Brain Correlates of the Interaction Between 5-HTTLPR and Psychosocial Stress Mediating Attention Deficit Hyperactivity Disorder Severity
Abstract
Objective:
The serotonin transporter 5-HTTLPR genotype has been found to moderate the effect of stress on severity of attention deficit hyperactivity disorder (ADHD), with stronger effects of stress in carriers of the short allele than in individuals homozygous for the long allele. The underlying neurobiological mechanism of this gene-environment interaction in ADHD is unknown. The authors aimed to determine whether 5-HTTLPR moderates the effect of stress on brain gray matter volume and, if so, which brain regions mediate the effect of this gene-environment interaction on ADHD severity.
Method:
Structural MRI, 5-HTTLPR genotype, and stress exposure questionnaire data were available for 701 adolescents and young adults participating in the multicenter ADHD cohort NeuroIMAGE study (from 385 families; 291 with ADHD, 78 with subthreshold ADHD, 332 healthy comparison subjects; 55.8% male; average age: 17.0 years). ADHD symptom count was determined through multi-informant questionnaires. For the analysis, a whole-brain voxel-based morphometry approach was combined with mediation analysis.
Results:
Stress exposure was associated with significantly less gray matter volume in the precentral gyrus, middle and superior frontal gyri, frontal pole, and cingulate gyrus in S-allele carriers compared with participants homozygous for the l-allele. The association of this gene-environment interaction with ADHD symptom count was mediated by gray matter volume in the frontal pole and anterior cingulate gyrus.
Conclusions:
5-HTTLPR genotype moderates the effect of stress on brain regions involved in social cognitive processing and cognitive control. Specifically, regions important for cognitive control link this gene-environment interaction to ADHD severity.
Attention deficit hyperactivity disorder (ADHD) results in most cases from the combined influence of multiple genetic and environmental risk factors of small effect size (1, 2). Research into the etiology of ADHD is further complicated by gene-environment interactions, whereby a person’s genetic makeup in part determines reactivity to environmental influences (3).
The serotonin transporter gene (SERT, also known as SLC6A4) is a gene implicated in ADHD (1). This gene contains a variable number tandem repeat polymorphism in its promoter region (5-HTTLPR), consisting of a 14-repeat short variant (S-allele) and a 16-repeat long variant (l-allele) (4). There is a large body of literature documenting that 5-HTTLPR may moderate the effects of stress exposure on mood disorders (5). Animal models have provided evidence of a causal relation between this gene-environment interaction and a range of pathological behaviors (6). It has also been shown to be involved in ADHD. We recently reported a stronger positive association between stress exposure and severity of ADHD in individuals carrying an S-allele than in those homozygous for the l-allele and found that this was independent of comorbid internalizing problems (7). In the present study, we aimed to further our understanding of these findings by investigating brain correlates of this gene-environment interaction in the same study cohort.
ADHD is characterized by a delay in brain maturation (8), and both 5-HTTLPR genotype and stress exposure have been shown to influence brain maturation (9, 10). MRI studies of both healthy individuals and those with internalizing problems have reported interaction effects between 5-HTTLPR genotype and stress exposure on limbic and frontal brain regions involved in (the regulation of) social and emotional behavior, including the amygdala and anterior cingulate cortex (11–13). S-allele carriers have been shown to have less connectivity between these regions, associated with higher levels of anxiety, suggesting that less top-down control of frontal regions over subcortical structures underlies part of the behavioral correlates of the 5-HTTLPR genotype (14). Hypofunctioning of frontal regions and connected subcortical structures is also a hallmark of both stress exposure (15) and ADHD (16), indicating overlap in neurobiological correlates of 5-HTTLPR, stress, and ADHD.
Knowledge of how 5-HTTLPR moderates environmental risk factors for ADHD may eventually lead to prevention and treatment being better adjusted to patients’ individual characteristics. The present study therefore aimed to determine 1) whether the interaction between stress and 5-HTTLPR genotype also affects brain gray matter volume and 2) which brain regions would mediate the effect of this gene-environment interaction on ADHD severity. To accomplish these aims, we performed a whole-brain voxel-based morphometry mediation analysis, with gene-environment interaction as predictor, gray matter volume as a mediator, and ADHD symptom count as outcome (see Figure 1). Based on previous literature (17), we expected that paralimbic regions would be most prominently involved in this gene-environment interaction. However, we are the first, to our knowledge, to study the brain correlates of this gene-environment interaction in relation to ADHD severity. For this reason, a whole-brain analysis was chosen above a region-of-interest approach, allowing for the identification of different or previously overlooked brain regions. The analyses were carried out in an adolescent and young adult sample (mean age=17.0 years [SD=3.6]) of individuals with ADHD, their unaffected siblings, and healthy comparison subjects, thus enabling analysis within a wide range of ADHD severity, in accordance with the continuous distribution of ADHD within the population (18).
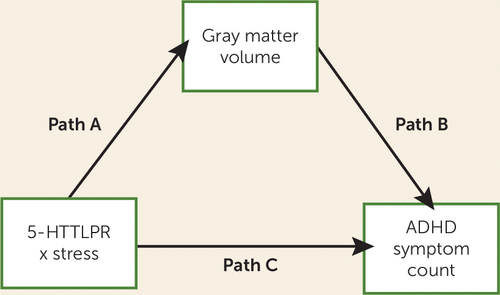
FIGURE 1. The Mediation Modela
a Path A represents the association between the gene-environment interaction and gray matter volume, consistent with aim 1. Path B represents the association between gray matter volume and attention deficit hyperactivity disorder (ADHD) symptom count, which, in combination with path A, is used to assess how gray matter volume mediates the effect of the gene-environment interaction on ADHD symptom count (aim 2). Path C represents the effect of the gene-environment interaction on ADHD symptom count, which we previously reported (7).
Method
Participants and Protocol
Participants were selected from the NeuroIMAGE study (19), a follow-up of the Dutch part of the International Multicenter ADHD Genetics (IMAGE) study. NeuroIMAGE included 365 families with at least one child with ADHD and at least one biological sibling (regardless of ADHD diagnosis) and 148 comparison families with at least one child and without any formal or suspected ADHD diagnosis in any of the first-degree family members. ADHD families were recruited through ADHD outpatient clinics in the regions of Amsterdam, Groningen, and Nijmegen (the Netherlands). Comparison families were recruited through primary schools and high schools in the same geographical regions. To be included in NeuroIMAGE, participants had to be of European Caucasian descent, to be between the ages of 5 and 30, to have an IQ ≥70, and to have no diagnosis of autism, epilepsy, general learning difficulties, brain disorders, or known genetic disorders. More information on the NeuroIMAGE study and its participants is available elsewhere (19).
All measurements were part of a comprehensive assessment protocol. Testing was carried out at either the Vrije Universiteit Amsterdam and Vrije Universiteit Medical Centre or the Radboud University Nijmegen Medical Centre and Donders Institute for Brain, Cognition, and Behavior in Nijmegen. Participants were motivated with short breaks and received €50.00 and a copy of their MRI scan at the end of the day. The study was approved by the regional ethics committee and the medical ethical committee of the Vrije Universiteit Medical Centre. All participants signed informed consent (parents signed informed consent for participants under age 12).
Assessment of ADHD
We constructed an ADHD symptom count based on the Conners’ ADHD Rating Scales questionnaires (20). These questionnaires were completed by the parents and either a teacher (for children <18 years old) or the participants themselves (for those ≥18 years old). The Conners’ Rating Scales provide operational definitions of each of the 18 ADHD symptoms defined in DSM-IV-TR. In this sample, the symptom count ranged from 0 to 18, with an average of 5.4. Crohnbach’s alpha for this measure was 0.91.
The 701 participants who met the inclusion criteria and had structural MRI data available came from 385 families. A total of 291 participants from 233 families had a diagnosis of ADHD, 78 participants had subthreshold ADHD (i.e., they had ADHD symptoms without meeting the criteria for a full ADHD diagnosis, of whom 56 were siblings of participants with ADHD), and 332 were healthy comparison participants (of whom 154 were unaffected siblings of participants with ADHD). ADHD diagnoses were made in accordance with DSM-IV-TR criteria on the basis of a combination of a semistructured diagnostic interview, the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (21), and the Conners’ Rating Scales. In this sample, 97 participants had an oppositional defiant disorder or conduct disorder, 23 had an internalizing disorder, and 79 had a reading disorder. An extensive description of the diagnostic algorithm for ADHD and comorbid disorders is provided in Appendix A in the data supplement accompanying the online version of this article.
Assessment of Stress Exposure
Two questionnaires were used to assess the amount of exposure to psychosocial stress. Parents completed the Long-Term Difficulties Inventory (22, 23), which contained 13 items measuring whether their children had been exposed to chronic stressors, such as a handicap, being bullied, having financial difficulties, or other persisting problems at home or school. They were asked to only report chronic, ongoing difficulties. In addition, participants themselves completed a Stressful Life Events Screening Questionnaire (22, 23), which contained 11 items on exposure to specific major stressful events in the past 5 years, such as death or serious illness of a loved one, physical or sexual abuse, or failure at something important to them. For the composite stress measure, the scores on the questionnaires were transformed to z values and averaged according to common practice for aggregating similar measures, as previously described elsewhere (7). For additional information on both questionnaires and an overview of the items, see Appendix B in the online data supplement.
Genotyping
Genotyping was performed as described by Brookes et al. (24). Briefly, DNA was extracted from blood samples at Rutgers University Cell and DNA Repository (Piscataway, N.J.). Standard polymerase chain reaction protocols were used for the determination of 5-HTTLPR genotype.
Socioeconomic Status
As a measure of socioeconomic status, the highest successfully completed education level of the parents was recoded into a measure reflecting years of education. This scale contained nine levels, ranging from 0 (no formal education) to 17 (university education) years of education (25). The average of both parents was used, which in this sample ranged from 5 to 17 years, with an average of 12.1 years.
MRI Data Acquisition and Preprocessing
Both scanning locations used two identical 1.5-T scanners. For each participant, two high-resolution T1-weighted magnetization-prepared rapid acquisition gradient echo anatomical scans were obtained (176 sagittal slices, repetition time=2,730 ms, echo time=2.95 ms, voxel size=1.0×1.0×1.0 mm, field of view=256 mm). Before processing, raw scans were manually evaluated for motion artifact and scan quality. Only scans with no or mild motion artifact were selected for further analysis. To increase signal-to-noise, scans from the same participant were averaged if they both contained no or mild motion. Three participants were excluded for further analysis because of severe motion in both scans, and 17 participants were excluded because of incidental morphologic abnormalities (e.g., enlarged ventricles).
Preprocessing of the MRI data was carried out with Statistical Parametric Mapping (SPM8; Wellcome Department of Cognitive Neurology, London; [http://www.fil.ion.ucl.ac.uk/spm/software/spm8/]), implemented in MATLAB 7.9 (Mathworks, Sherbourn, Mass.), using the VBM8 toolbox with standard settings. This included normalization to Montreal Neurological Institute (MNI) space, segmentation into tissue-specific maps, modulation by dividing the images through the nonlinear component of the Jacobian determinant of the warp, and smoothing with an 8-mm full width at half maximum Gaussian kernel.
Statistical Analysis
This study investigated a dominant genetic model of the 5-HTTLPRS-allele, wherein S-allele carriers were coded as “1” and l-allele homozygotes were coded as “0.” This is in accordance with the majority of studies investigating this gene-environment interaction (5) and is based on the functional effects of the S- and l-alleles (4). In addition, l-alleles with the rs25531 C-G single-nucleotide polymorphism were recoded as a functional S-allele, in accordance with previous studies (26). This led to 59 l-allele homozygotes being recoded as S-allele carriers. Compliance of genotype distribution with Hardy-Weinberg equilibrium was checked using standard methods.
All behavioral data were analyzed using R v3.1.1 (27). Differences between genotypes in sample demographic characteristics were checked for categorical variables with Pearson’s chi-square tests and for continuous variables with one-way analysis of variance. The model investigating the effect of the gene-environment interaction on ADHD symptom count consisted of 5-HTTLPR genotype, stress exposure, and their interaction, as well as age, gender, socioeconomic status, and location as covariates. In order to account for the within-family correlation because of the inclusion of siblings in the sample, we analyzed the data with linear mixed-effects models with family as a random factor, estimating a random intercept. The p values of the mixed models results were estimated through a Markov chain Monte Carlo algorithm, included in the languageR package.
Whole-Brain Voxel-Based Morphometry Mediation Analysis
We employed mediation effect parametric mapping (28) to determine the relationship between the gene-environment interaction, gray matter volume, and ADHD symptom count. This analysis technique is based on a standard three-variable mediation model, as shown in Figure 1. Here, path “a” represents the association of the predictor X with the mediator M; path “b” represents the effect of the mediator M on the dependent variable Y; and path “c” represents the total effect of the predictor X on the dependent variable Y. The mediation effect (i.e., the effect of X on Y mediated by M) is the product of path “a” and path “b,” the significance of which is determined through bootstrapping. This approach to mediation analysis is in line with the currently most accepted approach to mediation, which deviates from the classic “causal steps” approach to mediation, as the latter has been shown to be less powerful and rest on false assumptions (29).
Our whole-brain mediation model consisted of 5-HTTLPR genotype, amount of stress exposure, and their interaction as predictors, gray matter volume as a mediator, and ADHD symptom count as a dependent variable. Gender, age, socioeconomic status, and scanner location were added as covariates. All continuous predictors were mean-centered.
The whole-brain mediation analysis on the voxel-based morphometry data was performed in MATLAB with the Multilevel Mediation and Moderation Toolbox (28). As a mask, we used the average gray matter image of the sample with an absolute threshold value of 0.2 (number of voxels: 463,956). The toolbox performed a bootstrap test (5,000 samples), to estimate the significance of the effect on each voxel included in the mask, resulting in p value maps for paths “a,” “b,” and “ab.” Family-wise error correction was applied through the use of FSL’s [Functional Magnetic Resonance Imaging of the Brain Software Library] EasyThresh, which carries out cluster-based thresholding. A z value of 2.6 was used to define contiguous clusters, and subsequently each cluster’s significance level was estimated on the basis of Gaussian random field theory. Those clusters surviving a significance threshold of a p value set at 0.001 are reported. Localization was done with the Harvard-Oxford Atlas. All reported coordinates are in MNI space and in millimeters.
In order to further probe the interaction and mediation effects, as well as to correct for the nonindependence of the data, mean gray matter volume from significant clusters was extracted and analyzed with linear-mixed effects models in R, as described above for the behavioral data. Significance of the mediation effect was determined through bootstrapping with 5,000 samples. We further calculated Cohen’s f2, suitable for mixed models (30), as a measure of additional percentage variance explained by the gene-environment interaction term. For the mediation effects, we report κ2 as a ratio measure of the indirect effect compared with its maximal possible value (31).
Sensitivity Analyses
We conducted sensitivity analyses to check whether the findings were not biased as a result of methodological choices. We checked whether the findings were not driven by either a diagnostic subgroup or testing location, by rerunning the analyses with an interaction term between the gene-environment interaction and either diagnosis or testing location and checking whether these interaction terms had significant effects on gray matter volume. Additional information on the methods for these analyses is presented in Appendix C in the online data supplement. Furthermore, given the large age range, we checked whether age played a significant role in the association of the gene-environment interaction with ADHD symptom count by adding a three-way interaction to the model.
Results
Demographic Characteristics
No significant differences in gender distribution, age, stress exposure, socioeconomic status, or testing location were found between S-allele carriers and l-allele homozygotes, as summarized in Table 1. Genotyping frequencies did not deviate from Hardy-Weinberg equilibrium (p=0.11).
| Characteristic | S-Allele Carriers | l-Allele Homozygotes | Test Statistic | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | χ2 | df | p | |
| Covariates | |||||||
| Amsterdam location | 456 | 51.5 | 245 | 51.4 | 0.0007 | 1 | 0.98 |
| Male gender | 456 | 53.9 | 245 | 59.2 | 1.57 | 1 | 0.21 |
| Mean | SD | Mean | SD | F | df | p | |
| Age (years) | 16.93 | 3.56 | 17.14 | 3.63 | 0.54 | 1, 700 | 0.47 |
| Parents’ years of education | 12.00 | 2.51 | 12.18 | 2.46 | 0.78 | 1, 700 | 0.38 |
| Stress z score | –0.04 | 0.99 | 0.07 | 0.91 | 2.88 | 1, 700 | 0.09 |
| Number of stressful live events | 2.01 | 1.51 | 2.19 | 1.54 | 2.09 | 1, 700 | 0.15 |
| Number of long-term difficulties | 1.09 | 1.36 | 1.27 | 1.50 | 2.61 | 1, 688 | 0.11 |
TABLE 1. Demographic Characteristics of Participants by Genotypea
Association of the Interaction Between 5-HTTLPR and Stress With ADHD Symptom Count
There was no evidence of an association of genotype or stress with ADHD symptom count, as previously reported in a sample from which the present sample is a subset (7). The interaction effect was significant (B=0.80, SE=0.38, p=0.03), indicating that 5-HTTLPR genotype moderated the effect of stress exposure on ADHD symptom count. Within-group analysis confirmed that stress was highly and significantly correlated with ADHD symptom count in S-allele carriers (B=0.80, SE=0.24, p<0.001) but not in l-allele homozygotes.
Association of the Interaction Between 5-HTTLPR and Stress With Gray Matter Volume
The association between stress and gray matter volume was moderated by 5-HTTLPR genotype in the precentral gyrus, middle and superior frontal gyrus, frontal pole, and paracingulate gyrus (Figure 2). In these regions, S-allele carriers had a more pronounced negative correlation between stress and gray matter volume than l-allele homozygotes. Information on the clusters is presented in Table 2. Given our focus on the gene-environment interaction, significant clusters from the conditional main effects are presented in Appendix D in the online data supplement.
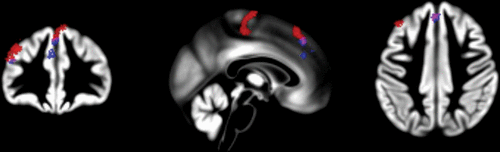
FIGURE 2. Association Between Stress and Gray Matter Volumea
a The images represent visualization of the location of the clusters where gray matter volume was significantly associated with the interaction between 5-HTTLPR and stress exposure (red) and those where gray matter volume mediated the effect of this gene-environment interaction on attention deficit hyperactivity disorder symptom count (blue). Overlap between the clusters is shown in purple. The thresholded z value maps are overlaid on the sample’s average gray matter image. The image is depicted in neurological convention, in Montreal Neurological Institute space (coordinates x=0, y=23, and z=−9 [mm]).
| Location (Peak, Other Regions in Cluster) | Montreal Neurological Institute Coordinates (x, y, z)b | Cluster Size | Coefficient | p | Cohen’s f2 |
|---|---|---|---|---|---|
| Frontal pole, middle frontal gyrus | –51, 21, 33 | 1232 | –0.022 | 0.0003 | 0.007 |
| Paracingulate gyrus, superior frontal gyrus | 0, 32, 50 | 334 | –0.015 | 0.001 | 0.001 |
| Precentral gyrus | 5, –23, 54 | 1097 | –0.017 | 0.00001 | 0.008 |
TABLE 2. Summary of the Clusters Where the Gene-Environment Interaction was Significantly Related to Gray Matter Volumea
The Whole-Brain Mediation Analysis
The mediation analysis revealed two clusters, one in the anterior cingulate and paracingulate gyrus and one in the frontal pole. These clusters are shown in Figure 2. Further inspection of our results revealed that the pattern was the same across both clusters: there was a negative correlation between the interaction term and gray matter volume (path a) and a negative correlation between gray matter volume and ADHD symptom count (path b), leading to a significant positive (path a by path b) mediation effect. That is, the stronger positive correlation between stress and ADHD symptom count found in S-allele carriers compared with l-allele homozygotes was in part statistically explained by less volume in these frontal brain regions. This, and further, information on the clusters is summarized in Table 3.
| Location (Peak, Other Regions in Cluster) | Montreal Neurological Institute Coordinates (x, y, z)b | Cluster Size | Path A | Path B | p | κ2 |
|---|---|---|---|---|---|---|
| Frontal pole, middle frontal gyrus | –30, 54, 20 | 390 | –0.019 | –8.46 | 0.004 | 0.025 |
| Anterior cingulate gyrus, paracingulate gyrus | 0, 35, 44 | 363 | –0.019 | –9.65 | 0.003 | 0.029 |
TABLE 3. Summary of the Clusters Where There was a Significant Mediation Effect of Gray Matter Volumea
Sensitivity Analyses
Age was not significantly correlated with symptom count. However, it was associated with stress, with older participants having experienced more stress (r=0.21, p<0.0001), and with total gray matter volume, with younger participants having more gray matter (r=−0.18, p<0.0001), as would be expected given that pruning of gray matter takes place with increasing age. We added a three-way interaction between the gene-environment interaction and age on ADHD symptom count to check whether this played a significant role in our findings. The gene-environment interaction effect remained significant (B=0.92, SE=0.39, p=0.02), whereas none of the other two-way or three-way interactions were significant. Results from the other sensitivity analyses are presented in Appendix C in the data supplement. Briefly, we found no evidence that diagnosis or testing location influenced the effects of the gene-environment interaction on gray matter volume in any of the significant clusters from the main analysis.
Discussion
We aimed to identify brain gray matter volume correlates of the interaction between 5-HTTLPR and stress exposure and to examine whether gray matter volume mediates the effect of this gene-environment interaction on ADHD severity. To achieve this, we combined a whole-brain voxel-based morphometry approach with mediation analysis. We found that stress exposure was associated with significantly less gray matter volume in the precentral gyrus, middle and superior frontal gyri, frontal pole, and paracingulate gyrus in S-allele carriers compared with participants homozygous for the l-allele. The association of this gene-environment interaction with ADHD symptom count was mediated by gray matter volume in the frontal pole and anterior cingulate gyrus.
Assuming that less gray matter volume is unfavorable, our findings would indicate that S-allele carriers are more sensitive to stress, in accordance with our previous findings on ADHD severity at the behavioral level (7), as well as with the majority of studies of the link between this gene-environment interaction with anxiety and depression (5). Because of the reported association between this gene-environment interaction and internalizing disorders, neuroimaging studies of 5-HTTLPR and its moderation of stress effects have mostly employed a region-of-interest approach focusing on limbic regions such as the amygdala (17). However, whole-brain structural and functional MRI studies have linked this gene-environment interaction to a brain network involved more broadly in social cognitive processing and emotion regulation (12, 32). This network includes the precentral gyrus and anterior cingulate and paracingulate gyri, regions also reported in the present study. We further found an association with gray matter volume in the frontal pole and superior and middle frontal gyri. These regions, together with the anterior cingulate gyrus, are essential for cognitive control, such as suppressing automatic emotional reactions in favor of more flexible goal-directed behavior (33). This includes control over the amygdala (33), which is in line with reports that top-down control of the anterior cingulate over the amygdala is central to the association of 5-HTTLPR with anxiety and depression (14).
The present findings speak to the idea that in the context of ADHD, 5-HTTLPR, and its moderation of the effects of stress, is more broadly involved in self-regulation problems above and beyond the regulation of anxiety and sad affect. We found that the stronger negative association between stress and gray matter volume in the frontal pole and anterior cingulate gyrus in S-allele carriers compared with l-allele homozygotes mediated the association between this gene-environment interaction and ADHD severity. Prefrontal cortex dysfunction, and associated problems with cognitive control, is a hallmark of ADHD (16); neuroimaging studies have repeatedly reported less volume and lower activity across the frontal lobes in individuals with ADHD compared with healthy comparison subjects (34). The results from the present study suggest that the interaction between 5-HTTLPR and stress exposure may contribute to these structural and functional deficits of the prefrontal cortex in ADHD. As described above, 5-HTTLPR and stress have been linked to social cognitive processing (35) and cognitive control, both the anterior cingulate and frontal pole are important for cognitive control in socioemotional situations (33, 36), and cognitive control problems in ADHD manifest in academic (37), social (38), or emotional (39) contexts. We therefore hypothesize that this gene-environment interaction may be linked to ADHD through its effect on broadly defined self-regulation problems.
Strengths of this study include a large sample size, use of multiple informants to determine ADHD phenotype, and the application of a whole-brain moderated mediation analysis that allowed for assessment of the neural pathways coupling the gene-environment interaction with ADHD severity. Limitations of this study are the retrospective assessment of stress exposure and the observational, cross-sectional design, the latter preventing strong inferences about causality. For instance, it could be the case that the reported gray matter volume differences are a causal factor in maladaptive behavior, which in turn may lead to the experience of more stressful live events. While animal studies have provided causal evidence that the brain of S-allele carriers is more affected by exposure to stress, longitudinal studies or studies making use of “natural experiments” (40) are needed to confirm this causality in humans.
In summary, we demonstrated that 5-HTTLPR moderates the effects of stress at the neural level, such that S-allele carriers show a more negative association between stress and gray matter volume than l-allele homozygotes. The implicated brain regions have been linked to social cognition, emotion regulation, and more broadly defined cognitive control functions. The anterior cingulate gyrus and frontal pole, regions important for cognitive control, statistically mediate the association between the gene-environment interaction and ADHD severity in adolescence and young adulthood. Our findings suggest that the interaction between 5-HTTLPR and stress may render individuals vulnerable to broadly defined self-regulation problems and that this mechanism is not only relevant for internalizing symptoms of anxiety and depression but also for ADHD symptoms. These findings have implications for both clinicians and researchers. Clinicians may eventually use information on the moderating effects of patients’ genotypes to shape their prevention and treatment strategies to individual patients’ needs; S-allele carriers may benefit more from preventing stressful experiences and treatments in order to better regulate their behavior, although more research is needed to confirm this. For researchers, these findings underline the fact that genetic and environmental factors do not operate in isolation and need to be studied in context. Such approaches are a step forward in resolving heterogeneity of ADHD and its underlying mechanisms. Our findings also suggest that future research may need to consider a broader role for this gene-environment interaction in shaping behavior than previously assumed, with effects on cognitive control. Because the brain regions reported in this study are complex and serve multiple functions, future studies should further specify how the interaction between 5-HTTLPR and stress exposure affects neurocognitive functioning and how this relates to ADHD.
1 : Candidate gene studies of ADHD: a meta-analytic review. Hum Genet 2009; 126:51–90Crossref, Medline, Google Scholar
2 : Environmental risk factors for attention-deficit hyperactivity disorder. Acta Paediatr 2007; 96:1269–1274Crossref, Medline, Google Scholar
3 : Measured gene-by-environment interaction in relation to attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2010; 49:863–873Crossref, Medline, Google Scholar
4 : Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Crossref, Medline, Google Scholar
5 : Genetic sensitivity to the environment: the case of the serotonin transporter gene and its implications for studying complex diseases and traits. Am J Psychiatry 2010; 167:509–527Link, Google Scholar
6 : Association between the recombinant human serotonin transporter linked promoter region polymorphism and behavior in rhesus macaques during a separation paradigm. Dev Psychopathol 2007; 19:977–987Crossref, Medline, Google Scholar
7 : The serotonin transporter gene polymorphism 5-HTTLPR moderates the effects of stress on attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry 2014; 55:1363–1371Crossref, Medline, Google Scholar
8 : Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci USA 2007; 104:19649–19654Crossref, Medline, Google Scholar
9 : Serotonin: a regulator of neuronal morphology and circuitry. Trends Neurosci 2010; 33:424–434Crossref, Medline, Google Scholar
10 : Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009; 10:434–445Crossref, Medline, Google Scholar
11 : Cognitive appraisal and life stress moderate the effects of the 5-HTTLPR polymorphism on amygdala reactivity. Hum Brain Mapp 2011; 32:1856–1867Crossref, Medline, Google Scholar
12 : Neural correlates of epigenesis. Proc Natl Acad Sci USA 2006; 103:16033–16038Crossref, Medline, Google Scholar
13 : Childhood stress, serotonin transporter gene and brain structures in major depression. Neuropsychopharmacology 2010; 35:1383–1390Crossref, Medline, Google Scholar
14 : 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci 2005; 8:828–834Crossref, Medline, Google Scholar
15 : Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry 2006; 59:975–982Crossref, Medline, Google Scholar
16 : Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry 2012; 51:356–367Crossref, Medline, Google Scholar
17 : Genetics of emotional regulation: the role of the serotonin transporter in neural function. Trends Cogn Sci 2006; 10:182–191Crossref, Medline, Google Scholar
18 : Attention-deficit hyperactivity disorder: a category or a continuum? genetic analysis of a large-scale twin study. J Am Acad Child Adolesc Psychiatry 1997; 36:737–744Crossref, Medline, Google Scholar
19 : The NeuroIMAGE study: a prospective phenotypic, cognitive, genetic and MRI study in children with attention-deficit/hyperactivity disorder: design and descriptives. Eur Child Adolesc Psychiatry 2014; 24:265–281Crossref, Medline, Google Scholar
20 : The revised Conners’ Parent Rating Scale (CPRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol 1998; 26:257–268Crossref, Medline, Google Scholar
21 : Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Crossref, Medline, Google Scholar
22 : Timing matters: long term effects of adversities from prenatal period up to adolescence on adolescents’ cortisol stress response: the TRAILS study. Psychoneuroendocrinology 2012; 37:1439–1447Crossref, Medline, Google Scholar
23 : Low heart rate: a marker of stress resilience: the TRAILS study. Biol Psychiatry 2008; 63:1141–1146Crossref, Medline, Google Scholar
24 : The analysis of 51 genes in DSM-IV combined type attention deficit hyperactivity disorder: association signals in DRD4, DAT1 and 16 other genes. Mol Psychiatry 2006; 11:934–953Crossref, Medline, Google Scholar
25 : Scaling Levels of Education. Amsterdam, Faculty of Social Sciences, VU-University Amsterdam, 2010Google Scholar
26 : Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet 2006; 78:815–826Crossref, Medline, Google Scholar
27 R Core Team: R: A Language and Environment for Statistical Computing. Vienna, Austria, R Foundation for Statistical Computing, 2012Google Scholar
28 : Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 2008; 59:1037–1050Crossref, Medline, Google Scholar
29 : Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York, Guilford Press, 2013Google Scholar
30 : A practical guide to calculating Cohen’s f(2): a measure of local effect size, from PROC MIXED. Front Psychol 2012; 3:111Crossref, Medline, Google Scholar
31 : Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods 2011; 16:93–115Crossref, Medline, Google Scholar
32 : Beyond affect: a role for genetic variation of the serotonin transporter in neural activation during a cognitive attention task. Proc Natl Acad Sci USA 2005; 102:12224–12229Crossref, Medline, Google Scholar
33 : Anterior prefrontal cortex inhibition impairs control over social emotional actions. Curr Biol 2011; 21:1766–1770Crossref, Medline, Google Scholar
34 : The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry 2006; 47:1051–1062Crossref, Medline, Google Scholar
35 : Long story short: the serotonin transporter in emotion regulation and social cognition. Nat Neurosci 2007; 10:1103–1109Crossref, Medline, Google Scholar
36 : Meeting of minds: the medial frontal cortex and social cognition. Nat Rev Neurosci 2006; 7:268–277Crossref, Medline, Google Scholar
37 : Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? a study of early academic skill deficits. J Child Psychol Psychiatry 2007; 48:1061–1070Crossref, Medline, Google Scholar
38 : Attention-deficit/hyperactivity disorder and social dysfunctioning. Clin Psychol Rev 2008; 28:692–708Crossref, Medline, Google Scholar
39 : Emotion dysregulation in attention deficit hyperactivity disorder. Am J Psychiatry 2014; 171:276–293Link, Google Scholar
40 : Proceeding from Observed Correlation to Causal Inference: the Use of Natural Experiments. Perspect Psychol Sci 2007; 2:377–395Crossref, Medline, Google Scholar



