The Neural Correlates of Anomalous Habituation to Negative Emotional Pictures in Borderline and Avoidant Personality Disorder Patients
Abstract
Objective
Extreme emotional reactivity is a defining feature of borderline personality disorder, yet the neural-behavioral mechanisms underlying this affective instability are poorly understood. One possible contributor is diminished ability to engage the mechanism of emotional habituation. The authors tested this hypothesis by examining behavioral and neural correlates of habituation in borderline patients, healthy comparison subjects, and a psychopathological comparison group of patients with avoidant personality disorder.
Method
During fMRI scanning, borderline patients, healthy subjects, and avoidant personality disorder patients viewed novel and repeated pictures, providing valence ratings at each presentation. Statistical parametric maps of the contrasts of activation during repeated versus novel negative picture viewing were compared between groups. Psychophysiological interaction analysis was employed to examine functional connectivity differences between groups.
Results
Unlike healthy subjects, neither borderline nor avoidant personality disorder patients exhibited increased activity in the dorsal anterior cingulate cortex when viewing repeated versus novel pictures. This lack of an increase in dorsal anterior cingulate activity was associated with greater affective instability in borderline patients. In addition, borderline and avoidant patients exhibited smaller increases in insula-amygdala functional connectivity than healthy subjects and, unlike healthy subjects, did not show habituation in ratings of the emotional intensity of the images. Borderline patients differed from avoidant patients in insula-ventral anterior cingulate functional connectivity during habituation.
Conclusions
Unlike healthy subjects, borderline patients fail to habituate to negative pictures, and they differ from both healthy subjects and avoidant patients in neural activity during habituation. A failure to effectively engage emotional habituation processes may contribute to affective instability in borderline patients.
Borderline personality disorder, an enduring condition that is present in an estimated 2.7% of the population (1), is characterized by severe affective instability, turbulent interpersonal relationships, and impulsive aggression (2). Approximately 10% of borderline patients commit suicide (2), and suicidality in borderline patients is associated with affective instability (3). Despite the clinical significance of affective instability, the processes that underlie it in this population are not understood (4). One factor that could contribute is an impaired ability to engage the adaptive emotional regulatory processes commonly used in healthy individuals. These include voluntary emotion regulation strategies such as cognitive reappraisal (5) and implicit processes such as habituation, whereby multiple exposures to a stimulus yield diminished responsivity (6). Anomalous neural activity has been identified in borderline patients during cognitive reappraisal (7, 8). In the present study, we extend this work to examine whether emotional habituation mechanisms are impaired as well.
Habituation of affect, which entails a reduction in subjective distress upon repeated exposure to an aversive stimulus, is a well established and highly adaptive psychophysiological phenomenon. Habituation plays a critical role as well in psychotherapeutic interventions such as desensitization (9). Neuroimaging studies in healthy individuals have demonstrated that habituation is associated with decreases in blood-oxygen-level-dependent (BOLD) signal in limbic regions such as the amygdala, the hippocampus, the insula, and the anterior cingulate cortex, as well as in cortical regions such as the left dorsolateral prefrontal cortex and temporal cortex (10–13). We have found that insula-amygdala connectivity increases in healthy individuals as they habituate to negative pictures (14). However, in borderline patients, little is known about limbic and cortical network response to repeated exposure to negative stimuli.
Functional neuroimaging studies of borderline patients engaged in emotion processing tasks have identified increased amygdala activity when viewing faces (15, 16) and increased amygdala, fusiform (17, 18), primary visual, anterior cingulate, and superior temporal gyrus activity (18) when viewing negative compared with neutral emotional pictures, as well as increased amygdala activity when viewing repeated images (19). During cognitive reappraisal by distancing, in borderline patients the dorsal anterior cingulate cortex and intraparietal sulci are not activated and amygdala activity is not down-regulated to the extent that these occur in healthy subjects (7). Moreover, studies of functional connectivity in borderline patients during viewing of fear faces (20) and during pain processing (21) have shown anomalous connectivity patterns involving the insula, amygdala, and prefrontal regions. However, there have been no studies of the relationship between functional connectivity and habituation in borderline patients.
We hypothesized that borderline patients would not be able to use habituation as effectively as healthy subjects and that this would be reflected in decreased behavioral habituation and anomalous activity in emotion regulatory networks when they viewed repeated versus novel aversive pictures. Specifically, we predicted that in borderline patients the dorsal anterior cingulate, a region implicated in emotion regulation, would not be activated and insula-amygdala connectivity would not increase to the degree that these occur in healthy subjects when confronting repeated negative pictures. We further hypothesized that impairment in the implicit regulatory mechanism of habituation would contribute to affective instability in borderline patients, and thus we predicted that affective instability in borderline patients would correlate with a deficit in dorsal anterior cingulate activity and insula-amygdala connectivity during habituation.
To examine the possibility that any differences we identified between borderline and healthy subjects could be the result of a low threshold for affective response not specific to borderline personality disorder, we included patients with avoidant personality disorder as a psychopathological comparison group. Avoidant personality disorder, which occurs in 1%−2% of the population, is characterized by hypersensitivity to negative evaluation, excessive fear of rejection, and avoidance of social relationships (22). To our knowledge, no functional imaging studies of avoidant personality disorder have been reported in the literature. Studies of generalized social anxiety disorder, however, a disorder thought to be related to avoidant personality disorder, have identified decreased insula-dorsal anterior cingulate functional connectivity relative to healthy subjects during viewing of fearful faces (23). Hence, we predicted decreased insula-dorsal anterior cingulate connectivity in avoidant patients relative to healthy subjects during habituation.
To accomplish these objectives, we examined changes in subjective affect self-reports and in the functional neural architecture of habituation when negative pictures were viewed a second compared with a first time in borderline patients, avoidant patients, and healthy subjects.
Method
Participants
We recruited 23 borderline patients, 27 avoidant patients, and 28 healthy subjects from outpatient clinics at the Mount Sinai Medical Center and the James J. Peters VA Medical Center in New York City and from newspaper and online advertisements. All participants provided written informed consent after procedures were fully explained to them. Four borderline patients, four avoidant patients, and three healthy subjects were excluded because of excessive head motion or inadequate response rates during scanning (for details, see the data supplement that accompanies the online edition of this article). Participants in the borderline group met DSM-IV criteria for borderline personality disorder, including the affective instability criterion, and did not meet criteria for schizotypal or avoidant personality disorder. Participants in the avoidant group met DSM-IV criteria for avoidant personality disorder but not criteria for borderline or schizotypal personality disorder. Participants with potentially confounding axis I diagnoses and medical conditions, as described in detail in the online data supplement, were excluded. Participants had to be free of psychotropic medications for 2 weeks (6 weeks in the case of fluoxetine). Healthy participants did not meet DSM-IV criteria for any axis I or axis II disorder. Diagnostic assessments were obtained using the Structured Clinical Interview for DSM-IV–Patient Edition and the Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Our group has achieved an interrater reliability of 0.81 for diagnosing borderline personality disorder. Participants were rated for affective instability by means of the Affective Lability Scale (24) and for depression using the Hamilton Depression Rating Scale (HAM-D). Affective Lability Scale scores were not available for one borderline patient.
The basic sample characteristics are presented in Table 1. The borderline and avoidant groups did not significantly differ in age from the healthy group. The avoidant group was slightly younger on average than the borderline group. The female-to-male ratio did not differ between groups. As expected, the borderline patients demonstrated greater affective instability as measured by the Affective Lability Scale than either comparison group. Depression as rated by the HAM-D was low for all groups, but as expected, the mean HAM-D score was highest for the borderline group, intermediate for the avoidant group, and lowest for the healthy group. Present and past axis I and axis II comorbidities are presented in the online data supplement.
| Characteristic | Patients With Borderline Personality Disorder (N=19) | Healthy Subjects (N=25) | Patients With Avoidant Personality Disorder (N=23) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Female | 11 | 58 | 13 | 52 | 13 | 57 |
| Mean | SD | Mean | SD | Mean | SD | |
| Agea (years) | 31.9 | 9.9 | 28.1 | 6.9 | 26.3 | 4.8 |
| Affective Lability Scale scoreb | 71.3 | 30.6 | 27.3 | 20.5 | 48.8 | 21.5 |
| Hamilton Depression Rating Scale scoreb | 7.2 | 2.9 | 1.1 | 1.1 | 4.9 | 2.9 |
Event-Related Task Design
BOLD signals were acquired as participants viewed a series of 48 negative and 48 neutral pictures drawn from the International Affective Pictures System (25) and the Empathy Picture System (26). The picture set is described in detail in the online data supplement. Two-thirds of the pictures were presented twice. The picture-repeat pairs were distributed uniformly throughout the 27 minutes of the task to control for the potential confounders of practice, fatigue, or scanner drift. Pictures that were repeated and those shown only once were counterbalanced across participants. The task design is illustrated in Figure 1. Neutral pictures were interspersed among the negative pictures so that participants would not become inured to viewing negative pictures. Negative pictures showed scenes reflecting conflict, abuse, or loss, and neutral pictures showed people at work or in street scenes. Participants were instructed to allow themselves to fully experience the emotion evoked by the picture and to indicate their emotional response to the picture they had just viewed on a scale of 1 (most negative) to 5 (most positive).

a Each picture is presented for 4 seconds and is followed by a 3-second rating interval when the subject rates the picture on a scale from 1 (most negative) to 5 (most positive) using a response button box. This is followed by a 3-second interstimulus interval. Pictures are shown in five consecutive blocks of 16 negative and 16 neutral pictures. Two-thirds of the pictures are shown a second time. A repeated picture is always shown 5.3 minutes after the first showing of that picture, and pictures and their repeats are distributed uniformly throughout the 27 minutes of the task. Novel and repeated pictures are counterbalanced across participants.
Image Acquisition and Analysis
BOLD images were obtained with a Siemens 3-T Allegra scanner using a gradient echo echo-planar sequence (the parameters used are described in the online data supplement). Preprocessing and statistical analyses were carried out using SPM8 (www.fil.ion.ucl.ac.uk/spm/software/spm8), and general linear modeling for each participant was carried out with NeuroElf (neuroelf.net). Preprocessing parameters and general linear modeling are described in the data supplement. For the trial-by-trial affect analyses, parametric weights corresponding to each participant’s affect rating per trial were specified. Contrast images for all participants were entered into a second-level random-effects group analysis carried out using NeuroElf. The principal contrast of interest was RepeatedNeg – NovelNeg. Thresholds for whole-brain family-wise error multiple comparison correction were determined using AlphaSim (27). In a priori regions of interest that included the amygdala, family-wise error extent thresholds were small-volume corrected using a bilateral Brodmann atlas-based anatomical amygdala mask. Reported coordinates refer to Montreal Neurological Institute space.
Functional Connectivity Analyses
Psychophysiological interaction (28) analyses were carried out using a seed region in the left middle-posterior insula, chosen as an affect-related (29, 30) region of interest whose activity correlated with trial-by-trial affect ratings as determined in the parametric analyses. The seed region was determined independently of the task-related connectivity changes for RepeatedNeg versus NovelNeg conditions. To protect against bias by group, we defined this region to be the conjunction of the NovelNeg and RepeatedNeg parametric maps for all three groups. A general linear model was then computed that included regressors for the coupling between this seed region and other brain areas, as well as a psychophysiological interaction term reflecting the coupling of the seed region and other brain regions modulated by the psychological context of interest, in this case the change between RepeatedNeg and NovelNeg conditions. Six motion parameters were also included, and data were high-pass filtered using a Fourier transform (cutoff=130 seconds). After general linear model estimation, random-effects analyses were performed as above, with contrasts in this case representing areas showing a significant psychophysiological interaction effect. Results were then statistically thresholded as described above.
Results
Behavioral Results
Figure 2 displays each group’s ratings of emotional valence for novel and repeated negative pictures. A repeated-measures analysis of variance of self-reported affect ratings with novelty (novel versus repeated) as a repeated measure and group (borderline, avoidant, healthy) as the between-subject measure showed a main effect of novelty (F=7.38, df=2, 64, p=0.008) but no group-by-novelty interaction. Planned comparisons examining our prediction that only healthy subjects would behaviorally habituate showed that healthy subjects did habituate to the negative pictures, rating repeated pictures less negatively than novel pictures (t=2.71, df=24, p<0.01, one-tailed), and that borderline and avoidant patients did not. (Neutral picture behavioral findings are presented in the online data supplement.)

a Affect is rated on a scale from 1 (most negative) to 5 (most positive).
b Significant difference in emotional valence between novel and repeated negative pictures for the healthy group (p<0.01, one-tailed).
Imaging Results
BOLD activation in borderline and avoidant patients compared with healthy subjects during repeat exposure to negative pictures.
When viewing repeated versus novel negative pictures, the borderline group in comparison to the healthy group exhibited a relative decrease in activation in the dorsal anterior cingulate cortex (156-voxel cluster, p<0.05, k=150, family-wise error corrected; Figure 3A and B), and the left superior, left middle, and right transverse temporal gyri (see Figure S1 and Table S1 in the online data supplement). In each region, this was accounted for by a relative increase in activity in the healthy group. The avoidant group also showed decreased dorsal anterior cingulate activity relative to the healthy group (162-voxel cluster, p<0.05, k=150, family-wise error corrected; Figure 3C and D), as well as in the right transverse temporal gyrus, right thalamus, left parahippocampal gyrus, left inferior frontal gyrus, left precuneus, and right cerebellar culmen (see Figure S1 and Table S1). The borderline and avoidant groups did not differ from each other in activation when viewing repeated versus novel negative pictures at the whole-brain corrected threshold (p<0.05, family-wise error corrected). Repeated versus novel contrasts within the groups are provided in Figure S2 and Table S2 in the online data supplement. Collapsing across novelty, we found that the borderline group showed greater amygdala and insula activity in response to negative pictures than either the healthy group or the avoidant group (see Figure S3 in the data supplement).

a In panel A, the map shows a 156-voxel dorsal anterior cingulate cluster indicating a significant difference between the healthy and borderline groups for RepeatedNeg versus NovelNeg activity (p<0.05, k=150, family-wise error corrected). Panel B shows extracted beta weights for each group during viewing of novel and repeated negative pictures. In panel C, the map shows a 162-voxel dorsal anterior cingulate cluster indicating a significant difference between the healthy and avoidant groups for RepeatedNeg versus NovelNeg activity (p<0.05, k=150, family-wise error corrected). Panel D shows extracted beta weights for each group during viewing of novel and repeated negative pictures. In panel E, the map shows an unbiased 65-voxel dorsal anterior cingulate region of interest derived from the conjunction of voxels showing significant between-group differences for repeated versus novel negative picture viewing for the contrasts between the borderline and healthy groups and between the avoidant and healthy groups (p<0.05). Panel F shows the correlation of repeated versus novel activation differences in the independently defined dorsal anterior cingulate region of interest (shown in panel E) with affective instability for each group.
b Significant within-group difference, p<0.05, two-tailed.
Association between decreased engagement of the dorsal anterior cingulate during habituation and affective instability.
To determine whether the degree to which deficits in engaging the dorsal anterior cingulate during repeated versus novel viewing was associated with affective instability, we determined correlations between Affective Lability Scale scores and BOLD signal changes in the dorsal anterior cingulate for each group.
Association between neural activity and subjective affect reports.
To identify the seed region for subsequent functional connectivity analyses, we examined the neural representation of the subjective experience of negative affect by conducting parametric analyses, examining the relationship between regional activation and trial-by-trial affect ratings of the negative pictures. For each of the three groups and for both novel and repeated picture viewings, the degree to which pictures were rated as negative was correlated with middle-posterior insula activity, with greater activity predicting greater subjective reports of negative affect in all six maps, a finding we previously made in this data set using the healthy group alone (14). Conjunction analysis of the six maps yielded a 27-voxel left mid-posterior insula cluster (p<0.05, two-tailed, peak at −42, 0, 6; see the axial inset in Figure 4), which became our insula seed region for subsequent functional connectivity analyses.
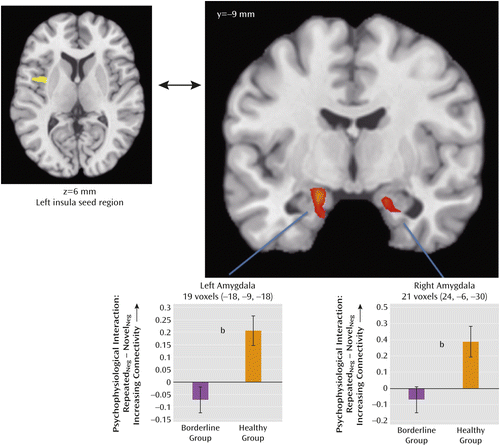
a Left and right amygdala regions showing significant group differences in functional connectivity to left insula seed region (healthy group > borderline group).
b Significant difference between groups, p<0.05, two-tailed.
Functional connectivity.
Montages showing significant changes in functional connectivity to the left insula seed region for repeated versus novel viewings within each group are provided in Figure S4 in the online data supplement. Significant change in functional connectivity between the left insula seed and the amygdala was observed only in the healthy group, where it was observed bilaterally. Group comparisons demonstrated that when viewing repeated versus novel pictures, the borderline group exhibited significantly less functional connectivity between the mid-posterior insula and the left and right amygdala relative to the healthy group (Figure 4; p<0.05, two-tailed, masked with a Brodmann atlas-based anatomical amygdala boundary).
Borderline patients also did not increase connectivity between the insula and the superior frontal gyrus (220 voxels, peak at 8, 45, 39; p=0.05, k=150, family-wise error corrected) to the degree that healthy participants did (see Figure S6 and Table S3 in the data supplement). Compared to the healthy group, the avoidant group showed less of an increase in connectivity between the insula and a broad region of the middle frontal gyrus (including the dorsolateral and medial prefrontal cortices) and posterior cingulate (Brodmann’s area 30) and more of an increase to the cerebellum (p=0.05, k=150, family-wise error corrected) (see Figure S6 and Table S3).
Discussion
We sought to determine whether borderline patients, who are emotionally dysregulated, show anomalous neural activity during the implicit emotion regulatory process of habituation and whether patients with a different personality disorder characterized by high emotional reactivity to interpersonal situations—namely, avoidant personality disorder—could be distinguished from borderline patients when presented with the same task. Behaviorally, we found that whereas healthy subjects habituated to the negative pictures, the borderline and avoidant patients did not. We also found that during viewing of repeated pictures, borderline and avoidant patients showed no significant change in dorsal anterior cingulate activity, while in healthy subjects activity in this region increased. Notably, the capacity to increase activation in this region on repeated viewing was associated with less affective instability in both the borderline and healthy groups. Taken together, these findings support the hypothesis that anomalies in habituation are associated with affective instability in borderline patients.
Furthermore, when viewing repeated negative emotional pictures, neither borderline nor avoidant patients exhibited the increased insula-amygdala functional connectivity seen in healthy subjects. Since increasing insula-amygdala connectivity was associated with increasing behavioral habituation in the healthy and borderline groups, the failure of this connectivity to increase adequately could additionally contribute to impaired behavioral habituation and consequent faulty emotion regulation in borderline patients.
In examining the subjective experience of negative emotion, this study demonstrated that left mid-posterior insula activity correlated with ratings of negative affect in borderline, avoidant, and healthy participants during both novel and repeated negative picture viewing. This finding is consistent with the proposal that the insula broadly participates in self-awareness of feeling state and affective integration (31, 32), with a particular role for the mid and posterior insula in visceral awareness (30, 33, 34). In addition to these novel findings, we replicated the observation that borderline patients exhibit greater amygdala activation than healthy subjects when viewing negative social pictures (7, 15–17), and we extended these observations to show that the increased amygdala activation in borderline patients was also greater than that seen in avoidant patients. In addition, we demonstrated greater insula activation to negative pictures in borderline patients compared with healthy subjects and avoidant patients.
A strength of this study is its inclusion of avoidant patients as a psychopathological comparison group to explore whether differences in functional patterns identified in borderline patients could be distinguished from those seen in other personality disorders. Although displaying a number of features in common with borderline patients, the avoidant and borderline groups differed in several respects. The borderline patients exhibited greater insula-ventral anterior cingulate functional connectivity with repeated viewing of negative pictures compared with the avoidant patients. The insula and anterior cingulate have been posited as key nodes in a network dedicated to assessing the salience of external and internal stimuli, allocating attentional and control resources and preparing for action (35). Thus, decreased connectivity to this region in avoidant patients suggests the possibility of a distinct mechanism accounting for the affective dysregulation in these patients. The borderline and avoidant groups also differed in the degree to which increasing activation of the dorsal anterior cingulate to repeated versus novel pictures predicted less affective instability.
In the avoidant patients, the thalamus, parahippocampal gyrus, ventrolateral prefrontal cortex, and dorsal anterior cingulate were not activated as strongly as in the healthy subjects during viewing of repeated versus novel negative pictures. In addition, insula connectivity to extensive cortical regions, including the rostral anterior cingulate, the medial and dorsolateral prefrontal cortex, and the posterior cingulate, did not increase to the extent that it did in the healthy subjects. Decreased insula connectivity to the rostral anterior cingulate when viewing threat pictures has been reported in generalized social anxiety disorder (23), suggesting the possibility of some functional similarities between these disorders.
Although the healthy group showed significant behavioral habituation and the borderline and avoidant groups did not, the magnitude of the habituation effect in the healthy group was not large, and in the absence of a group-by-novelty interaction, we cannot conclude that there are group differences in behavioral habituation. The small behavioral effect and consequent absence of the interaction effect may be a consequence of the fact that pictures were repeated only once, which is a limitation of our study. Nevertheless, rather robust group differences in activation and functional connectivity were observed. The finding of significant neural group differences alongside blunted behavioral differences is common to a number of neurophysiological studies comparing borderline patients to healthy subjects (8, 17, 18, 36, 37). New et al. have suggested (38) that this may be a consequence of borderline patients’ alexithymia and difficulty self-identifying emotion.
This study demonstrates that when presented with repeated negative pictures, borderline patients show anomalies in limbic-cortical functional connectivity and in engagement of cortical regulatory regions that are associated with affective instability. This suggests that impairment in the implicit emotion regulatory mechanism of habituation may contribute to the affective instability that characterizes borderline patients. The anomalous patterns of activity and connectivity in borderline patients are distinct from those in avoidant patients, indicating that the neural features of habituation in borderline patients are not simply characteristics of personality disorders in general. Understanding the processing deficits underlying anomalous habituation in borderline and avoidant personality disorders may help guide the development of novel psychotherapeutic or pharmacologic interventions to enhance adaptive habituation in these disorders.
1 : Characteristics of borderline personality disorder in a community sample: comorbidity, treatment utilization, and general functioning. J Pers Disord (Epub ahead of print, Feb 27, 2013)Google Scholar
2 : The borderline diagnosis, I: psychopathology, comorbidity, and personality structure. Biol Psychiatry 2002; 51:936–950Crossref, Medline, Google Scholar
3 : Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Pers Disord 2001; 15:358–370Crossref, Medline, Google Scholar
4 : Components of emotion dysregulation in borderline personality disorder: a review. Curr Psychiatry Rep 2013; 15:335Crossref, Medline, Google Scholar
5 : The emerging field of emotion regulation: an integrative review. Rev Gen Psychol 1998; 2:271–299Crossref, Google Scholar
6 : Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev 1966; 73:16–43Crossref, Medline, Google Scholar
7 : Neural correlates of the use of psychological distancing to regulate responses to negative social cues: a study of patients with borderline personality disorder. Biol Psychiatry 2009; 66:854–863Crossref, Medline, Google Scholar
8 : Neuronal correlates of cognitive reappraisal in borderline patients with affective instability. Biol Psychiatry 2011; 69:564–573Crossref, Medline, Google Scholar
9 : Emotional processing of fear: exposure to corrective information. Psychol Bull 1986; 99:20–35Crossref, Medline, Google Scholar
10 : Habituation of attentional networks during emotion processing. Neuroreport 2002; 13:1255–1258Crossref, Medline, Google Scholar
11 : Brain habituation during repeated exposure to fearful and neutral faces: a functional MRI study. Brain Res Bull 2003; 59:387–392Crossref, Medline, Google Scholar
12 : Habituation of rostral anterior cingulate cortex to repeated emotionally salient pictures. Neuropsychopharmacology 2003; 28:1344–1350Crossref, Medline, Google Scholar
13 : Differential prefrontal cortex and amygdala habituation to repeatedly presented emotional stimuli. Neuroreport 2001; 12:379–383Crossref, Medline, Google Scholar
14 : Insula-amygdala functional connectivity is correlated with habituation to repeated negative images. Soc Cogn Affect Neurosci (in press)Google Scholar
15 : Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biol Psychiatry 2003; 54:1284–1293Crossref, Medline, Google Scholar
16 : Fronto-limbic dysfunction in response to facial emotion in borderline personality disorder: an event-related fMRI study. Psychiatry Res 2007; 155:231–243Crossref, Medline, Google Scholar
17 : Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biol Psychiatry 2001; 50:292–298Crossref, Medline, Google Scholar
18 : Neural correlates of emotion processing in borderline personality disorder. Psychiatry Res 2009; 172:192–199Crossref, Medline, Google Scholar
19 : Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol Psychiatry 2012; 72:448–456Crossref, Medline, Google Scholar
20 : Amygdala functional connectivity in young women with borderline personality disorder. Brain Connect 2011; 1:61–71Crossref, Medline, Google Scholar
21 : Alterations in default mode network connectivity during pain processing in borderline personality disorder. Arch Gen Psychiatry 2012; 69:993–1002Crossref, Medline, Google Scholar
22 : Avoidant personality disorder, in Encyclopedia of Human Behavior, 2nd ed. Edited by Ramachandran V. San Diego, Academic Press, 2012, pp 257–266Crossref, Google Scholar
23 : Insula reactivity and connectivity to anterior cingulate cortex when processing threat in generalized social anxiety disorder. Biol Psychol 2012; 89:273–276Crossref, Medline, Google Scholar
24 : The affective lability scales: development, reliability, and validity. J Clin Psychol 1989; 45:786–793Crossref, Medline, Google Scholar
25 : Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology 1993; 30:261–273Crossref, Medline, Google Scholar
26 : Emotional valence modulates activity in the posterior fusiform gyrus and inferior medial prefrontal cortex in social perception. Neuroimage 2003; 18:675–684Crossref, Medline, Google Scholar
27 Ward BD: Simultaneous inference for fMRI data. Milwaukee, Medical College of Wisconsin, Biophysics Research Institute, June 19, 2000 (http://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf)Google Scholar
28 : Psychophysiological and modulatory interactions in neuroimaging. Neuroimage 1997; 6:218–229Crossref, Medline, Google Scholar
29 : Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp (Epub ahead of print, Jun 13, 2012)Google Scholar
30 : Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex 2011; 21:1498–1506Crossref, Medline, Google Scholar
31 : The sentient self. Brain Struct Funct 2010; 214:563–577Crossref, Medline, Google Scholar
32 : Attention to aversive emotion and specific activation of the right insula and right somatosensory cortex. Neuroimage 2011; 54:2534–2538Crossref, Medline, Google Scholar
33 Wager TD, Barrett LF: From affect to control: functional specialization of the insula in motivation and regulation. Online publication at PsycExtra, 2004 (available at http://affective-science.org/pubs/2004/Wager_Edfest_submitted_copy.pdf)Google Scholar
34 : Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex 2013; 23:739–749Crossref, Medline, Google Scholar
35 : Saliency, switching, attention, and control: a network model of insula function. Brain Struct Funct 2010; 214:655–667Crossref, Medline, Google Scholar
36 : Script-driven imagery of self-injurious behavior in patients with borderline personality disorder: a pilot fMRI study. Acta Psychiatr Scand 2010; 121:41–51Crossref, Medline, Google Scholar
37 : Exaggerated affect-modulated startle during unpleasant stimuli in borderline personality disorder. Biol Psychiatry 2007; 62:250–255Crossref, Medline, Google Scholar
38 : Empathy and alexithymia in borderline personality disorder: clinical and laboratory measures. J Pers Disord 2012; 26:660–675Crossref, Medline, Google Scholar



