatment-related changes in brain function, and differences in brain function related to clinical indices beyond either diagnosis or PARS treatment response.
Across all analyses, significant clusters were identified using both whole brain and region-of-interest approaches. With an initial threshold of p<0.005 followed by a gray matter–masked cluster correction, a whole brain cluster threshold of 1,063 mm3 was needed for a correction of p<0.05. This threshold was determined using 10,000 Monte Carlo simulations in AFNI’s 3dClustSim tool with the autocorrelation function correction. Based on findings from previous imaging studies with the dot-probe task, a region-of-interest approach was used to test for significant results specifically in the prefrontal cortex and the insula (14–16, 24). The cluster-wise threshold for the prefrontal cortex was based on a single prefrontal cortex mask, used in a previous study with the dot-probe task (24), that encompassed gray matter voxels anterior to a plane drawn at y=0 perpendicular to the anterior commissure-posterior commissure line. Also as in previous studies with the dot-probe task (15), the threshold for the right and left insulae was defined based on the insula Talairach Daemon atlas in AFNI. 3dClustSim produced a cluster-wise threshold size of 734 mm3 for the prefrontal cortex and 203 mm3 for each insula, for a correction of p<0.05. The group maps were also thresholded to include only data for which 90% of participants had valid data. All Talairach coordinates are presented in the left-posterior-inferior convention.
Pretreatment amygdala connectivity:
The first imaging analyses examined amygdala-based connectivity using individual-level connectivity values (PPI coefficients) for 105 participants (54 patients and 51 healthy comparison subjects). Connectivity values were subjected to a linear mixed-effects model using AFNI’s 3dMVM program (25) with baseline group (patients, healthy comparison subjects) as a between-subject variable and task condition (congruent, incongruent, neutral) as the within-subject variable.
Amygdala connectivity and treatment response:
The next imaging analyses examined relationships between connectivity and treatment response in 40 patients who had both usable pretreatment dot-probe fMRI data and a posttreatment clinical assessment. This set of analyses also used 3dMVM; posttreatment PARS rating was entered as a continuous variable, ABMT group (active, placebo) as a between-subject variable, and PPI coefficients for task condition (congruent, incongruent, neutral) as the within-subject variable. To control for baseline anxiety, pretreatment PARS rating was entered as a covariate.
Two interactions were tested within one model to yield two sets of results. First, the two-way condition-by-posttreatment PARS interaction was examined in patients as a group; this result maps connectivity related to overall CBT response, controlling for ABMT group and pretreatment PARS rating. Significant interactions were decomposed using partial correlation analyses between connectivity levels and posttreatment PARS rating. The second result considered connectivity related specifically to ABMT treatment response. This result pertained to the three-way condition-by-ABMT-by-posttreatment PARS rating interaction, mapping connectivity uniquely related to treatment differences in either the active or placebo ABMT group relative to the other group. Post hoc visualization relied on correlations between connectivity levels and posttreatment PARS rating for each of the two ABMT groups. The Fisher r-to-z transformation test was used to test for significant ABMT group differences between correlation coefficient magnitudes.
Results
Clinical Effects of CBT and ABMT
The treatment groups did not differ significantly in demographic characteristics or pretreatment anxiety severity (Table 2). CBT produced marked decreases in anxiety across the two groups (p<0.001), but patients in the active ABMT group had lower posttreatment PARS ratings than patients in the placebo ABMT group, with a medium effect size (t=2.05, df=111.14, p=0.043; Cohen’s d=0.51) (see Figure 3). There were no significant ABMT group differences on posttreatment CGI-I ratings. There were no interactions with ABMT group and age or sex.
| Characteristic or Measure | Active ABMT Group (N=43) | Placebo ABMT Group (N=42) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Female | 26 | 60.5 | 24 | 57.1 |
| Mean | SD | Mean | SD | |
| Age (years) | 11.62 | 2.78 | 11.79 | 2.73 |
| IQ | 110.42 | 14.66 | 114.00 | 15.50 |
| PARS rating | ||||
| Pretreatment assessment | 17.03 | 2.56 | 16.84 | 3.03 |
| Midtreatment assessment | 15.26 | 3.86 | 15.38 | 2.85 |
| Posttreatment assessmentb | 11.97 | 4.69 | 13.67 | 3.25 |
| CGI-I | ||||
| Midtreatment assessment | 3.86 | 0.80 | 4.23 | 0.69 |
| Posttreatment assessment | 3.35 | 0.88 | 3.29 | 0.97 |
TABLE 2. Demographic Characteristics and Treatment Ratings for Youths With Pediatric Anxiety, by ABMT Group, for Treatment Analysisa
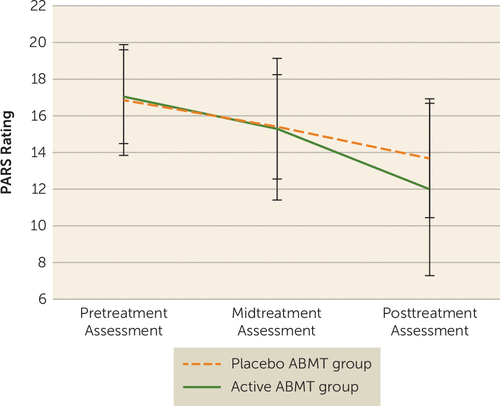
FIGURE 3. Anxiety Ratings in Youths With Pediatric Anxiety Receiving Cognitive-Behavioral Therapy Plus Active or Placebo Attention Bias Modification Therapy (ABMT)a
a PARS=Pediatric Anxiety Rating Scale. Significant difference between groups, p<0.05. Error bars indicate standard deviation.
Pretreatment Amygdala Connectivity
The first analysis compared patients and healthy comparison subjects on baseline amygdala connectivity, where no clusters surpassed the whole-brain-corrected threshold. However, a significant right amygdala–right insula cluster surpassed the region-of-interest threshold (cluster size=1,031 mm3; peak activation=41, −6, 14; F=8.29, df=2, 206; p<0.001) (Figure 4A). Post hoc tests revealed that the patient and healthy comparison groups did not differ significantly in connectivity on the neutral condition. However, the groups displayed opposite patterns of amygdala-insula connectivity on both the congruent and the incongruent trials (Figure 4B). Thus, on the attention bias contrast (incongruent-congruent), the groups showed a significant difference. The patient group showed greater positive right amygdala-insula connectivity during congruent trials, whereas the healthy comparison group showed greater positive connectivity during incongruent trials.
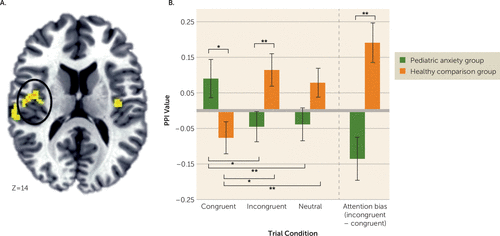
FIGURE 4. Amygdala-Insula Connectivity on a Dot-Probe Task in Youths With Pediatric Anxiety and Healthy Comparison Subjectsa
a Whole brain random-effects analyses indicated a condition (congruent, incongruent, neutral)-by-anxiety group interaction for connectivity between the right amygdala and insula (panel A; the image is displayed in radiological convention [left=right]; cluster size=1,031 mm3, peak activation=41, −6, 14). Post hoc analyses were conducted (panel B) to examine group differences in connectivity on each task condition as well as on the difference between the incongruent and congruent conditions (i.e., attention bias contrast). PPI=psychophysiological interaction. Error bars indicate standard error.
*p≤0.05. **p≤0.01.
No findings approached significance for group differences in left amygdala connectivity; however, several significant age-by-diagnosis-by-condition interactions emerged (see Table S1 in the online data supplement). An interaction emerged for connectivity between the left amygdala and the left insula (cluster size=563 mm3; peak activation=−32, 13, 1; F=12.18, df=2, 202, p<0.001) (see Figure S1A in the data supplement). This interaction reflected distinct associations with age in the patient and healthy comparison groups, as further described in the Supplemental Results section of the data supplement. There were no significant interactions with sex and diagnostic group. Descriptions of between-group differences in amygdala activation, which emerged in the left but not the right amygdala, also appear in the data supplement.
Amygdala Connectivity and Overall Treatment Response
The second analysis, which examined relationships between baseline amygdala-based connectivity and overall treatment response in patients, revealed several findings that surpassed the whole-brain-corrected threshold (see Table S2 in the data supplement). A significant condition-by-PARS rating interaction was detected for connectivity between the right amygdala and a cluster in the right insula that extended into the superior temporal gyrus (Figure 5A) (cluster size=1,859 mm3; peak activation=54, −24, 9; F=12.02, df=2, 70, p<0.001). Post hoc correlation analyses showed that the level of baseline amygdala-insula connectivity during congruent trials was positively related to higher posttreatment symptoms on the PARS (Figure 5B). No significant correlation emerged for incongruent trials (Figure 5C). Therefore, on the attention bias contrast (incongruent-congruent), a strongly negative correlation between amygdala-insula connectivity emerged with posttreatment PARS ratings (Figure 5D).
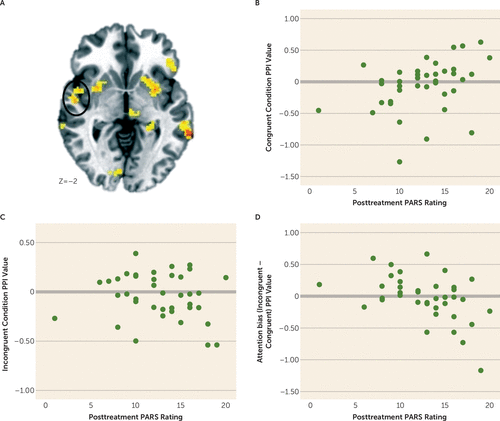
FIGURE 5. Amygdala-Insula Connectivity on a Dot-Probe Task Related to Overall Treatment Response in Youths With Pediatric Anxietya
a Whole brain random-effects analyses controlling for baseline Pediatric Anxiety Rating Scale (PARS) ratings and attention bias modification therapy (ABMT) indicated a condition (congruent, incongruent, neutral)-by-posttreatment PARS ratings interaction for connectivity between the right amygdala and insula (panel A; the image is displayed in radiological convention [left=right]) (cluster size=1,859 mm3, peak activation=54, 24, 9). To probe the interaction, correlations between posttreatment PARS ratings and condition were examined. Panels B–D are scatterplots between posttreatment PARS rating and congruent (r=0.43, p<0.01), incongruent (r=−0.11, n.s.), and attention bias (incongruent – congruent; r=−0.48, p<0.01) conditions. PPI=psychophysiological interaction.
The additional significant findings for right and left amygdala connectivity (see Table S2 in the data supplement) differentiated patients on treatment response; however, they did not differentiate patients from healthy comparison subjects.
Several noteworthy interaction effects emerged with age (see Table S1 in the data supplement). Similar to findings comparing patients with healthy comparison subjects, left amygdala connectivity differed as a function of age and treatment outcome on the PARS in the left insula (see Figure S1B in the data supplement; cluster size=1,391 mm3; peak activation=−51, 11, 11; F=17.02, df=2, 62, p<0.001). In patients above the median age, the connectivity difference on the attention bias contrast (incongruent-congruent) was negatively associated with posttreatment outcome ratings. This pattern was also seen in patients as a group for the right amygdala, as noted above. There were no significant interactions with sex and overall treatment response.
Amygdala Connectivity and ABMT-Specific Response
The final analysis examined relationships between baseline amygdala connectivity and treatment response as a function of ABMT group assignment. No findings surpassed the whole-brain-corrected or prefrontal cortex–corrected thresholds. However, there was a significant condition-by-ABMT-by-PARS rating interaction in the right insula extending into the superior temporal gyrus (Figure 6A) (cluster size=516 mm3; peak activation=51, −4, −4, F=9.57, df=2, 70, p<0.001) that surpassed the insula cluster correction. Correlations between posttreatment PARS rating and connectivity values (Figure 6B-C) revealed no significant relationships within the active ABMT group. However, for patients in the placebo ABMT group, there was a strong relationship between posttreatment symptoms and amygdala-insula connectivity across conditions. Specifically, there was a negative correlation for the attention bias contrast. Moreover, Fisher r-to-z transformation revealed that for the placebo ABMT group, the correlation coefficients for PARS rating and connectivity on congruent (Z=2.15, p=0.03) and incongruent (Z=−1.99, p=0.05) conditions were significantly stronger than those observed in the active ABMT group.
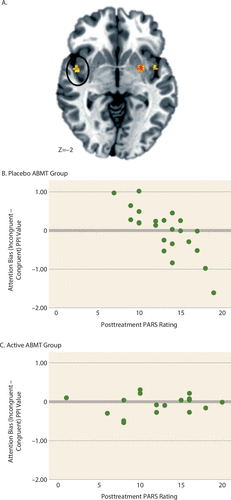
FIGURE 6. Amygdala-Insula Connectivity on a Dot-Probe Task Related to ABMT-Specific Treatment Response in Youths With Pediatric Anxietya
a Whole brain random-effects analyses controlling for baseline Pediatric Anxiety Rating Scale (PARS) ratings indicated an attention bias modification therapy (ABMT) group (active, placebo)-by-condition (congruent, incongruent, neutral)-by-posttreatment PARS ratings interaction for connectivity between the right amygdala and insula (panel A; the image is displayed in radiological convention [left=right]; cluster size=615 mm3, peak activation=45, 0, −4). To probe the interaction, correlations between posttreatment PARS ratings and the attention bias contrast (incongruent – congruent) were examined for each of the ABMT groups. The scatterplots in panels B and C show the association between posttreatment PARS rating and attention bias contrast for the placebo ABMT group (r=−0.78, p<0.001) and the active ABMT group (r=0.15, n.s.). PPI=psychophysiological interaction.
No findings approached significance for the condition-by-ABMT-by-PARS rating interaction for the left amygdala seed. Significant interactions with age and sex appear in Table S1 in the data supplement.
Discussion
This study produced three main findings. First, active ABMT enhanced the clinical response to CBT in children and adolescents with anxiety disorders. Second, at study entry, patients differed from healthy comparison subjects in level of amygdala connectivity elicited by an fMRI version of the same task used in ABMT. Third, baseline amygdala functional connectivity differentiated patients’ level of treatment response. Some indirect evidence also suggests that active ABMT may correct aspects of perturbed amygdala-based connectivity not targeted by placebo ABMT. These aspects reflect a tendency in patients entering the study to exhibit dysfunctional amygdala-insula connectivity.
ABMT Augmentation of Clinical Response
In this study, we tested whether ABMT augments clinical response to CBT in pediatric anxiety disorders, a question that has been addressed previously in only two randomized controlled trials (5, 26). Augmentation could occur if ABMT targets implicit components of perturbed threat processing that are less directly targeted by CBT (5), reflecting the heterogeneous nature of threat responding (9). We found lower clinician-rated anxiety in patients receiving CBT plus active ABMT compared with patients receiving CBT plus placebo ABMT, which differs from findings in the two previous studies comparing different forms of ABMT added to CBT in pediatric anxiety disorders (5, 26). Several methodological factors may explain the differences; for example, one study included a CBT-alone condition, which produced weak effects (5); the other utilized a different form of ABMT than the one we used (26). Moreover, both of those studies possessed limited statistical power because of small sample sizes. In the present study, group differences emerged on the primary continuous outcome measure, with an effect size of 0.51. While not a large effect, it may represent a clinically meaningful one, comparable in magnitude to the effect of adding a selective serotonin reuptake inhibitor to CBT (12). Nevertheless, no group differences were observed for the categorical outcome measure.
Amygdala-Based Functional Connectivity and Anxiety Disorders
Using imaging data, we also compared healthy subjects and anxiety patients on levels of amygdala connectivity, a measure that has adequate test-retest reliability (24). At study entry, the patient group differed from the healthy group in patterns of functional connectivity between the amygdala and the insula elicited by the dot-probe task, the same task adapted for ABMT.
This finding is similar to findings in other imaging work with the dot-probe paradigm (14–16). In the present study, the patient group differed from the healthy comparison group in both types of angry-face trials, with higher connectivity in the patient group for congruent trials and higher connectivity in the healthy group for incongruent trials. This pattern suggests that youths with anxiety disorders fail to deploy this circuitry effectively when salient, task-irrelevant threats appear as either proximal or distal threats.
Beyond research employing the dot-probe task, previous studies using various imaging techniques have also linked anxiety to perturbed functional amygdala-insula connectivity (27–29). The previous findings suggest that connectivity with the insula allows the amygdala to engage the salience network to deploy attention when threats appear (30, 31). Both imaging and basic research identify the insula, a hub in the salience network, as a region of interest for understanding anxiety and threat processing. The insula connectivity findings in this study arose in the mid to posterior insular cortex.
Amygdala-Based Connectivity and Treatment Outcome
We found that amygdala-based functional connectivity was related to both overall and ABMT-specific treatment effects. Overall clinical effects occurred in patients as a group receiving CBT, regardless of ABMT condition. For this first effect, amygdala connectivity in those patients who had an increasingly poor treatment response appeared increasingly different from that of the healthy comparison group. This dysfunctional pattern of amygdala connectivity appeared for the contrast of congruent and incongruent threat trials on the dot-probe task. Thus, at study entry, the patients who appeared most different from the healthy group on measures of amygdala-insula function exhibited the poorest treatment response.
We also found that patterns of amygdala connectivity differentiated treatment response between the two ABMT groups in a region adjacent to the insula region associated with overall treatment response. Specifically, an association emerged between pretreatment amygdala-insula connectivity and posttreatment anxiety in the group receiving CBT plus placebo ABMT. Of note, no such correlation was seen in the group receiving CBT plus active ABMT. Thus, these preliminary findings suggest that connectivity was related to both the presence of an anxiety disorder and response to a specific type of treatment.
The observed relationships between poor treatment outcome and deficient circuitry functioning in the placebo ABMT group can be understood in the context of the above-mentioned two-process model (9). This pattern could arise if ABMT targets functions associated with perturbed threat reactions that are unaffected by either CBT or placebo ABMT. Such an effect could attenuate the relationship between connectivity at baseline and clinical response after treatment.
Limitations and Conclusions
This study has several limitations. First, the sample size is modest, particularly for the analyses comparing subgroups, such as the fMRI analyses examining ABMT-specific treatment effects. Second, all patients received CBT with either active or placebo ABMT, so our findings may not be generalizable to other effective treatments, such as medication. This design also prevented us from directly comparing ABMT and CBT or isolating key components of the two treatments. A direct comparison would require a design that included both combined treatments and each treatment delivered as a monotherapy. As a result, we could only partially test a two-factor model of anxiety. Third, limitations arise in the analyses that collapse across ABMT conditions because of heterogeneity that is introduced when using a sample of patients who received both forms of ABMT. Fourth, the study found no behavioral group difference in attention bias and thus failed to demonstrate any relevance of disorder in behavior evoked by the dot-probe task. However, the absence of such differences also removes a potential task performance confounder. Lastly, the generalizability of our findings may be affected by the study’s exclusionary criteria (e.g., depression, OCD), as well as by the fact that not all participants had usable imaging data.
Despite these limitations, this proof-of-concept study clinically extends a new two-process model (9) regarding treatment complementarity and a new therapeutic modality. Findings generated by adding a brain imaging component to a clinical test of efficacy suggest that ABMT may target processes that are less directly targeted by CBT. Thus, combining ABMT and CBT may produce benefits in youths who might otherwise not fully respond to CBT monotherapy.
In summary, ABMT and CBT may have complementary effects in the treatment of pediatric anxiety disorders. This study produced evidence of enhanced clinical effect for the primary continuous outcome measure, and the clinical effect was related to pretreatment amygdala-insula functional connectivity. Taken together, these clinical and imaging data suggest that ABMT and CBT may target distinct circuitry components, with enhanced clinical effects in combined therapy, possibly arising through influences of ABMT on implicit processes that are less directly targeted by CBT.
1 : Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychol Bull 2007; 133:1–24Crossref, Medline, Google Scholar
2 : Attentional bias for emotional faces in children with generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 2008; 47:435–442Crossref, Medline, Google Scholar
3 : Isolating neural components of threat bias in pediatric anxiety. J Child Psychol Psychiatry 2012; 53:678–686Crossref, Medline, Google Scholar
4 : Attention bias modification for youth with social anxiety disorder. J Child Psychol Psychiatry 2016; 57:1317–1325Crossref, Medline, Google Scholar
5 : Attention bias modification treatment augmenting effects on cognitive behavioral therapy in children with anxiety: randomized controlled trial. J Am Acad Child Adolesc Psychiatry 2014; 53:61–71Crossref, Medline, Google Scholar
6 : Attention bias modification for social anxiety: a systematic review and meta-analysis. Clin Psychol Rev 2015; 40:76–90Crossref, Medline, Google Scholar
7 : Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry 2010; 68:982–990Crossref, Medline, Google Scholar
8 : Treatment of separation, generalized, and social anxiety disorders in youths. Am J Psychiatry 2014; 171:741–748Link, Google Scholar
9 : Using neuroscience to help understand fear and anxiety: a two-system framework. Am J Psychiatry 2016; 173:1083–1093Link, Google Scholar
10 : Prefrontal reactivity to social signals of threat as a predictor of treatment response in anxious youth. Neuropsychopharmacology 2016; 41:1983–1990Crossref, Medline, Google Scholar
11 : fMRI predictors of treatment outcome in pediatric anxiety disorders. Psychopharmacology (Berl) 2007; 191:97–105Crossref, Medline, Google Scholar
12 : Cognitive behavioral therapy, sertraline, or a combination in childhood anxiety. N Engl J Med 2008; 359:2753–2766Crossref, Medline, Google Scholar
13 : Predictors and moderators of treatment response in childhood anxiety disorders: results from the CAMS trial. J Consult Clin Psychol 2014; 82:212–224Crossref, Medline, Google Scholar
14 : Amygdala and ventrolateral prefrontal cortex activation to masked angry faces in children and adolescents with generalized anxiety disorder. Arch Gen Psychiatry 2008; 65:568–576Crossref, Medline, Google Scholar
15 : Patterns of neural connectivity during an attention bias task moderate associations between early childhood temperament and internalizing symptoms in young adulthood. Biol Psychiatry 2013; 74:273–279Crossref, Medline, Google Scholar
16 : Vigilance in the laboratory predicts avoidance in the real world: a dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Dev Cogn Neurosci 2016; 19:128–136Crossref, Medline, Google Scholar
17 : Common and distinct amygdala-function perturbations in depressed vs anxious adolescents. Arch Gen Psychiatry 2009; 66:275–285Crossref, Medline, Google Scholar
18 : Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
19 Abend R, Pine DS, Bar-haim Y: The TAU-NIMH Attention Bias Measurement Toolbox. Tel Aviv, Tel Aviv University, School of Psychological Sciences, Laboratory for Research on Anxiety and Trauma, 2014. http://people.socsci.tau.ac.il/mu/anxietytrauma/research/Google Scholar
20 : Child/Adolescent Anxiety Multimodal Study (CAMS): rationale, design, and methods. Child Adolesc Psychiatry Ment Health 2010; 4:1Crossref, Medline, Google Scholar
21 : Fluvoxamine for the treatment of anxiety disorders in children and adolescents. N Engl J Med 2001; 344:1279–1285Crossref, Medline, Google Scholar
22 : The Pediatric Anxiety Rating Scale (PARS): development and psychometric properties. J Am Acad Child Adolesc Psychiatry 2002; 41:1061–1069Crossref, Medline, Google Scholar
23 : A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage 2012; 61:1277–1286Crossref, Medline, Google Scholar
24 : Behavioral and neural stability of attention bias to threat in healthy adolescents. Neuroimage 2016; 136:84–93Crossref, Medline, Google Scholar
25 : Linear mixed-effects modeling approach to fMRI group analysis. Neuroimage 2013; 73:176–190Crossref, Medline, Google Scholar
26 : Training-associated changes and stability of attention bias in youth: implications for attention bias modification treatment for pediatric anxiety. Dev Cogn Neurosci 2013; 4:52–64Crossref, Medline, Google Scholar
27 : Intrinsic functional connectivity of amygdala-based networks in adolescent generalized anxiety disorder. J Am Acad Child Adolesc Psychiatry 2013; 52:290–299.e2Crossref, Medline, Google Scholar
28 : Functional network dysfunction in anxiety and anxiety disorders. Trends Neurosci 2012; 35:527–535Crossref, Medline, Google Scholar
29 : Amygdala-prefrontal cortex functional connectivity during threat-induced anxiety and goal distraction. Biol Psychiatry 2015; 77:394–403Crossref, Medline, Google Scholar
30 : Salience processing and insular cortical function and dysfunction. Nat Rev Neurosci 2015; 16:55–61Crossref, Medline, Google Scholar
31 : The insular cortex: a review. Prog Brain Res 2012; 195:123–163Crossref, Medline, Google Scholar



