Fetal and Sociocultural Environments and Autism
Autism spectrum disorder (ASD) has received intense scrutiny as a genetic condition, but there is also considerable reason to believe that environmental factors interact with genetic propensities, as Abel et al. (1) propose in this issue of the Journal. Identical twins concordant for ASD often have marked differences in IQ, language skill, and patterns of severity in ASD core features such as social deficits and repetitive behaviors, as do affected fraternal twins and other siblings (2). Environmental effects can occur in utero, in the air, and in the sociocultural opportunities afforded to different children. Yet these effects are difficult to study; consistent evidence is only beginning to emerge about their role or lack of role in ASD. Consider how difficult it has been to show that the most well-known environmental hypothesis—increased ASD risk associated with vaccines—is not supported. Given that many genetic aspects of brain development are expressed in utero, the fetal environment is of obvious interest (1, 3, 4). The Abel et al. study, as part of a series of studies using the Swedish Youth Cohort, also raises questions about broader sociocultural environmental effects on prevalence.
Emerging evidence, primarily from clinical samples, has indicated a number of prenatal and perinatal factors related to ASD (3). However, the need to recruit samples from sources associated with other risks, problems with ASD screening in children with other significant disabilities, and imprecise measures of fetal growth have limited the strength of conclusions. The large population-based study of the Swedish Youth Cohort by Abel et al. offers a clearer picture of these relationships. Both preterm birth and fetal growth (1.5 to two standard deviations below the mean) were associated with increased risk for ASD, particularly with comorbid intellectual disability. ASD both with and without intellectual disability was associated with fetal growth two standard deviations above the mean. To find measurable and potentially treatable environmental risk factors for ASD in a well-controlled study is very promising. These findings offer an impetus for researchers to include measures such as preterm birth and fetal growth in studies of how genetic and environmental factors come to affect brain function and cognitive development in ASD.
The Swedish Youth Cohort studies build on near-universal access to public health services by Swedish children, 99.8% of whom receive regular developmental screenings across infancy and the preschool years. Yet the median age of ASD diagnosis in the Swedish cohort was 11 years for children without comorbid intellectual disability and 6 years for children with comorbid intellectual disability (5). Median ages of diagnoses in the United States are notably lower, ranging from 4 to 6 years, even though the consistency and quality of well-baby care and pediatric follow-up in the United States are more variable (6). With evidence showing that autism can be diagnosed reliably at 2 or 3 years and that autism-specific early intervention can make a difference in the trajectories of intellectual and adaptive development, these ages of diagnosis in Sweden and in the United States are a source of concern (7).
The research on ASD from the Swedish Youth Cohort provides data not only about associations with the fetal environment but also epidemiological associations—with broader aspects of the social environment linked to ASD in Sweden, including parental psychiatric history, household income, occupation (5, 6), and emigration status (8), which was particularly associated with ASD with intellectual disability. However, a validation study of the intellectual disability determination in the Swedish sample indicated much less consistency than in the ASD diagnoses. On the one hand, this dichotomous grouping of children into presence or absence of intellectual disability on the basis of a single assessment may underestimate relationships that might be evident with a more valid and continuous measure of intelligence. On the other hand, limitations in this designation make the interpretation of results contrasting children with ASD with and without intellectual disability more questionable (6).
For example, epidemiological studies in the United States typically find a lower rather than higher overall rate of ASD in families with fewer financial or educational resources or who are from ethnic or racial minority groups; the decrease is clearest when children with ASD without intellectual disability in families with lower socioeconomic status are compared to other children, and specifically when proportionately increased rates of ASD and intellectual disability in immigrants and minority racial and ethnic groups are compared to proportionately decreased rates in white families (9, 10). In the United States, lower prevalence in particular ethnic or socioeconomic groups has been interpreted as lack of access to services or as an indication of possible biases in health and educational diagnostic systems. Children with milder impairments in families with fewer resources are less likely to receive a timely diagnosis of ASD (6, 8). Moreover, there is marked variability in the proportions of children who receive ASD diagnoses in different U.S. clinics and states (6), suggesting regional and clinical differences as to where the boundaries of ASD are set and who is seen for evaluation (Figures 1, 2, and 3).
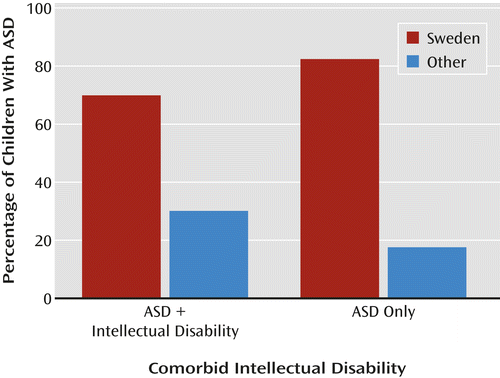
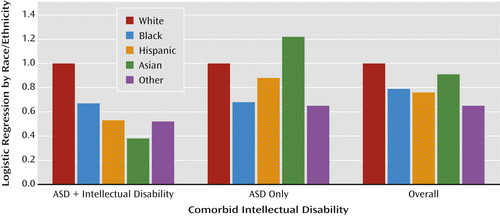
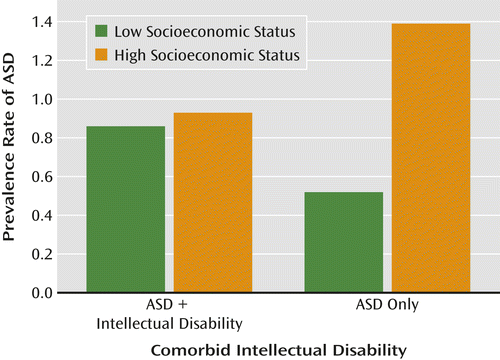
Although the results from the Swedish Youth Cohort and the U.S. studies are not directly comparable, it is striking that different perspectives are represented in interpreting the environmental factors contributing to “true” rates of ASD. In the U.S. studies, less advantaged and nonwhite families were shown to have different interactions with health services, including clinician bias for making or not making a diagnosis; these differences could occur in Sweden as well, particularly given that diagnoses are occurring so late. For example, clinicians might be biased not to make a diagnosis of intellectual disability in milder cases of ASD in families with more resources; less-advantaged families might be more likely to seek help for children with intellectual disability than for more able children (6, 7). The series of studies from both Sweden and the United States offer opportunities to address the intersection between political and public health factors related to assumptions about minorities, immigrants, and other typically underserved populations. These studies challenge us to better understand which children receive a diagnosis in both countries, when they receive it, and the degree to which intellectual disability is attributed (7).
Beyond this, the results from Abel et al. (1) have clear and important clinical implications. Children who have restricted or unusually large fetal growth and those with preterm births have significantly greater risk for ASD. ASD symptoms should be monitored and addressed in the context of neonatal follow-up clinics and well-baby care in order to decrease the age at diagnosis. Care needs to be taken that children from families of less-advantaged backgrounds receive the same attention as other children. We now have evidence of measurable fetal-environmental risks that can be considered in the search for genotype-phenotype associations, and we can move a step closer to using individualized medical and behavioral profiles to provide better and ideally earlier treatments in both Sweden and the United States (3, 5).
1 : Deviance in fetal growth and risk of autism spectrum disorder. Am J Psychiatry 2013; 170:391–398Link, Google Scholar
2 : A broader phenotype of autism: the clinical spectrum in twins. J Child Psychol Psychiatry 1996; 37:785–801Crossref, Medline, Google Scholar
3 : Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 2011; 128:344–355Crossref, Medline, Google Scholar
4 : Functional and evolutionary insights into human brain development through global transcriptome analysis. Neuron 2009; 62:494–509Crossref, Medline, Google Scholar
5 : Autism spectrum disorders in the Stockholm Youth Cohort: design, prevalence and validity. PLoS One 2012; 7:e41280Crossref, Medline, Google Scholar
6
7 : Randomized, controlled trial of an intervention for toddlers with autism: the Early Start Denver Model. Pediatrics 2010; 125:e17–e23Crossref, Medline, Google Scholar
8 : Migration and autism spectrum disorder: population-based study. Br J Psychiatry 2012; 201:109–115Crossref, Medline, Google Scholar
9 : Socioeconomic inequality in the prevalence of autism spectrum disorder: evidence from a US cross-sectional study. PLoS One 2010; 5:e1551Crossref, Google Scholar
10 : Autism spectrum disorders and developmental disabilities in children from immigrant families in the United States. Pediatrics 2012; 130(suppl 2):S191Crossref, Medline, Google Scholar



