Evaluation of the FDA Warning Against Prescribing Citalopram at Doses Exceeding 40 mg
Abstract
Objective
A recent Food and Drug Administration (FDA) warning cautioned that citalopram dosages exceeding 40 mg/day may cause abnormal heart rhythms, including torsade de pointes. The authors assessed relationships between citalopram use and ventricular arrhythmias and mortality.
Method
A cohort study was conducted using Veterans Health Administration data between 2004 and 2009 from depressed patients who received a prescription for citalopram (N=618,450) or for sertraline (N=365,898), a comparison medication with no FDA warning. Cox regression models, adjusted for demographic and clinical characteristics, were used to examine associations of antidepressant dosing with ventricular arrhythmia and cardiac, noncardiac, and all-cause mortality.
Results
Citalopram daily doses >40 mg were associated with lower risks of ventricular arrhythmia (adjusted hazard ratio=0.68, 95% CI=0.61–0.76), all-cause mortality (adjusted hazard ratio=0.94, 95% CI=0.90–0.99), and noncardiac mortality (adjusted hazard ratio=0.90, 95% CI=0.86–0.96) compared with daily doses of 1–20 mg. No increased risks of cardiac mortality were found. Citalopram daily doses of 21–40 mg were associated with lower risks of ventricular arrhythmia (adjusted hazard ratio=0.80, 95% CI=0.74–0.86) compared with dosages of 1–20 mg/day but did not have significantly different risks of any cause of mortality. The sertraline cohort revealed similar findings, except there were no significant associations between daily dose and either all-cause or noncardiac mortality.
Conclusions
This large study found no elevated risks of ventricular arrhythmia or all-cause, cardiac, or noncardiac mortality associated with citalopram dosages >40 mg/day. Higher dosages were associated with fewer adverse outcomes, and similar findings were observed for a comparison medication, sertraline, not subject to the FDA warning. These results raise questions regarding the continued merit of the FDA warning.
On August 24, 2011, the Food and Drug Administration (FDA) issued a drug safety “MedWatch” communication regarding abnormal heart rhythms associated with high dosages of citalopram hydrobromide (1). This FDA warning informed health care professionals that citalopram should no longer be prescribed at dosages above 40 mg/day because of potential abnormal changes in electrical activity of the heart. The warning indicated that the citalopram drug label had previously stated that certain patients may require dosages of up to 60 mg/day, yet studies had not shown benefits for dosages above 40 mg/day. The drug label was subsequently revised to include information regarding potential negative cardiac outcomes, specifically QT interval prolongation (also known as long QT syndrome) (2) and torsade de pointes, which can be fatal. The warning recommended avoiding higher dosages, specifically among patients with congenital long QT syndrome, congestive heart failure, bradyarrhythmias, or predisposition to hypokalemia or hypomagnesemia. Concerns about risks of citalopram use were revised and further elucidated by the FDA on March 27, 2012 (3). The updated FDA warning recommended that dosages >20 mg/day should not be used in adults over age 60.
The stated bases for the warning were twofold: 1) postmarketing reports of long QT syndrome and torsade de pointes associated with citalopram and 2) the FDA’s analysis of an unpublished study assessing the effects of 20-mg and 60-mg daily doses of citalopram on QT intervals in adults. According to the description provided in the warning, the study was a multicenter double-blind placebo-controlled crossover study with 119 subjects. Compared with placebo, corrected QT (QTc) intervals were 8.5 ms and 18.5 ms for 20 mg/day and 60 mg/day of citalopram, respectively. QTc intervals for 40 mg/day of citalopram were estimated to be 12.6 ms.
While this FDA warning provided data regarding adverse electrophysiologic changes observed in patients receiving citalopram in a single study, risks of negative patient-centered health outcomes, including ventricular arrhythmia and mortality, have not been systematically assessed to fully ascertain population risks. This is critical to do because citalopram has been well tolerated by many patients over a long time, is available in a low-cost generic formulation, and is an antidepressant of choice on many prescription formularies. All of these issues imply that the warning could have substantial implications, particularly for integrated health care delivery systems, such as the Veterans Health Administration (VHA), where citalopram is one of four selective serotonin reuptake inhibitors (SSRIs) on the formulary (4). Therefore, we sought to evaluate potential dose-dependent risks associated with citalopram use in the VHA. We identified all depressed VHA patients who received a prescription for citalopram since its generic form became available in 2004. We examined relationships between citalopram and both ventricular arrhythmia and death, and we conducted all study analyses using a comparison medication, sertraline, which was not subject to the FDA warning.
Method
Design, Data Sources, and Sample
This study employed a cohort design using data from the VHA National Registry for Depression (NARDEP), collected and maintained by the Serious Mental Illness Treatment Resource and Evaluation Center (5). NARDEP has been described in detail elsewhere (6). NARDEP provides data on medical records (inpatient and outpatient) and pharmacy data for all VHA patients with a depression diagnosis from 1997 through the present. Patients are included in NARDEP if they have any diagnosis of depression (ICD-9 codes 293.83, 296.2, 296.3, 296.90, 296.99, 298.0, 300.4, 301.12, 309.0, 309.1, and 311). We linked NARDEP data to cause of death indicators from the National Death Index using mortality data from the most recently available year (2009). The National Death Index compiles death certificates from all state vital statistics offices and is considered the gold standard of mortality ascertainment data (7). The use of data from this index in VHA studies has been described elsewhere (8). Our analyses included data from this index as well as from NARDEP from 2004 through 2009.
We restricted our analyses to the period after generic citalopram became available to exclude potential biases associated with changes in prescribing trends due to patent expiration. Study cohorts consisted of patients in NARDEP with at least one citalopram (or sertraline) prescription fill during the study period. The citalopram cohort sample size was 618,450, and the sertraline cohort sample size was 365,898.
The Serious Mental Illness Treatment Resource and Evaluation Center maintains 5% annual random samples of data for all VHA patients treated nationwide, including linked Medicare and Medicaid (Centers for Medicare and Medicaid Services) data for patients treated between 2002 and 2009. We found that 160,023 patients treated with citalopram (25.9% of the total citalopram cohort) and 96,968 patients treated with sertraline (26.5% of the total sertraline cohort) were included in at least one random sample. Sensitivity analyses that assessed patient ventricular arrhythmias treated outside the VHA system included data from NARDEP, the National Death Index, and the Centers for Medicare and Medicaid Services.
To avoid potential immortal person-time, observation time began on the date of the first citalopram (or sertraline) prescription fill that occurred after the first depression diagnosis within the study period (9). Observation time for each analysis ended on the day of each outcome (discussed below) or at the end of the study period (2009), whichever came first. Individuals remained in the study cohorts even if they had no citalopram (or sertraline) prescription fills after the first one. This study was approved by the Veterans Affairs Ann Arbor Human Studies Subcommittee.
Clinical and Mortality Outcomes
We examined negative health outcomes thought to be associated with citalopram use. We identified cases of ventricular arrhythmia (using an approach validated by Hennessy et al. [10]) that occurred after the first citalopram prescription was filled, including ICD-9 codes for paroxysmal ventricular tachycardia (427.1), ventricular fibrillation and flutter (427.4), ventricular fibrillation (427.41), ventricular flutter (427.42), and cardiac arrest (427.5). We examined all-cause, cardiac, and noncardiac mortality using data from the National Death Index, where cardiac death was indicated using ICD-10 codes I00–I99, and noncardiac death comprised all other ICD-10 codes.
Medication Prescribing
Maximum daily medication dose was measured as a time-varying covariate. We used an “as prescribed” approach, assuming that all patients take the maximum prescribed daily doses indicated on the clinician-recommended schedule (6, 11). As in our previous studies (6, 11), patients who received refills or new prescriptions for citalopram (or sertraline) at the same dosage while having medication on hand from a prior fill were assumed to have taken the medication from the previous fill as prescribed before taking medication from the second fill. However, patients taking citalopram (or sertraline) who received prescriptions for a different dosage were assumed to have begun taking the new prescription on the fill date.
Next, each patient’s total maximum daily dose for each day of the study period was calculated by adding daily doses of all fills that covered that particular day. Specific daily dose contributed by each fill was determined by dividing the total milligrams dispensed in that fill by the number of days supplied. This measurement reflects the maximum daily dose prescribed and not necessarily the actual amount consumed. Maximum daily dose of citalopram was converted into a categorical variable with values of 1–20 mg, 21–40 mg, and >40 mg. Comparable sertraline daily doses (12) were categorized as 1–50 mg, 51–100 mg, and >100 mg. Dose ranges were selected in accordance with available doses of citalopram and sertraline, as well as doses discussed in the FDA warning (citalopram dosages >40 mg/day were considered potentially risky overall, and dosages >20 mg/day were considered potentially risky for older adults) (1). Using this definition of maximum daily dose means that there could be periods in which individuals do not take any doses on a particular day (94% of participants in our study had at least one such period during the study follow-up). We did not exclude individuals with these periods; however, we report on findings that occurred during the periods of medication use.
Independent Variables
Demographic covariates included sex, race (white, black, other, or unknown), Hispanic ethnicity (yes, no, or unknown), and age. Several additional clinical factors were included based on administrative data recorded in the year prior to and including the date of the first citalopram fill during the study period. These included an indicator of overall medical comorbidity using the Elixhauser coding algorithm (Agency for Healthcare Research and Quality–Web version) (13), modified to exclude depression (i.e., ICD-9 codes 300.4, 301.12, 309.0, 309.1, and 311 were removed). Elixhauser comorbidity conditions were collapsed into a continuous comorbidity score for each patient using a previously validated point system (14). We also used an indicator of use of any medication identified with risks or possible risks of torsade de pointes (15, 16), an indicator of any hospitalization, and the number of outpatient visits in the previous year as additional signifiers of illness severity.
Data Analyses
We calculated unadjusted rates for study outcomes by daily dose. We used Cox proportional hazards models to examine relationships between prescribing and risks of all study outcomes, adjusted for demographic and clinical covariates. We also conducted multivariable competing risks analyses using Cox proportional hazards regression analysis to examine predictors of deaths from cardiac disease or other causes simultaneously, using the Lunn–McNeil approach (method B) (17). This method allowed us to estimate hazard ratios for each covariate for each cause of death separately and to test whether estimated hazard ratios for each covariate differed significantly between the two mortality outcomes (17). In adjusted models, zero-dose periods were excluded. All individuals were included in modeling when possible; 95 individuals were excluded because of missing Elixhauser coding algorithm data. Analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, N.C.). The statistical significance threshold was set at a p value <0.05.
Results
Of 618,450 individuals who received a prescription for citalopram between 2004 and 2009, 6,754 (1.1%) experienced ventricular arrhythmia. Of 64,970 (10.5% of the total) patient deaths that occurred during the study, 20,509 (3.3%) were from cardiac causes. Of 365,898 individuals who received a prescription for sertraline, 4,198 (1.1%) experienced ventricular arrhythmia. Of 45,768 (12.5% of the total) patient deaths, 14,808 (4.0%) were from cardiac causes.
Clinical and demographic characteristics of the study patients are summarized in Table 1. Similar to the overall VHA patient population, those included were primarily white (72.5%), non-Hispanic (84.0%), and male (90.4%), with a mean age of 56.9 years (SD=15.2). Patients receiving medications associated with risk of torsade de pointes comprised 36.1% of the sample.
| Characteristic | Citalopram Cohort (N=618,450) | Sertraline Cohort (N=365,898) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Age (years) | ||||
| <40 | 83,040 | 13.4 | 47,269 | 12.9 |
| 40–49 | 88,750 | 14.4 | 50,869 | 13.9 |
| 50–59 | 191,899 | 31.0 | 112,896 | 30.9 |
| 60–69 | 129,017 | 20.9 | 74,792 | 20.4 |
| 70–79 | 71,187 | 11.5 | 46,585 | 12.7 |
| ≥80 | 54,557 | 8.8 | 33,487 | 9.2 |
| Male | 559,062 | 90.4 | 329,563 | 90.1 |
| Race | ||||
| Black | 94,769 | 15.3 | 55,149 | 15.1 |
| White | 448,571 | 72.5 | 267,462 | 73.1 |
| Other | 15,764 | 2.6 | 8,742 | 2.4 |
| Unknown | 59,346 | 9.6 | 34,545 | 9.4 |
| Ethnicity | ||||
| Hispanic | 33,841 | 5.5 | 19,944 | 5.5 |
| Non-Hispanic | 519,241 | 84.0 | 304,129 | 83.1 |
| Unknown | 65,368 | 10.6 | 41,825 | 11.4 |
| Received medication with risk or possible risk of torsade de pointes | ||||
| Yes | 222,999 | 36.1 | 132,661 | 36.3 |
| No | 395,451 | 63.9 | 233,237 | 63.7 |
| Hospitalization in year prior to first fill | ||||
| Yes | 114,334 | 18.5 | 66,495 | 18.2 |
| No | 504,116 | 81.5 | 299,403 | 81.8 |
| Mean | SD | Mean | SD | |
| Modified Elixhauser comorbidity scorea | 2.0 | 5.7 | 2.0 | 5.6 |
| Number of outpatient visits in year prior to fill | 16.4 | 19.1 | 16.8 | 19.9 |
Unadjusted rates of outcomes by daily dose are presented in Table 2. Rates of each outcome decreased with higher doses; for citalopram, 18.6% of patients received dosages >40 mg/day (comprising 6.9% of total person-time). Of the total ventricular arrhythmia cases, 44.6% occurred when the prescribed dosage of citalopram was 1–40 mg/day, and 6.1% occurred at dosages >40 mg/day. Of all deaths from any cause, 29.6% occurred when the dosage was 1–40 mg/day, and 3.9% occurred at dosages >40 mg/day. Of all deaths from cardiac causes, 31.6% occurred when the dosage was 1–40 mg/day, and 4.1% occurred at dosages >40 mg/day. Of all deaths from noncardiac causes, 28.7% occurred when the dosage was 1–40 mg/day, and 3.8% occurred at dosages >40 mg/day. For sertraline, 35.9% of patients received dosages >100 mg/day; 35.8% of ventricular arrhythmia cases occurred when the dosage was 1–100 mg/day, and 14.5% occurred at dosages >100 mg/day. Of all deaths from any cause, 25.0% occurred when the dosage was 1–100 mg/day, and 8.9% occurred at dosages >100 mg/day. Of all deaths from cardiac causes, 27.2% occurred when the dosage was 1–100 mg/day, and 9.4% occurred at dosages >100 mg/day. Of all deaths from noncardiac causes, 23.9% occurred when the dosage was 1–100 mg/day, and 8.7% occurred at dosages >100 mg/day.
| Outcome and Dosage (mg) | Citalopram | Sertraline | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total N | Event N | Person-Years | Rate/100,000 Person-Years | 95% CI | Total N | Event N | Person-Years | Rate/100,000 Person-Years | 95% CI | |
| Ventricular arrhythmia | ||||||||||
| Low | 478,025 | 1,827 | 286,312.0 | 638.1 | 609.2–667.7 | 206,866 | 745 | 131,170.4 | 568.0 | 527.9–609.5 |
| Medium | 318,333 | 1,187 | 270,937.8 | 438.4 | 413.5–463.4 | 205,940 | 758 | 176,607.3 | 429.2 | 399.2–460.3 |
| High | 114,594 | 412 | 120,612.6 | 341.6 | 309.4–375.4 | 131,193 | 609 | 181,204.4 | 336.1 | 309.9–363.3 |
| All-cause mortality | ||||||||||
| Low | 478,205 | 11,559 | 287,887.2 | 4,015.1 | 3,942.3–4,088.6 | 206,993 | 5,650 | 131,840.9 | 4,285.5 | 4,174.5–4,397.9 |
| Medium | 318,922 | 7,686 | 272,517.2 | 2,820.4 | 2,757.7–2,883.8 | 206,242 | 5,774 | 177,731.4 | 3,248.7 | 3,165.5–3,333.1 |
| High | 114,965 | 2,519 | 121,439.3 | 2,074.3 | 1994.1–2,156.1 | 131,439 | 4,088 | 182,251.8 | 2,243.1 | 2,174.8–2,312.3 |
| Cardiac mortality | ||||||||||
| Low | 478,205 | 3,962 | 287,887.2 | 1,376.2 | 1,333.7–1,419.4 | 206,993 | 2,017 | 131,840.9 | 1,529.9 | 1,463.8–1,597.4 |
| Medium | 318,922 | 2,523 | 272,517.2 | 925.8 | 890.0–962.3 | 206,242 | 2,009 | 177,731.4 | 1,130.4 | 1,081.5–1,180.3 |
| High | 114,965 | 832 | 121,439.3 | 685.1 | 639.4–732.4 | 131,439 | 1,386 | 182,251.8 | 760.5 | 721.0–801.0 |
| Noncardiac mortality | ||||||||||
| Low | 478,205 | 7,597 | 287,887.2 | 2,638.9 | 2,579.9–2,698.6 | 206,993 | 3,633 | 131,840.9 | 2,755.6 | 2,666.7–2,845.9 |
| Medium | 318,922 | 5,163 | 272,517.2 | 1,894.6 | 1843.2–1,946.6 | 206,242 | 3,765 | 177,731.4 | 2,118.4 | 2,051.2–2,186.6 |
| High | 114,965 | 1,687 | 121,439.3 | 1,389.2 | 1,323.7–1,456.2 | 131,439 | 2,702 | 182,251.8 | 1,482.6 | 1,427.2–1,539.0 |
Findings from the adjusted Cox models, controlling for covariates, for each outcome variable are reported in Tables 3 and 4 and in Figure 1. Citalopram daily doses >40 mg were associated with lower risks of ventricular arrhythmia (adjusted hazard ratio=0.68, 95% confidence interval [CI]=0.61–0.76), all-cause mortality (adjusted hazard ratio=0.94, 95% CI=0.90–0.99), and noncardiac mortality (adjusted hazard ratio=0.90, 95% CI=0.86–0.96) compared with daily doses of 1–20 mg; no elevation in risks of cardiac mortality were found. Daily doses of 21–40 mg were associated with lower risks of ventricular arrhythmia (adjusted hazard ratio=0.80, 95% CI=0.74–0.86) compared with doses of 1–20 mg/day but did not have significantly different risks of any cause of mortality. The sertraline analyses resulted in similar findings, except there were no significant associations with all-cause mortality or noncardiac mortality.
| Variable | Ventricular Arrhythmias | All-Cause Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Citalopram | Sertraline | Citalopram | Sertraline | |||||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Dosageb | ||||||||
| Low | Referent | Referent | Referent | Referent | ||||
| Medium | 0.80 | 0.74–0.86 | 0.83 | 0.75–0.93 | 0.97 | 0.94–1.00 | 1.03 | 0.99–1.07 |
| High | 0.68 | 0.61–0.76 | 0.70 | 0.62–0.78 | 0.94 | 0.90–0.99 | 1.00 | 0.96–1.04 |
| Male | 1.70 | 1.40–2.05 | 1.75 | 1.37–2.24 | 1.93 | 1.78–2.10 | 2.03 | 1.83–2.26 |
| Age (years) | ||||||||
| <40 | Referent | Referent | Referent | Referent | ||||
| 40–49 | 2.54 | 1.80–3.60 | 1.27 | 0.87–1.86 | 1.82 | 1.59–2.08 | 2.09 | 1.73–2.54 |
| 50–59 | 4.04 | 2.92–5.58 | 2.33 | 1.67–3.25 | 2.41 | 2.13–2.72 | 2.83 | 2.37–3.38 |
| 60–69 | 5.31 | 3.83–7.35 | 3.22 | 2.30–4.50 | 3.29 | 2.91–3.72 | 4.33 | 3.62–5.17 |
| 70–79 | 5.52 | 3.97–7.66 | 2.99 | 2.13–4.21 | 5.99 | 5.30–6.77 | 8.22 | 6.89–9.82 |
| ≥80 | 4.59 | 3.28–6.41 | 2.75 | 1.94–3.90 | 9.96 | 8.81–11.25 | 13.57 | 11.36–16.20 |
| Race | ||||||||
| Black | 0.92 | 0.82–1.02 | 0.86 | 0.74–0.99 | 0.78 | 0.74–0.83 | 0.83 | 0.78–0.89 |
| White | Referent | Referent | Referent | Referent | ||||
| Other | 0.84 | 0.66–1.08 | 0.95 | 0.70–1.29 | 0.78 | 0.70–0.87 | 0.78 | 0.68–0.89 |
| Unknown | 0.55 | 0.45–0.67 | 0.51 | 0.39–0.67 | 0.84 | 0.80–0.89 | 0.88 | 0.83–0.93 |
| Ethnicity | ||||||||
| Hispanic | 0.66 | 0.55–0.80 | 0.69 | 0.55–0.88 | 0.86 | 0.80–0.93 | 0.93 | 0.85–1.02 |
| Non-Hispanic | Referent | Referent | Referent | Referent | ||||
| Unknown | 0.90 | 0.78–1.05 | 0.70 | 0.57–0.85 | 2.05 | 1.97–2.14 | 2.24 | 2.14–2.34 |
| Elixhauser comorbidity score | 1.09 | 1.09–1.10 | 1.09 | 1.09–1.10 | 1.08 | 1.07–1.08 | 1.08 | 1.07–1.08 |
| Received medication with risk or possible risk of torsade de pointes | 1.32 | 1.22–1.42 | 1.42 | 1.29–1.56 | 1.22 | 1.19–1.26 | 1.23 | 1.19–1.28 |
| Hospitalization | 2.16 | 1.99–2.35 | 2.10 | 1.89–2.34 | 1.55 | 1.50–1.61 | 1.53 | 1.46–1.60 |
| Number of outpatient visits | 1.00 | 1.00–1.01 | 1.00 | 1.00–1.01 | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 |
| Variable | Cardiac Mortality | Noncardiac Mortality | ||||||
|---|---|---|---|---|---|---|---|---|
| Citalopram | Sertraline | Citalopram | Sertraline | |||||
| Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | |
| Dosageb | ||||||||
| Low | Referent | Referent | Referent | Referent | ||||
| Medium | 0.98 | 0.94–1.04 | 1.04 | 0.98–1.11 | 0.97 | 0.93–1.00 | 1.03 | 0.98–1.08 |
| High† | 1.03 | 0.95–1.11 | 1.03 | 0.96–1.11 | 0.90 | 0.86–0.96 | 0.98 | 0.93–1.03 |
| Male† | 2.57 | 2.16–3.05 | 2.13 | 1.76–2.57 | 1.74 | 1.58–1.92 | 1.99 | 1.75–2.25 |
| Age (years) | ||||||||
| <40 | Referent | Referent | Referent | Referent | ||||
| 40–49†‡ | 5.73 | 3.67–8.96 | 4.04 | 2.44–6.69 | 1.50 | 1.31–1.73 | 1.80 | 1.46–2.21 |
| 50–59†‡ | 9.53 | 6.19–14.67 | 6.86 | 4.24–11.10 | 1.83 | 1.61–2.08 | 2.21 | 1.83–2.68 |
| 60–69†‡ | 13.66 | 8.87–21.04 | 10.69 | 6.61–17.30 | 2.45 | 2.15–2.78 | 3.36 | 2.77–4.07 |
| 70–79†‡ | 28.60 | 18.58–44.03 | 23.06 | 14.27–37.25 | 4.16 | 3.66–4.73 | 5.98 | 4.94–7.24 |
| ≥80†‡ | 54.63 | 35.50–84.05 | 41.81 | 25.88–67.54 | 6.38 | 5.62–7.26 | 9.33 | 7.71–11.3 |
| Race | ||||||||
| Black†‡ | 0.91 | 0.83–0.99 | 0.97 | 0.87–1.08 | 0.73 | 0.68–0.78 | 0.77 | 0.70–0.83 |
| White | Referent | Referent | Referent | Referent | ||||
| Other | 0.89 | 0.74–1.07 | 0.83 | 0.66–1.04 | 0.73 | 0.64–0.84 | 0.75 | 0.64–0.89 |
| Unknown | 0.79 | 0.73–0.86 | 0.89 | 0.81–0.98 | 0.87 | 0.82–0.92 | 0.87 | 0.81–0.94 |
| Ethnicity | ||||||||
| Hispanic‡ | 0.83 | 0.73–0.95 | 0.80 | 0.68–0.95 | 0.88 | 0.80–0.96 | 1.00 | 0.90–1.11 |
| Non-Hispanic | Referent | Referent | Referent | Referent | ||||
| Unknown | 2.12 | 1.98–2.26 | 2.27 | 2.11–2.45 | 2.02 | 1.92–2.12 | 2.22 | 2.10–2.34 |
| Elixhauser comorbidity score† | 1.07 | 1.07–1.07 | 1.07 | 1.07–1.08 | 1.08 | 1.08–1.08 | 1.08 | 1.08–1.08 |
| Received medication with risk or possible risk of torsade de pointes | 1.19 | 1.13–1.25 | 1.18 | 1.12–1.26 | 1.24 | 1.20–1.29 | 1.26 | 1.21–1.32 |
| Hospitalization | 1.50 | 1.42–1.60 | 1.52 | 1.41–1.64 | 1.58 | 1.52–1.65 | 1.53 | 1.45–1.62 |
| Number of outpatient visits | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 | 1.00 | 1.00–1.00 |
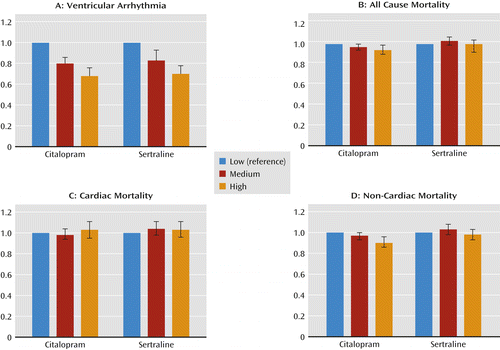
a Low dosages for citalopram and sertraline were 1–20 mg/day and 1–50 mg/day, respectively; medium dosages were 21–40 mg/day and 51–100 mg/day, respectively; and high dosages were >40 mg/day and >100 mg/day, respectively.
Sensitivity Analyses
To address the potential concern that VHA patients experiencing ventricular arrhythmias were treated outside the VHA system, we examined ventricular arrhythmia outcomes for patients with non-VHA utilization (i.e., the random samples from data from the Centers for Medicare and Medicaid Services). In these samples of approximately one-quarter of patients from both the citalopram and sertraline cohorts, we found results similar to those in our primary analyses: higher daily doses of citalopram and sertraline were associated with lower risks of ventricular arrhythmia (>40 mg/day of citalopram, hazard ratio=0.77, 95% CI=0.67–0.89; >100 mg/day of sertraline, hazard ratio=0.68, 95% CI=0.59–0.78).
Discussion
This study provides evidence on outcomes associated with citalopram (and sertraline) use in the largest samples, to our knowledge, ever analyzed. Unlike previous studies, we evaluated long-term risks and outcomes by daily dose across a large patient population, controlling for sociodemographic characteristics and clinical features implicated in negative events associated with citalopram use. We also used a comparison medication not subject to the FDA warning. Time-varying measurement that captured changes in dosing as they occurred indicated no elevation in risks of ventricular arrhythmia, nor of all-cause, cardiac, and noncardiac mortality, from higher dosages of either medication. Higher dosages of citalopram and sertraline were associated with significantly lower risks of ventricular arrhythmia. Furthermore, citalopram dosages >40 mg/day were associated with lower risks of all-cause and noncardiac mortality, yet there were no significantly lower risks in the sertraline cohort. These findings raise questions regarding the continued merit of the FDA warning and provide support for the question of whether the warning itself will cause more harm than good (18).
These findings add to another recent pharmacoepidemiology study that did not find evidence that citalopram is associated with elevations in the risks of QTc prolongation and torsade de pointes (19). The link between citalopram and torsade de pointes is primarily derived from case reports (20). Citalopram was the third most common suspected drug (9% of cases) out of 27 different medications in an assessment of simultaneous torsade de pointes cases between 1991 and 2006 in Sweden (21). Case reports of overdose have described adverse cardiac outcomes associated with citalopram and torsade de pointes in an elderly man with multiple medical problems (22, 23). Conversely, several existing national and international studies found essentially benign outcomes associated with citalopram use. A meta-analysis published in 1999 examining 40 prospective and retrospective studies conducted between 1978 and 1996 (including almost 2,000 patients and more than 6,000 ECGs) found that there were no significant effects of citalopram on cardiac conduction during short- or long-term treatment among adults (including older adults) (24). Another study that reviewed 10 years of data from 30 randomized clinical trials in European and American patients found that citalopram was well tolerated in the therapeutic dosage range (20–60 mg/day) (25).
Citalopram was approved by the FDA in 1998 (26), and its patent expired in 2004. By 1999, it had been used by more than 8 million people in 60 countries (27). However, previous experience with other medications indicates that awareness of serious adverse events may come only after many years of use, with a probability of 20% that a drug will acquire a “black box” warning or be withdrawn from the market within 20 years (28). Furthermore, prolonged QT intervals have been the single most common cause of a drug being withdrawn or restricted in the past decade (29). The warning regarding citalopram dosing arose after many years of use by a large number of patients without widespread reports of serious adverse events. Yet postmarketing FDA warnings in general, and for this specific adverse outcome, are neither new nor rare.
FDA warnings have historically changed provider and patient behavior, with intended and unintended effects (30). After the 2003 FDA advisory regarding potential suicidality among children receiving SSRIs for depression, rates of antidepressant use decreased among both children and adults, and there were no compensatory increases in psychotherapy or use of other psychotropic medications (30). It is likely that some inappropriate use of these medications decreased, but it is also likely that a substantial portion of appropriate use decreased as well. The FDA warning has already begun to limit citalopram prescribing practices (18), even though some high-risk patients may benefit from higher dosages of citalopram (31).
Several investigators have questioned the thoroughness of the “thorough QTc study” cited in the FDA warning (20, 31) and the warning more broadly (18). There are limited data available to assess risks of these outcomes among patients receiving approved citalopram dosages (as opposed to overdoses) (32, 33). There are questions regarding the clinical significance of the findings in the unpublished FDA data (31). The FDA recently responded to criticism of the citalopram warnings in a letter to the editor (34). The FDA maintained that the warning was based on worrisome increases in QTc intervals and that there was no evidence of patients responding better to dosages of 40 mg/day than to doses of 60 mg/day. Yet the FDA acknowledged that the relationship between the magnitude of QT prolongation and torsade de pointes is unknown and that there are no data available demonstrating the benefit or harm of dosages of 40 mg/day compared with 60 mg/day (34).
Our results, taken together with the FDA warning, raise a number of questions regarding the prescribing of citalopram. Should patients treated with more than 40 mg/day of citalopram have their dosage reduced? Should dosage be modified for those with the risk factors outlined in the warning? Should health care providers alter how they prescribe citalopram for new users? Should providers order ECGs for patients thought to be at potential risk of negative cardiac outcomes before writing new prescriptions or for those currently taking citalopram at high dosages, or any dosage? Should patients be switched to other antidepressants that may have similar risk profiles but are not subject to specific FDA warnings? Additional research and other data sources may be needed to examine and validate potential mechanisms of the link between citalopram and cardiac mortality to provide further guidance to clinicians.
While we conducted the largest study to date regarding the relationship between citalopram dosing and negative health outcomes, there were factors that we did not examine. Although we could not determine whether more patients with treatment-resistant depression received higher dosages, we found that both older patients and patients with higher comorbidity levels received lower dosages of both citalopram and sertraline. This may mean that there could have been residual confounding by comorbidity; patients with more physical health problems were less likely to receive high dosages but were more likely to die, and the degree of physical health problems may not have been fully captured by the selected control variables. This could potentially explain the appearance of a protective mortality effect of higher dosages. However, the results indicate that even if this were the case and there was a relationship between citalopram use and adverse cardiac outcomes that could not be detected with these data, patient selection and prescribing practices prior to the FDA warning appear to have offset this issue. Furthermore, while physicians may have been cautious before the FDA warning was issued, they also prescribed lower dosages of sertraline for older patients and patients with more comorbidities.
We did not examine patients without a depression diagnosis who received prescriptions of citalopram for off-label purposes, that is, for indications other than depression (e.g., to relieve pain from diabetic neuropathy, for headache syndromes, or for premenstrual dysphoric disorder) (35). We did not measure guideline-concordant antidepressant treatment, degree of adverse event monitoring, or use of other psychiatric or medical services that may moderate the relationship between antidepressant use and outcomes (36). We examined medication use as prescribed, but as with all pharmacoepidemiology studies, we are unable to verify actual ingestion of medication. We focused on daily dose prescribed (the focus of the warning) rather than cumulative exposure; long-term use of higher dosages may potentiate higher risks. Cardiac outcomes may be misclassified, and administrative data may underreport actual events. National Death Index data may misreport causes of death, although it is the gold standard for documenting mortality (7). While we found lower risks of noncardiac and all-cause mortality associated with higher dosages of citalopram (and not sertraline), we cannot determine whether curtailing the use of citalopram would increase the risk of mortality. Finally, while we studied a patient population that may not be generalizable to other adult populations of depressed patients, it is uncertain how the effect of medication dosage would vary based on demographic characteristics or site of care. In fact, a strength of VHA data is that the patient population largely comprises men and older adults with higher risks of negative cardiac outcomes (37). Although it is unclear how these limitations might influence our findings, the fact that our results were so similar for both the citalopram and sertraline cohorts bolsters our confidence that these findings were not unique to patients receiving citalopram. However, we do not make any claims regarding citalopram’s safety; all medications have some risks, and we did not include a nondrug comparison group. Rather, we have documented the lack of negative outcomes associated with daily doses >40 mg, or even >20 mg (which was the focus of the March 2012 FDA warning regarding adults over age 60), compared with lower doses.
Conclusions
This study did not find elevations in risks of negative patient-centered health outcomes associated with citalopram dosages >40 mg/day. Currently, clinicians and patients who perceive benefits from high-dosage citalopram must make a trade-off between adhering to the FDA warning and possible worsening depression if patients receive too low a dosage (31). Given the strength of the methods used in our study and the low likelihood of a definitive controlled trial, we question the continued merit of the warning.
1 FDA: FDA Drug Safety Communication: Abnormal Heart Rhythms Associated with High Doses of Celexa (citalopram hydrobromide). Silver Spring, Md, US Food and Drug Administration, 2012. http://www.fda.gov/Drugs/DrugSafety/ucm269086.htmGoogle Scholar
2 : Long QT Syndrome. JAMA 2003; 289:2041–2044Crossref, Medline, Google Scholar
3 US Department of Health and Human Services: FDA Drug Safety Communication: Revised Recommendations for Celexa (citalopram hydrobromide) Related to a Potential Risk of Abnormal Heart Rhythms with High Doses. Silver Spring, Md, US Food and Drug Administration, 2012. http://www.fda.gov/Drugs/DrugSafety/ucm297391.htmGoogle Scholar
4 Department of Veterans Affairs Pharmacy Benefits Management: Recommendation for Initial Choice of Selective Serotonin Reuptake Inhibitor (SSRI) in Treatment Naïve Veterans with Major Depression in the Primary Care Setting. Washington, DC, US Department of Veterans Affairs, 2005Google Scholar
5 : Specialty Care for Veterans with Depression in the VHA 2009 National Registry for Depression (NARDEP) Report. Ann Arbor, Mich, VA National Serious Mental Illness Treatment Resource and Evaluation Center, Veterans Health Administration Health Services Research and Development, 2011Google Scholar
6 : Benzodiazepine use among depressed patients treated in mental health settings. Am J Psychiatry 2004; 161:654–661Link, Google Scholar
7 : A primer and comparative review of major US mortality databases. Ann Epidemiol 2002; 12:462–468Crossref, Medline, Google Scholar
8 : Suicide mortality among patients receiving care in the veterans health administration health system. Am J Epidemiol 2009; 169:1033–1038Crossref, Medline, Google Scholar
9 : Immortal time bias in pharmaco-epidemiology. Am J Epidemiol 2008; 167:492–499Crossref, Medline, Google Scholar
10 : Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol Drug Saf 2010; 19:555–562Crossref, Medline, Google Scholar
11 : Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA 2011; 305:1315–1321Crossref, Medline, Google Scholar
12 : Costs of antidepressant medications associated with inadequate treatment. Am J Manag Care 2004; 10:357–365Medline, Google Scholar
13 : Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005; 43:1130–1139Crossref, Medline, Google Scholar
14 : A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care 2009; 47:626–633Crossref, Medline, Google Scholar
15 Arizona CERT: Drugs with possible risk of torsades de Pointes. http://www.azcert.org/medical-pros/drug-lists/list-02.cfm?sort=Brand_nameGoogle Scholar
16 Arizona CERT: Drugs with risk of torsades de Pointes. http://www.azcert.org/medical-pros/drug-lists/list-01.cfm?sort=Brand_nameGoogle Scholar
17 : Applying Cox regression to competing risks. Biometrics 1995; 51:524–532Crossref, Medline, Google Scholar
18 : Reconciling the risk of QT interval prolongation in antidepressants. Pharmacoepidemiol Drug Saf 2012; 21:329–330, author reply 331–332Crossref, Medline, Google Scholar
19 : Antidepressants and the risk of sudden cardiac death and ventricular arrhythmia. Pharmacoepidemiol Drug Saf 2011; 20:903–913Medline, Google Scholar
20 : Citalopram, QTc interval prolongation, and torsade de pointes: how should we apply the recent FDA ruling? Am J Med 2012; 125:859–868Google Scholar
21 : Drug-induced torsades de pointes: a review of the Swedish pharmacovigilance database. Pharmacoepidemiol Drug Saf 2008; 17:587–592Crossref, Medline, Google Scholar
22 : QTc interval prolongation associated with citalopram overdose: a case report and literature review. Clin Neuropharmacol 2001; 24:158–162Crossref, Medline, Google Scholar
23 : Clinical toxicology of citalopram after acute intoxication with the sole drug or in combination with other drugs: overview of 26 cases. Ther Drug Monit 2008; 30:365–371Crossref, Medline, Google Scholar
24 : Cardiac safety of citalopram: prospective trials and retrospective analyses. J Clin Psychopharmacol 1999; 19:407–415Crossref, Medline, Google Scholar
25 : Citalopram therapy for depression: a review of 10 years of European experience and data from U.S. clinical trials. J Clin Psychiatry 2000; 61:896–908Crossref, Medline, Google Scholar
26 Center for Drug Evaluation and Research. Celexa approval package. Silver Spring, Md, US Food and Drug Administration, 1998Google Scholar
27 : Citalopram in the treatment of depression and other potential uses in psychiatry. Pharmacotherapy 1999; 19:675–689Crossref, Medline, Google Scholar
28 : Timing of new black box warnings and withdrawals for prescription medications. JAMA 2002; 287:2215–2220Crossref, Medline, Google Scholar
29 : Drug-induced prolongation of the QT interval. N Engl J Med 2004; 350:1013–1022Crossref, Medline, Google Scholar
30 : Spillover effects on treatment of adult depression in primary care after FDA advisory on risk of pediatric suicidality with SSRIs. Am J Psychiatry 2007; 164:1198–1205Link, Google Scholar
31 . FDA drug safety communications: a narrative review and clinical considerations for older adults. Am J Geriatr Pharmacother 2012; 10:264–271Google Scholar
32 : A critical evaluation of the cardiac toxicity of citalopram: part 2. J Psychosoc Nurs Ment Health Serv 2011; 49:13–16Google Scholar
33 : A critical evaluation of the cardiac toxicity of citalopram: part 1. J Psychosoc Nurs Ment Health Serv 2011; 49:13–16Google Scholar
34 : Removal from labeling of 60-mg citalopram dose. Pharmacoepidemiol Drug Saf 2012; 21:784–786Crossref, Medline, Google Scholar
35 : Beyond depression: Evaluation of newer indications and off-label uses for SSRIs. Formulary 2002; 37:312Google Scholar
36 : Does consideration of Medicare use affect VA evaluations of treatment for new episodes of depression? Adm Policy Ment Health Ment Health Serv Res 2008; 35:468–476Crossref, Medline, Google Scholar
37 : Lifetime risk of developing coronary heart disease. Lancet 1999; 353:89–92Crossref, Medline, Google Scholar



