Effect of Early-Life Fluoxetine on Anxiety-Like Behaviors in BDNF Val66Met Mice
Abstract
Objective:
Adolescence is a developmental stage in which the incidence of psychiatric disorders, such as anxiety disorders, peaks. Selective serotonin reuptake inhibitors (SSRIs) are the main class of agents used to treat anxiety disorders. However, the impact of SSRIs on the developing brain during adolescence remains unknown. The authors assessed the impact of developmentally timed SSRI administration in a genetic mouse model displaying elevated anxiety-like behaviors.
Method:
Knock-in mice containing a common human single-nucleotide polymorphism (Val66Met; rs6265) in brain-derived neurotrophic factor (BDNF), a growth factor implicated in the mechanism of action of SSRIs, were studied based on their established phenotype of increased anxiety-like behavior. Timed administration of fluoxetine was delivered during one of three developmental periods (postnatal days 21–42, 40–61, or 60–81), spanning the transition from childhood to adulthood. Neurochemical and anxiety-like behavioral analyses were performed.
Results:
We identified a “sensitive period” during periadolescence (postnatal days 21–42) in which developmentally timed fluoxetine administration rescued anxiety-like phenotypes in BDNF Val66Met mice in adulthood. Compared with littermate controls, BDNFMet/Met mice exhibited diminished maturation of serotonergic fibers projecting particularly to the prefrontal cortex, as well as decreased expression of the serotonergic trophic factor S100B in the dorsal raphe. Interestingly, deficient serotonergic innervation, as well as S100B levels, were rescued with fluoxetine administration during periadolescence.
Conclusions:
These findings suggest that SSRI administration during a “sensitive period” during periadolescence leads to long-lasting anxiolytic effects in a genetic mouse model of elevated anxiety-like behaviors. These persistent effects highlight the role of BDNF in the maturation of the serotonin system and the capacity to enhance its development through a pharmacological intervention.
The incidence of anxiety disorders peaks during adolescence (1). More than 75% of adults with anxiety disorders met diagnostic criteria as children or adolescents (2, 3). However, because of a lack of sufficient diagnoses or specialized therapeutics, less than one in five children or adolescents with anxiety will receive treatment (4). In 2004, the U.S. Food and Drug Administration (FDA) issued a black-box warning for selective serotonin reuptake inhibitors (SSRIs), the main class of pharmacological agents used to treat depression and anxiety disorders, for children and adolescents because of a risk of suicidality (5). Within 2 years after the FDA advisory was issued, SSRI prescription rates for pediatric populations decreased (6, 7). However, the impact of SSRIs on brain development during adolescence remains unknown.
Preclinical studies in rodents and nonhuman primates have shown that administration of SSRIs during periadolescence leads to persistent neurochemical changes into adulthood. In both rodent and nonhuman primates, a prolonged up-regulation of the serotonin transporter (SERT) has been found in the cortex and the hippocampus after fluoxetine treatment during the juvenile or periadolescent stages of life (8–10). Interestingly, in primates, no effects were observed on fear-related or social behaviors (8). In rodents, early-life fluoxetine was found not to change anxiety-like or fear extinction behaviors in adulthood (11–13), although conflicting reports exist (14, 15). Notably, all preclinical studies were performed in wild-type animals that did not exhibit elevated depressive or anxiety-like phenotypes, for which SSRIs are indicated in human populations.
In this context, genetically engineered mice expressing reduced levels of brain-derived neurotrophic factor (BDNF), a neurotrophin involved in neuronal growth and plasticity, display increased anxiety-like and depressive-like behaviors (16, 17). Additionally, SSRI-associated up-regulation of BDNF is thought to be a key mechanism by which the long-term effects of SSRIs on neuronal plasticity are mediated (18, 19). This genetic knock-in mouse model of a common single-nucleotide polymorphism (SNP) in the human BDNF gene (rs6265) may hold particular relevance to human populations, as this SNP in humans is associated with altered susceptibility to anxiety and depressive pathology (20–22). This SNP leads to substitution of the conserved valine with a methionine at position 66 in the BDNF polypeptide (17), resulting in decreased BDNF bioavailability (23, 24). Of note, BDNF Val66Met mice reproduce the phenotypic hallmarks of human carriers, including altered anxiety- and fear-related behaviors (17, 25), and this phenotype is not responsive to fluoxetine administered in adulthood (17, 26).
In this study, we sought to determine whether developmentally timed SSRI administration in BDNFMet/Met mice during periadolescence would lead to persistent neurochemical and behavioral changes in adulthood. The periadolescent period, which corresponds to the transition from childhood to adolescence, is when BDNF levels rise significantly (27, 28). Thus, we hypothesized that pharmacological intervention during periadolescence, which would further elevate both serotonin and BDNF levels, may alter subsequent developmental trajectories for the neuronal populations dependent on these neuromodulators and alter the emergence of anxiety and fear-related behavioral phenotypes in the BDNFMet/Met mice.
Method
Animals
Separate cohorts of male BDNF Val66Met mice (17), backcrossed (≥10 generations) onto C57BL/6N background, were used for each experiment. Animal care was in accordance with Weill Cornell Medical College’s Institutional Animal Care and Use Committee and NIH’s Guide for the Care and Use of Laboratory Animals.
Fluoxetine Administration
Fluoxetine was dissolved in drinking water, delivered ad libitum (29). Fresh drug was delivered twice a week, and intake was monitored by bottle weight before and after consumption. Fluoxetine was dissolved in drinking water at a concentration of 160 mg/L, which has been established to correspond to therapeutic serum levels of 18 mg/kg per day (30–32). Control animals received tap water.
Novelty-Induced Hypophagia
Mice received 3 consecutive days of training (days 1–3) in dim lighting, acclimating to milk delivery (30). A dual-bearing sipper tube (5-oz. bottle) was inserted in the home cage’s wire lid, containing 90% sweetened condensed milk diluted in tap water for several hours a day. On day 4 (home cage test), mice were individually placed in the home cage for testing in dim lighting. Latency to drink was scored over a 10-minute period. On day 5, in the novel cage test, mice were placed in a clean cage with no bedding, under bright light conditions, with white paper underneath. Excess light was directed at the sipper bottle. Latency to drink was scored over a 10-minute period by a rater blind to genotype.
Elevated Plus Maze
The elevated plus maze consisted of two opposite enclosed arms with 14-cm opaque walls and two open arms of the same size (30 cm × 5 cm), made of white polycarbonate. An infrared digital camera with the video recorder under the control of the Ethovision software program (Noldus, Leesburg, Va.) recorded and analyzed mouse movement in 10-minute sessions in low light (∼4 lux). Anxiety-like behavior was reported as entries into and time spent in the open arms.
Cued Fear Extinction
The fear conditioning apparatus consisted of a mouse shock chamber (Coulbourn Instruments, Whitehall, Pa.) in a sound-attenuated box (33). Briefly, mice were fear conditioned with three tone-shock pairings, consisting of 30-second presentation of 5 kHz, 70 dB tones (conditioned stimulus; CS) coterminating with a 0.7 mA foot shock (unconditioned stimulus; US) during the last 1.0 second of the tone, with an intertrial interval of 30 seconds. Twenty-four hours after fear conditioning, the extinction procedure began, in which mice were exposed to five presentations of the CS in the absence of the US in context B, a cylindrical arena. Extinction trials were repeated daily for 4 days. Experiments were controlled by a computer, using the Graphic State software program (Coulbourn Instruments). Mice were videotaped for subsequent analysis by raters blind to genotype. Freezing was defined as the absence of visible movement except that required for respiration (34). The percentage time spent freezing was calculated by dividing the amount of time spent freezing during the 30-second tone presentations by the duration of the tone. Early trials represent the average of the trials on day 1 of extinction (24 hours after conditioning), and late trials represent the average of the trials on day 4 of extinction (96 hours after conditioning).
BDNF ELISA
BDNF enzyme-linked immunosorbent assay (ELISA) was used (G7611; BDNF Emax Immunoassay System, Promega, Madison, Wisc.) according to the manufacturer’s instructions. Mice were sacrificed by carbon dioxide followed by decapitation. The left and right hippocampi were lysed in 800 μL of lysis buffer (1×Tris buffered saline [TBS], 1% NP-40, 1% Triton-100, 1 mM PMSF, 10% glycerol, and a protease inhibitor cocktail [Sigma]) (35). Lysates were rotated for 30 minutes at 4°C and then centrifuged for 10 minutes at 4°C at maximum speed, and the clarified supernatant was collected. Using the Bradford method, protein levels were quantified, and 150 μg were loaded per well.
Immunohistochemistry
Mice were sacrificed and coronal brain sections were cut as described elsewhere (33). Free-floating serial sections were incubated for 30 minutes in blocking solution containing 4% normal horse serum and 1% bovine serum albumin in TBS with 0.2% Triton X-100 (TBS-Tx), then incubated overnight at 4°C with a mixture of goat anti-S100B (1:800, Sigma) and rabbit anti-SERT primary antibody (1:1000, Sigma) in blocking solution. Sections were then washed in TBS and incubated for 2 hours with a cocktail of Alexa Fluor labeled donkey antigoat and antirabbit IgG secondary antibodies (1:500, Alexa Fluor 555 and 488) in TBS-Tx. Slides were coverslipped by mounting medium containing DAPI.
Stereological Estimation of Cell and Fiber Density
Stereological estimation of cell density was performed using the StereoInvestigator software program (MicroBrightField, Williston, Vt.). After systemic random sampling as described elsewhere (33), all sampled sections containing brain regions of interest were included and contours of brain regions (the medial prefrontal cortex, the hippocampus, and the dorsal raphe) were drawn. Systematic random sampling was applied to each individual contour. The total area of brain regions was estimated by drawn contours. Total cell numbers were estimated using a fractionator, with a counting frame size of 25×25×40 μm and a sampling grid size of 100×100 μm. Detection of fiber density was performed using a perimetrics method. Briefly, sections were traced under a 4× lens and then perimetrics probe analysis was done under a 40× lens. The counting frame was set to 25×25 μm, and the radius of the Merz coherent test system was set to 5 μm. The total length of all sampling sites was automatically calculated. For each animal, fiber density was obtained from the sum of the lengths divided by the sum of the area for all included sections.
Statistics
When two means were compared, statistical significance was calculated using two-tailed Student’s t tests with p values ≤0.05 considered significant. For comparisons with two factors, two-way analysis of variance with Bonferroni post hoc correction was used.
Results
Developmental Onset of Anxiety-Like Behaviors in BDNFMet/Met Mice
When placed in conflict settings, adult BDNFMet/Met mice exhibit elevated anxiety-like behavior compared with littermate controls (BDNFVal/Val) (17, 36). However, developmental emergence of this phenotype has not been investigated in male BDNFMet/Met mice. To this end, adolescent (postnatal day [P] 30) and adult (P60) male BDNF Val66Met mice were tested on two standard measures of anxiety-related behavior, the elevated plus maze and novelty-induced hypophagia paradigms. At P30, there was no effect of genotype on entries into and time spent in the open arms of the elevated plus maze (Figure 1A,B) or on latency to drink sweetened milk in a stressful environment (Figure 1C). However, exploration of the open arms was significantly lower (percent of time in open arm, F=124.3, df=1, 56, p<0.0001; percent entries in open arm, F=141.0, df=1, 56, p<0.0001) (Figure 1A,B), while latency to drink milk was greater (F=20.53, df=1, 35, p<0.0001) (Figure 1C) for adult BDNFMet/Met mice compared with age-matched BDNFVal/Val controls. Thus, as previously described in females (36), male BDNFMet/Met mice exhibit a late developmental onset of anxiety-like phenotypes.
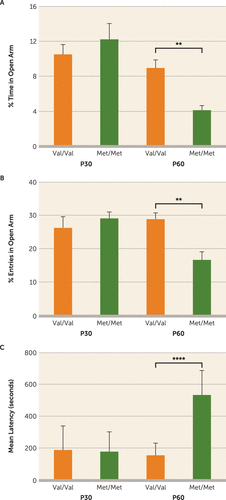
FIGURE 1. Anxiety-Like Behaviors in Adolescent and Adult Wild-Type (Val/Val) and BDNFMet/Met (Met/Met) Micea
a Adolescent (postnatal day 30; P30) and adult (postnatal day 60; P60) wild-type (Val/Val) mice and BDNFMet/Met (Met/Met) littermates were assessed on the elevated plus maze. As shown in panels A and B, there was no effect of genotype at P30 on entries into and time spent in the open arms of the elevated plus maze, whereas both measures were significantly lower at P60 in BDNFMet/Met mice compared with BDNFVal/Val controls. Similarly, in panel C, latency to first event of drinking milk in a stressful environment (the novelty-induced hypophagia task) showed no effect of genotype at P30 but was significantly longer at P60 in BDNFMet/Met mice compared with BDNFVal/Val controls. Means and standard deviations (error bars) are based on the analysis of eight to 15 mice per group.
**p<0.01. ****p<0.0001.
Effects of Early-Life Fluoxetine on Anxiety-Like Behaviors
We hypothesized that while adult BDNFMet/Met mice exhibit a blunted behavioral response to chronic fluoxetine treatment (17, 26) compared with BDNFVal/Val littermates, early-life pharmacological intervention, prior to phenotype onset, may have long-term anxiolytic effects. To test this, we administered fluoxetine (18 mg/kg per day) to BDNFMet/Met mice in drinking water during one of three developmental periods (P21–P42, P40–P61, or P60–P81), with controls receiving water alone. Anxiety-related behaviors were assessed 28 days after the last day of fluoxetine administration (Figure 2A). A significant decrease in latency to drink milk was observed in BDNFMet/Met mice treated with early-life (P21–P42) fluoxetine compared with control BDNFMet/Met mice (F=8.2, df=1, 60, p<0.01) (Figure 2B), suggesting that developmental SSRI intervention rescues anxiety-like phenotypes in a genetic mouse model of diminished BDNF bioavailability. This effect of fluoxetine treatment was also confirmed on the elevated plus maze test with treated BDNFMet/Met mice displaying a significant increase in entries (F=13.84, 1, 64, p<0.001) and time spent (F=18.19, df=1, 64, p<0.001) in the open arm compared with BDNFMet/Met controls (Figure 2C,D). Additionally, an interaction (genotype by treatment) was observed for entries into (F=34.92, 1, 64, p<0.0001) and time spent (F=34.86, 1, 64, p<0.0001) in the open arm. A significant effect of genotype for the water-alone groups (Figure 2B–D) confirmed the previously observed anxiety-related phenotype in BDNFMet/Met mice (17).
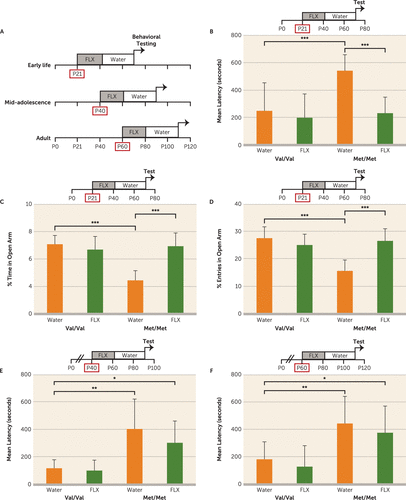
FIGURE 2. Effect of Early-Life Fluoxetine on Anxiety-Like Behavior in BDNFMet/Met Micea
a Panel A is a diagram of fluoxetine (FLX) intervention at three developmental periods in BDNF Val66Met mice followed by behavioral testing. Littermate controls of both genotypes received water alone. Anxiety-like behaviors were assessed in BDNFMet/Met (Met/Met) mice and littermate controls (Val/Val) treated with fluoxetine or water alone at postnatal days 21–42 (P21–P42) in the novelty-induced hypophagia task (panel B) and the elevated plus maze (panels C and D). Latency to drink milk in the novelty-induced hypophagia task was assessed in wild-type (Val/Val) and BDNF knock-in (Met/Met) mice treated with fluoxetine or water alone at postnatal days 40–61 (P40–P61) (panel E) and postnatal days 60–81 (P60–P81) (panel F). Means and standard deviations (error bars) are based on the analysis of 22 mice per group.
*p<0.05. **p<0.01. ***p<0.001.
No significant changes in behavior were observed for BDNFMet/Met mice treated with fluoxetine at P40–P61 (F=0.9, 1, 44, p>0.05) (Figure 2E) or P60–P81 (F=0.02, 1, 45, p>0.05) (Figure 2F) compared with water-alone controls of the same genotype. As expected, there was no long-term effect of fluoxetine in BDNFVal/Val mice (Figure 2B–F) (11, 13). Taken together, these results suggest that a pharmacological intervention during a defined time frame in postnatal development has long-term anxiolytic effects in BDNFMet/Met but not wild-type mice.
Effects of Early-Life Fluoxetine on Fear-Learning
BDNFMet/Met mice have been shown to exhibit deficits in cued fear extinction, where the contingencies between a cue (tone) and a paired aversive outcome (shock) no longer hold (37). In wild-type mice, effects of fluoxetine on fear learning depend on the developmental stage of treatment (11, 12, 38); however, there are no persistent effects of drug on fear conditioning and extinction in wild-type mice treated at P21–P49 (11). To determine whether early-life (P21–P42) fluoxetine attenuates fear-extinction deficits associated with the Met allele, Val66Met mice treated at P21–P42 underwent fear conditioning in adulthood followed by an extinction paradigm. We found no effect of early-life fluoxetine on freezing behavior in either BDNFMet/Met or BDNFVal/Val mice compared with water-alone controls of these respective genotypes (F=0.4, df=1, 42, p>0.05) (see Figure S1 in the data supplement that accompanies the online edition of this article). There was only the expected effect of genotype on extinction learning (F=61.56, df=1, 42, p<0.0001) (see Figure S1). Thus, our findings suggest that the effects of early-life fluoxetine in BDNFMet/Met mice are restricted to anxiety-like phenotypes.
Effect of Early-Life Fluoxetine on ProBDNF and Mature BDNF Levels
Fluoxetine functions through augmenting BDNF levels (18, 19, 39–41). When fluoxetine was administered at P21–P42, P40–P61, or P60–P81 to mice expressing C-terminal HA-tagged BDNF (BDNF-HA) (35), hippocampal BDNF levels increased for all treatment periods but normalized after a 3-week drug washout (effect of treatment, F=35.14, df=1, 85, p<0.0001) (see Figure S2A in the online data supplement). This effect was also confirmed using Western blot analysis of proBDNF and mature BDNF levels (see Figure S2B). Interestingly, the ratio of proBDNF to mature BDNF is highest after early-life fluoxetine exposure (see Figure S2B). Additionally, fluoxetine treatment did not affect levels of the proBDNF receptor, p75, whose expression is higher at P42 compared with later time points (see Figure S2C). These results indicate that proBDNF signaling is more robust after fluoxetine treatment early in life compared with later developmental stages.
Interestingly, early-life fluoxetine also did not change hippocampal BDNF levels in BDNFMet/Met mice, even though levels in BDNFVal/Val mice were significantly increased (F=9.11, df=3, 19, p<0.01) (see Figure S2D). These results suggest that the mechanism of behavioral rescue in BDNFMet/Met mice did not depend on increasing hippocampal BDNF levels.
Effects of Early-Life Fluoxetine on Serotonergic Fiber Density
Serotonergic neurons from the dorsal raphe project to cortico-limbic structures involved in regulation of anxiety and depend on BDNF for trophic support (42, 43). We hypothesized that serotonergic innervation is impaired in BDNFMet/Met mice. We performed immunohistochemical analysis for serotonin transporter immunoreactive fibers in the infralimbic prefrontal cortex of BDNFVal/Val and BDNFMet/Met mice at P21 and P42. We observed that at P21 there was no effect of genotype on fiber density (Figure 3A). At P42 there was a significant increase in fiber density for BDNFVal/Val mice compared with the P21 time point (effect of genotype, F=19.76, df=1, 16, p<0.001; effect of age, F=14.60, df=1, 16, p<0.01); however, this effect of age was not observed in BDNFMet/Met mice, where the fiber density was not significantly different from at the P21 time point. This deficit persists into adulthood (effect of genotype, F=30.43, df=1, 16, p<0.0001) (Figure 3B; water-alone group). These results suggest that the adolescent P21–P42 period is crucial for the development of the serotonergic system in mice and that the BDNF Val66Met SNP is associated with impaired serotonergic development and innervation of the infralimbic prefrontal cortex. These observations are in accordance with previous human and rodent studies showing that BDNF deficiency leads to deficits in serotonergic neuron development (44–49).
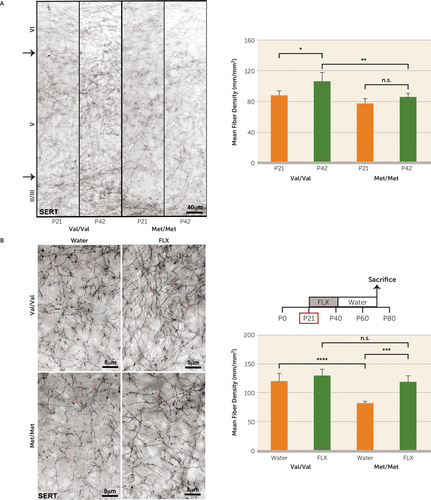
FIGURE 3. Effect of Development and Early-Life Fluoxetine on Serotonergic Fiber Density in BDNFMet/Met Micea
a In panel A, serotonin transporter (SERT) immunoreactive fibers were assessed in the infralimbic prefrontal cortex of BDNFMet/Met mice and wild-type controls at postnatal days 21 and 42 (P21 and P42). Panel B shows density of SERT immunoreactive fibers in the infralimbic prefrontal cortex of BDNFMet/Met mice and wild-type controls treated with or without fluoxetine (FLX) at postnatal days 21–42. Means and standard deviations (error bars) are based on the analysis of five mice per group.
*p<0.05. **p<0.01. ***p<0.001. ****p<0.0001.
Interestingly, early-life fluoxetine ameliorated the deficient serotonergic innervation of the infralimbic prefrontal cortex in BDNFMet/Met mice and had no significant effects in BDNFVal/Val controls (effect of treatment, F=26.55, df=1, 16, p<0.0001) (Figure 3B). This effect was also observed in the ventral CA1 of the hippocampus (effect of genotype, F=41.54, df=1, 16, p<0.0001; effect of treatment, F=22.05, df=1, 16, p<0.001) (see Figure S3A in the online data supplement). However, when fluoxetine was administered at P60, there were no changes in serotonergic fiber density in the infralimbic prefrontal cortex of treated BDNFMet/Met mice compared with controls of the same genotype (F=4.0, df=1, 16, p>0.05) (see Figure S3B). This suggested that increasing serotonergic tone during adolescence, a period when the serotonergic system begins to decline in BDNFMet/Met mice, is crucial for long-term anxiety-related behaviors. Furthermore, no effects of genotype or treatment were observed in number of serotonergic cell bodies in the dorsal raphe (F=0.27, df=1, 16, p>0.05) (see Figure S3C), suggesting altered morphology but no loss of serotonergic neuron numbers in BDNFMet/Met mice.
Effects of Early-Life Fluoxetine on S100B Levels
S100B has established functions as an astrocyte-derived trophic factor for serotonergic neurons, and its expression is altered by change in serotonergic tone (50, 51). To determine whether the BDNF Val66Met SNP is associated with changes in S100B expression, we analyzed S100B immunoreactive cell density in the dorsal raphe nucleus of BDNF Val66Met mice. We found a significant decrease in S100B-expressing cells in BDNFMet/Met mice compared with controls (effect of genotype, F=24.83, df=1, 16, p<0.0001), which was rescued with fluoxetine treatment in early life (effect of treatment, F=31.45, df=1, 16, p<0.0001) (Figure 4) but not fluoxetine administered at P60 (F=2.59, df=1, 16, p>0.05) (see Figure S4A in the data supplement). An effect of early-life fluoxetine on S100B expression, but not of genotype, was also observed in the hippocampus (effect of treatment, F=16.11, df=1, 16, p<0.01; effect of genotype, F=2.91, df=1, 16, p>0.05) (see Figure S4B). Finally, since the time points of behavioral and immunohistochemical analyses differed for the three treatment groups (P70, P89, and P109), we assessed whether there was an effect of age in adult mice who were treated with water alone. We performed statistical analyses comparing expression of S100B, serotonergic fiber density, and novelty-induced hypophagia latency across age groups within each genotype group and found no significant effects of age (see Table S1 in the data supplement).
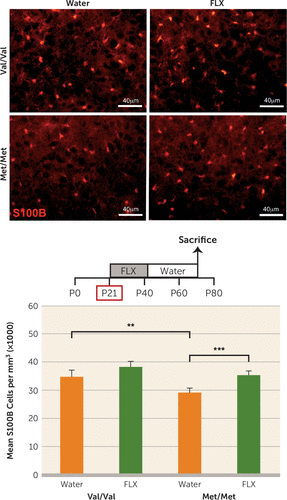
FIGURE 4. Effect of Early-Life Fluoxetine on S100B Expression in BDNFMet/Met Micea
a The figure shows the analysis of S100B immunoreactive cell density in the dorsal raphe of BDNFMet/Met mice (Met/Met) and wild-type controls (Val/Val) treated with fluoxetine (FLX) or water alone at postnatal days 21–42. Means and standard deviations (error bars) are based on the analysis of five mice per group.
**p<0.01. ***p<0.001.
Our findings suggest that deficits in trophic support of serotonergic neurons in BDNFMet/Met mice lead to altered serotonergic innervation of brain areas regulating anxiety-related outputs in these mice. Pharmacologically elevating serotonergic tone and S100B expression during a “sensitive period” in development persistently ameliorates deficiencies in the serotonin system and lowers anxiety-like phenotypes in BDNFMet/Met mice.
Discussion
Anxiety disorders emerge in childhood and adolescence (52–55), a developmental time frame characterized by transitional changes in emotional behavior as well as cortico-limbic neurocircuit maturation (56). Here, we identify in a genetic mouse model of the common human BDNF SNP a “sensitive period” during the transition into adolescence (P21–P42) in which a timed pharmacological intervention rescued subsequent expression of elevated anxiety-like phenotypes. We found that the anxiety-like behaviors in BDNFMet/Met mice, which emerge in late adolescence (Figure 1) (36), were rescued with early-life (P21–P42) fluoxetine treatment, which persistently elevated a serotonergic trophic factor, S100B, and enhanced maturation of serotonergic projections particularly to the infralimbic prefrontal cortex. To our knowledge, this is the first report of a developmentally timed pharmacological treatment that fully rescues a genetically induced anxiety-related behavioral phenotype during a discrete developmental window.
This P21–P42 “sensitive period” in BDNFMet/Met mice is well outside the limits of a previously identified early postnatal window when environmental perturbation of serotonin signaling leads to persistently altered emotional behaviors. Postnatal (P4–P21) exposure to the serotonin inhibitors fluoxetine, clomipramine, or citalopram but not desipramine, a norepinephrine transporter inhibitor, has been shown to elevate anxiety-like behaviors in adult wild-type animals (12, 57–59). However, there are conflicting reports on whether SSRI exposure in periadolescence and adolescence has effects on adult emotional behavior. Mice receiving fluoxetine during the P14–P42 period exhibit an anxiogenic response when behavior is assessed immediately after treatment, although the effect is lost in adulthood (13). In addition, adolescent (P12–P21, P21–P49, P22–P41) or adult (P56–P84) fluoxetine administration did not lead to long-term changes in anxiety-like or fear extinction behaviors in wild-type mice (11, 12). On the other hand, rats receiving fluoxetine during the P25–P46, P35–P49, or P67–P88 periods spend significantly less time in the open arm of the elevated plus maze at 1–3 weeks after drug cessation (14, 15, 60), which suggests species-specific effects of SERT blockade across development (61). The reported discrepancies could be due to a number of methodological factors, such as the dosage, delivery route, and duration of fluoxetine treatment. Our study shows that wild-type mice do not exhibit long-term alterations in anxiety and fear learning behaviors when treated during the P21–P42 period. These findings are in accordance with previous studies showing that fluoxetine administration during adolescence, including the P21–P49 period, or during adulthood does not lead to long-term changes in anxiety-like or fear extinction behaviors in wild-type mice (11, 12). Furthermore, it is important to point out that the anxiolytic fluoxetine effects we observed in BDNFMet/Met mice are due to developmental compensation of trophic support.
Additionally, it has been shown that early-life exposure to fluoxetine or ketamine may have persistent antidepressant effects in rodents. Fluoxetine exposure at P35–P49 in mice resulted in suppressed depression-like behavior (14). Repeated ketamine exposure in rats at P35–P49 resulted in anxiolytic- and antidepressant-like responses 2 months after drug exposure (62). Furthermore, fluoxetine exposure during adolescence (P35–P49) in rats resulted in long-lasting decreases in behavioral reactivity to forced swimming stress (60). These reports highlight the need for further research into the enduring impact antidepressants may have on the developing nervous system.
Previously, the variant BDNF Met allele has been associated with decreased 5-HT1A receptor binding as well as SERT density in the cortex and hippocampus in human carriers (44, 45). In accordance, BDNFMet/Met mice exhibit perturbations in the serotonergic system as a result of reduced trophic support (Figure 3A,B). Our results are also consistent with a report from a study in BDNF haploinsufficient (BDNF+/−) mice indicating a reduction in serotonergic fibers in the cortex and hippocampus of these mice (46).
Interestingly, our results indicate that early-life fluoxetine alters the density and complexity of serotonergic projections in BDNFMet/Met mice (Figure 3A,B), suggesting that at P21–P42 serotonergic neurons in BDNFMet/Met mice, but not in wild-type mice, are still responsive to changes in environmental stimuli. Administration of fluoxetine to rats during periadolescence (P21–P35) results in significant increases in S100B in multiple brain regions that persist into adulthood (63). In vitro and in vivo studies indicate that S100B has trophic effects on serotonergic neurons (50, 64, 65). Indeed, 10-week-old mice overexpressing S100B exhibit increased density of serotonergic fibers in the hippocampus (66). Persistent elevation in S100B expression induced by fluoxetine administered during periadolescence (Figure 4) may mediate the rescue of anatomical and behavioral phenotypes related to the serotonin system in the BDNFMet/Met mice.
An unexpected finding in our study was that fluoxetine administration at P21–P42 failed to increase hippocampal BDNF levels in BDNFMet/Met mice (see Figure S2D in the data supplement), whereas it robustly increases BDNF levels in wild-type and BDNF-HA reporter mice (see Figure S2A–D). A previous report in BDNF Val66Met heterozygous mice showed that while there is a significant reduction in BDNF protein levels in the hippocampus of BDNFVal/Met mice compared with wild-type mice, there is no effect of genotype on mRNA levels (26). As the variant BDNFMet polypeptide is inadequately trafficked into secretory vesicles (23, 67), anterograde delivery of BDNF to the hippocampus from other brain regions could account for diminished total levels.
Another possible explanation for the effects of early-life fluoxetine in BDNFMet/Met mice could be that fluoxetine pharmacokinetics differ with age. However, previously it has been shown that continuous administration of a fixed fluoxetine dosage provides clinically relevant steady-state plasma drug levels in juvenile mice that do not vary significantly with age.
Conclusions
This study, to our knowledge, is the first to demonstrate that timed SSRI administration during a “sensitive period” in periadolescence leads to long-lasting anxiolytic effects in a genetic mouse model displaying elevated anxiety-like behaviors. Our results highlight the role of BDNF in maturation of the serotonin system and the capacity to enhance its development through a timed pharmacological intervention. While the clinical implications on the effects of SSRIs administered during periadolescence cannot be made directly, these findings suggest that transiently elevating serotonergic tone during a “sensitive period” affects anxiety-related behaviors, particularly when the serotonergic system is underdeveloped. There is evidence that including environmental exposures as well as developmental stage in genetic association studies may enhance the ability to identify clinically relevant genetic effects (68). We recently applied a similar mouse-human translational approach to identify developmental stage–specific effects of a common human polymorphism in an endocannabinoid-regulating gene (33, 69).
1 : Mental health: adolescent mental health: opportunity and obligation. Science 2014; 346:547–549Crossref, Medline, Google Scholar
2 : Prior juvenile diagnoses in adults with mental disorder: developmental follow-back of a prospective-longitudinal cohort. Arch Gen Psychiatry 2003; 60:709–717Crossref, Medline, Google Scholar
3 : Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am J Psychiatry 1996; 153:376–381Link, Google Scholar
4 : Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2010; 49:980–989Crossref, Medline, Google Scholar
5 US Food and Drug Administration (FDA): Public Health Advisory: Suicidality in children and adolescents being treated with antidepressant medications. Rockville, Md, 2004Google Scholar
6 : Spillover effects on treatment of adult depression in primary care after FDA advisory on risk of pediatric suicidality with SSRIs. Am J Psychiatry 2007; 164:1198–1205Link, Google Scholar
7 : Changes in antidepressant use by young people and suicidal behavior after FDA warnings and media coverage: quasi-experimental study. BMJ 2014; 348:g3596Crossref, Medline, Google Scholar
8 : Fluoxetine administered to juvenile monkeys: effects on the serotonin transporter and behavior. Am J Psychiatry 2014; 171:323–331Link, Google Scholar
9 : Persistently increased density of serotonin transporters in the frontal cortex of rats treated with fluoxetine during early juvenile life. J Child Adolesc Psychopharmacol 1999; 9:13-24Crossref, Medline, Google Scholar
10 : Age-dependent effects of chronic fluoxetine treatment on the serotonergic system one week following treatment. Psychopharmacology (Berl) 2012; 221:329–339Crossref, Medline, Google Scholar
11 : Effects of adolescent fluoxetine treatment on fear-, anxiety-, or stress-related behaviors in C57BL/6J or BALB/cJ mice. Psychopharmacology (Berl) 2008; 200:413–424Crossref, Medline, Google Scholar
12 : Postnatal day 2 to 11 constitutes a 5-HT-sensitive period impacting adult mPFC function. J Neurosci 2014; 34:12379–12393Crossref, Medline, Google Scholar
13 : Paradoxical anxiogenic response of juvenile mice to fluoxetine. Neuropsychopharmacology 2009; 34:2197–2207Crossref, Medline, Google Scholar
14 : Fluoxetine exposure during adolescence alters responses to aversive stimuli in adulthood. J Neurosci 2014; 34:1007–1021Crossref, Medline, Google Scholar
15 : Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One 2011; 6:e16646Crossref, Medline, Google Scholar
16 : [Progress in the study of response surface modeling in investigation of drug-drug interaction in anesthetic drugs]. Yao Xue Xue Bao 2008; 43:1171–1178 (Chinese)Medline, Google Scholar
17 : Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science 2006; 314:140–143Crossref, Medline, Google Scholar
18 : Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol Psychiatry 2008; 63:642–649Crossref, Medline, Google Scholar
19 : A neurotrophic model for stress-related mood disorders. Biol Psychiatry 2006; 59:1116–1127Crossref, Medline, Google Scholar
20 : Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry 2010; 15:260–271Crossref, Medline, Google Scholar
21 : The brain-derived neurotrophic factor Val66Met polymorphism predicts response to exposure therapy in posttraumatic stress disorder. Biol Psychiatry 2013; 73:1059–1063Crossref, Medline, Google Scholar
22 : Interactions between BDNF Val66Met polymorphism and early life stress predict brain and arousal pathways to syndromal depression and anxiety. Mol Psychiatry 2009; 14:681–695Crossref, Medline, Google Scholar
23 : Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J Neurosci 2005; 25:6156–6166Crossref, Medline, Google Scholar
24 : The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 2003; 112:257–269Crossref, Medline, Google Scholar
25 : A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science 2010; 327:863–866Crossref, Medline, Google Scholar
26 : Variant brain-derived neurotrophic factor Val66Met polymorphism alters vulnerability to stress and response to antidepressants. J Neurosci 2012; 32:4092–4101Crossref, Medline, Google Scholar
27 : Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem 1997; 69:34–42Crossref, Medline, Google Scholar
28 : Brain-derived neurotrophic factor levels in the nervous system of wild-type and neurotrophin gene mutant mice. J Neurochem 1999; 72:1930–1938Crossref, Medline, Google Scholar
29 : BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology 2012; 37:1297–1304Crossref, Medline, Google Scholar
30 : Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 2005; 29:771–783Crossref, Medline, Google Scholar
31 : Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 2004; 29:1321–1330Crossref, Medline, Google Scholar
32 : Sensitive and selective liquid-chromatographic assay of fluoxetine and norfluoxetine in plasma with fluorescence detection after precolumn derivatization. Clin Chem 1992; 38:1756–1761Medline, Google Scholar
33 : FAAH genetic variation enhances fronto-amygdala function in mouse and human. Nat Commun 2015; 6:6395Crossref, Medline, Google Scholar
34 : Conditioned and unconditional components of post-shock freezing. Pavlov J Biol Sci 1980; 15:177–182Medline, Google Scholar
35 : Neuronal release of proBDNF. Nat Neurosci 2009; 12:113–115Crossref, Medline, Google Scholar
36 : Variant brain-derived neurotrophic factor (Valine66Methionine) polymorphism contributes to developmental and estrous stage–specific expression of anxiety-like behavior in female mice. Biol Psychiatry 2012; 72:499–504Crossref, Medline, Google Scholar
37 : The BDNF Val66Met polymorphism impairs synaptic transmission and plasticity in the infralimbic medial prefrontal cortex. J Neurosci 2012; 32:2410–2421Crossref, Medline, Google Scholar
38 : Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science 2011; 334:1731–1734Crossref, Medline, Google Scholar
39 : Activation of the TrkB neurotrophin receptor is induced by antidepressant drugs and is required for antidepressant-induced behavioral effects. J Neurosci 2003; 23:349–357Crossref, Medline, Google Scholar
40 : Deficit in BDNF does not increase vulnerability to stress but dampens antidepressant-like effects in the unpredictable chronic mild stress. Behav Brain Res 2009; 202:245–251Crossref, Medline, Google Scholar
41 : Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci 2002; 22:3251–3261Crossref, Medline, Google Scholar
42 : The developmental role of serotonin: news from mouse molecular genetics. Nat Rev Neurosci 2003; 4:1002–1012Crossref, Medline, Google Scholar
43 : Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology 2008; 33:73–83Crossref, Medline, Google Scholar
44 : Genetic variation in brain-derived neurotrophic factor is associated with serotonin transporter but not serotonin-1A receptor availability in men. Biol Psychiatry 2009; 66:477–485Crossref, Medline, Google Scholar
45 : Genetic variation in brain-derived neurotrophic factor val66met allele is associated with altered serotonin-1A receptor binding in human brain. Neuroimage 2014; 94:33–39Crossref, Medline, Google Scholar
46 : Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc Natl Acad Sci USA 1999; 96:15239–15244Crossref, Medline, Google Scholar
47 : Serotonin transporter function, but not expression, is dependent on brain-derived neurotrophic factor (BDNF): in vivo studies in BDNF-deficient mice. J Neurochem 2007; 101:641–651Crossref, Medline, Google Scholar
48 : Severe deficits in 5-HT2A -mediated neurotransmission in BDNF conditional mutant mice. J Neurobiol 2006; 66:408–420Crossref, Medline, Google Scholar
49 : Regulation of serotonin-1A receptor function in inducible brain-derived neurotrophic factor knockout mice after administration of corticosterone. Biol Psychiatry 2007; 62:521–529Crossref, Medline, Google Scholar
50 : Stimulation of astroglial 5-HT1A receptors releases the serotonergic growth factor, protein S-100, and alters astroglial morphology. Brain Res 1990; 528:155–158Crossref, Medline, Google Scholar
51 : S-100B but not NGF, EGF, insulin, or calmodulin is a CNS serotonergic growth factor. Brain Res 1990; 516:354–356Crossref, Medline, Google Scholar
52 : Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage 2003; 20:420–428Crossref, Medline, Google Scholar
53 : Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med 2005; 352:2515–2523Crossref, Medline, Google Scholar
54 : Service utilization for lifetime mental disorders in US adolescents: results of the National Comorbidity Survey–Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry 2011; 50:32–45Crossref, Medline, Google Scholar
55 : Psychiatric disorder in a birth cohort of young adults: prevalence, comorbidity, clinical significance, and new case incidence from ages 11 to 21. J Consult Clin Psychol 1996; 64:552–562Crossref, Medline, Google Scholar
56 : Adolescent brain development and animal models. Ann N Y Acad Sci 2004; 1021:23–26Crossref, Medline, Google Scholar
57 : Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 2004; 306:879–881Crossref, Medline, Google Scholar
58 : Inhibition of serotonin but not norepinephrine transport during development produces delayed, persistent perturbations of emotional behaviors in mice. J Neurosci 2008; 28:199–207Crossref, Medline, Google Scholar
59 : Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur Neuropsychopharmacol 2009; 19:97–108Crossref, Medline, Google Scholar
60 : Short- and long-term functional consequences of fluoxetine exposure during adolescence in male rats. Biol Psychiatry 2010; 67:1057–1066Crossref, Medline, Google Scholar
61 : Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology 2015; 40:88–112Crossref, Medline, Google Scholar
62 : Repeated ketamine exposure induces an enduring resilient phenotype in adolescent and adult rats. Biol Psychiatry 2013; 74:750–759Crossref, Medline, Google Scholar
63 : Chronic fluoxetine treatment changes S100B expression during postnatal rat brain development. J Child Adolesc Psychopharmacol 2013; 23:481–489Crossref, Medline, Google Scholar
64 : S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001; 33:637–668Crossref, Medline, Google Scholar
65 : S100B in brain damage and neurodegeneration. Microsc Res Tech 2003; 60:614–632Crossref, Medline, Google Scholar
66 : Effects of S100B on serotonergic plasticity and neuroinflammation in the hippocampus in Down syndrome and Alzheimer’s disease: studies in an S100B overexpressing mouse model. Cardiovasc Psychiatry Neurol 2010; article ID 153657Crossref, Medline, Google Scholar
67 : Variant brain-derived neurotrophic factor (BDNF) (Met66) alters the intracellular trafficking and activity-dependent secretion of wild-type BDNF in neurosecretory cells and cortical neurons. J Neurosci 2004; 24:4401–4411Crossref, Medline, Google Scholar
68 : Common polymorphisms in the age of Research Domain Criteria (RDoC): integration and translation. Biol Psychiatry 2016; 79:25–31Crossref, Medline, Google Scholar
69 : Individual differences in frontolimbic circuitry and anxiety emerge with adolescent changes in endocannabinoid signaling across species. Proc Natl Acad Sci USA 2016; 113:4500–4505Crossref, Medline, Google Scholar



