Comorbidities and Mortality in Persons With Schizophrenia: A Swedish National Cohort Study
Abstract
Objective
Schizophrenia is associated with premature mortality, but the specific causes and pathways are unclear. The authors used outpatient and inpatient data for a national population to examine the association between schizophrenia and mortality and comorbidities.
Method
This was a national cohort study of 6,097,834 Swedish adults, including 8,277 with schizophrenia, followed for 7 years (2003–2009) for mortality and comorbidities diagnosed in any outpatient or inpatient setting nationwide.
Results
On average, men with schizophrenia died 15 years earlier, and women 12 years earlier, than the rest of the population, and this was not accounted for by unnatural deaths. The leading causes were ischemic heart disease and cancer. Despite having twice as many health care system contacts, schizophrenia patients had no increased risk of nonfatal ischemic heart disease or cancer diagnoses, but they had an elevated mortality from ischemic heart disease (adjusted hazard ratio for women, 3.33 [95% CI=2.73–4.05]; for men, 2.20 [95% CI=1.83–2.65]) and cancer (adjusted hazard ratio for women, 1.71 [95% CI=1.38–2.10; for men, 1.44 [95% CI=1.15–1.80]). Among all people who died from ischemic heart disease or cancer, schizophrenia patients were less likely than others to have been diagnosed previously with these conditions (for ischemic heart disease, 26.3% compared with 43.7%; for cancer, 73.9% compared with 82.3%). The association between schizophrenia and mortality was stronger among women and the employed. Lack of antipsychotic treatment was also associated with elevated mortality.
Conclusions
Schizophrenia patients had markedly premature mortality, and the leading causes were ischemic heart disease and cancer, which appeared to be underdiagnosed. Preventive interventions should prioritize primary health care tailored to this population, including more effective risk modification and screening for cardiovascular disease and cancer.
Schizophrenia affects ∼1% of the world’s population and is a highly debilitating disease (1). It has been consistently associated with a life expectancy 10–25 years lower than that of the general population (2). Efforts to reduce this disparity have been largely ineffectual, partly because the underlying etiologies and pathways remain unclear (3–6). Research from the past 20 years has shown that people with schizophrenia have an elevated mortality from cardiovascular disease and suicide (6–9). However, the relative importance of these risks, the effect of other comorbidities, and the underlying pathways remain unknown. Previous studies have had important limitations, including an overreliance on hospital or secondary care data, community-based samples, or insufficient sample sizes. No study has comprehensively examined the somatic health effects of schizophrenia using complete outpatient as well as inpatient data for a national population. The use of outpatient diagnoses would allow the inclusion of cases of schizophrenia and comorbidities less severe than those included in studies that are limited to hospitalized patients. This is important for avoiding the potential bias that may result from the sole use of hospital-based data, enabling more reliable risk estimates for comorbidities among schizophrenia patients and permitting examination of underdiagnosis of these comorbidities. Such information would advance our understanding of the causes of premature mortality among schizophrenia patients and help facilitate more effective strategies for improving the health of this vulnerable population.
We conducted a national cohort study of some 6 million Swedish adults to examine 1) the association between schizophrenia and somatic comorbidities, 2) the association between schizophrenia and all-cause and cause-specific mortality, and 3) the association between specific antipsychotic medications and mortality. Schizophrenia, comorbidities, mortality, and antipsychotics were ascertained using registry data obtained from all outpatient and inpatient health care settings nationwide.
Method
Schizophrenia and Antipsychotic Ascertainment
The study population consisted of all persons age 25 or older who had lived in Sweden for at least 2 years as of January 1, 2003 (N=6,097,834). This age limit was chosen because most young adults in Sweden are working by this age and no longer living with their parents, and hence their socioeconomic data are more complete and accurate.
The primary predictor was schizophrenia (ICD-10 code F20), which was identified by any outpatient or inpatient diagnosis during the preceding 2 years (January 1, 2001, through December 31, 2002) using the Swedish Outpatient Registry and the Swedish Hospital Registry (10). The Swedish Outpatient Registry contains all primary and secondary outpatient diagnoses nationwide starting in 2001, and the Swedish Hospital Registry contains all primary and secondary hospital discharge diagnoses with nationwide coverage since 1987. These registries are estimated to be >99% complete (11, 12). This study was approved by the Ethics Committee of Lund University. Informed consent was waived as a requirement by the Ethics Committee.
A secondary predictor of interest was specific antipsychotic medications, which were identified from all prescriptions nationwide using the Swedish Pharmacy Registry from its inception in July 1, 2005, through December 31, 2009. This registry contains records of all prescriptions dispensed by all outpatient and inpatient pharmacies in Sweden. Medications are classified according to the Anatomical Therapeutic Chemical system (13, 14). Information was not available for dosages. We examined both “any use” (defined as any prescription during the follow-up period) and “sole use” (defined as any prescription and no other antipsychotics prescribed during the follow-up period). Sole use of perphenazine was used as the reference category because it is commonly prescribed in Sweden and to facilitate comparisons with previous research (15).
Comorbidity and Mortality Ascertainment
The study population was followed for comorbidities and mortality for 7 years, from January 1, 2003, through December 31, 2009. Study outcomes included the following specific comorbidities, identified by any primary or secondary diagnosis in the Swedish Outpatient Registry or the Swedish Hospital Registry and classified according to ICD-10 codes: hypertension (I10), ischemic heart disease (I20–I25), stroke (I60–I66), cancer (C00–C97), diabetes mellitus (E10–E14), lipid disorders (E78), influenza or pneumonia (J09–J18), chronic obstructive pulmonary disease (COPD) (J41–J44), and liver disease (K70–K77).
We also examined all-cause mortality during the same follow-up period (7 years) and cause-specific mortality for the first 5 years (January 1, 2003, through December 31, 2007; this was the latest that cause-specific data were available at the time of the analysis). Deaths were identified using the Swedish Death Registry, which contains a record of all deaths in Sweden, with compulsory reporting nationwide. Cause of death was based on the primary cause and classified according to ICD-10 codes.
Adjustment Variables
Sociodemographic characteristics that may be associated with schizophrenia and mortality were identified using national census data for 2000–2001 and were linked to the registry data using an anonymous personal identification number (16). The following were used as adjustment variables: age (modeled as a continuous variable by birthdate), marital status (married or cohabiting, never married, divorced or widowed, or unknown), education level (compulsory high school or less [≤9 years], practical high school or some theoretical high school [10–11 years], theoretical high school and/or college [≥12 years], or unknown), employment status (employed or nonemployed; “nonemployed” includes students and homemakers), and income (categorical variable in quartiles, or unknown). In a separate model, we examined the potential mediating effect of substance use disorders by further adjusting for any outpatient or inpatient diagnosis of alcohol use disorder (ICD-10 code F10) or other substance use disorders (F11–F19) during the study period (entered separately as categorical variables).
Statistical Analysis
The Kruskal-Wallis nonparametric test was used to assess for differences in the number of health care system contacts between schizophrenia patients and the rest of the population and for sociodemographic differences between schizophrenia patients who were diagnosed with comorbidities and those who were not. Cox proportional hazards regression was used to estimate hazard ratios and 95% confidence intervals (CIs) for the association between schizophrenia and specific comorbidities, all-cause mortality, and cause-specific mortality. The hazard ratio in this context is an estimate of the risk of the outcome among schizophrenia patients relative to the rest of the population. These analyses were stratified by sex because of differences in risk estimates. In the comorbidity analyses, individuals were followed up to the earliest outpatient or inpatient diagnosis of the comorbidity. In all analyses, individuals were censored at the time of emigration (N=85,631; 1.4%), as determined by the absence of a Swedish residential address in census data. We used three different adjusted models. The first was adjusted for age, the second for age and other sociodemographic variables (marital status, education, employment status, and income), and the third for age, the same additional sociodemographic variables, and alcohol or other substance use disorders. In addition, Cox proportional hazards regression was used to estimate hazard ratios and 95% CIs for the association between specific antipsychotic medications and mortality among persons with any outpatient or inpatient diagnosis of schizophrenia between 2001 and 2009 (N=23,971). The proportional hazards assumption was evaluated by graphical assessment of log-log plots (17) and was met in each of the models. We also assessed for first-order interactions between schizophrenia and sociodemographic variables or substance use disorders with respect to all-cause mortality by repeating the main analyses after stratifying by these variables and using likelihood ratio tests to formally test for interaction. All statistical tests were two-sided and used an alpha level of 0.05. All analyses were conducted using Stata, version 11.0 (18).
Individual data on tobacco smoking were unavailable for this cohort. We assessed the sensitivity of the results to the potential mediating effect of smoking using previously reported population prevalences of smoking. In this sensitivity analysis, we calculated standardized mortality ratios and 95% CIs for smoking-related cause-specific mortality (ischemic heart disease, stroke, lung cancer, influenza/pneumonia, and COPD) as the ratio of observed to expected numbers of cases, assuming a Poisson distribution. The expected numbers of cases were calculated on the basis of mortality rates for the general Swedish population during this study period, standardized by age and sex, using the following formula to adjust for smoking (19, 20): where a and b, respectively, denote the prevalence of smoking and nonsmoking among persons with schizophrenia; c and d, respectively, denote the corresponding prevalences in the general population; and RR denotes the relative risk of the respective outcome in smokers compared with nonsmokers. We used 70% as the estimated smoking prevalence among persons with schizophrenia (21) and 25% as the estimated smoking prevalence in the general population (22). We used a relative risk of 2 for ischemic heart disease, stroke, or influenza/pneumonia mortality and a relative risk of 15 for lung cancer or COPD mortality among smokers relative to nonsmokers (23, 24).
where a and b, respectively, denote the prevalence of smoking and nonsmoking among persons with schizophrenia; c and d, respectively, denote the corresponding prevalences in the general population; and RR denotes the relative risk of the respective outcome in smokers compared with nonsmokers. We used 70% as the estimated smoking prevalence among persons with schizophrenia (21) and 25% as the estimated smoking prevalence in the general population (22). We used a relative risk of 2 for ischemic heart disease, stroke, or influenza/pneumonia mortality and a relative risk of 15 for lung cancer or COPD mortality among smokers relative to nonsmokers (23, 24).
Results
In this population of 6,097,834 Swedish adults, 3,490 women (0.11% of all women) and 4,787 men (0.16% of all men) were diagnosed with schizophrenia in any outpatient or inpatient setting in 2001 and 2002. Most schizophrenia patients were between 35 and 55 years old, and 57.8% were male. Only 8.1% were currently married or cohabiting, and 72.1% had never been married. They were disproportionately less educated (37.5% had completed only compulsory high school or less, compared with 20.8% in the general population), and only 7.1% were currently employed (Table 1).
| Total Population (N=6,097,834) | Schizophrenia Patients (N=8,277) | |||
|---|---|---|---|---|
| Characteristic | N | % | N | % |
| Sex | ||||
| Female | 3,126,984 | 51.3 | 3,490 | 42.2 |
| Male | 2,970,850 | 48.7 | 4,787 | 57.8 |
| Age (years) | ||||
| 25–34 | 1,109,807 | 18.2 | 1,154 | 13.9 |
| 35–44 | 1,194,972 | 19.6 | 2,218 | 26.8 |
| 45–54 | 1,152,871 | 18.9 | 2,207 | 26.7 |
| 55–64 | 1,088,442 | 17.9 | 1,453 | 17.6 |
| 65–74 | 733,378 | 12.0 | 723 | 8.7 |
| ≥75 | 818,364 | 13.4 | 522 | 6.3 |
| Marital status | ||||
| Married or cohabiting | 2,968,842 | 48.7 | 672 | 8.1 |
| Never married | 1,835,392 | 30.1 | 5,966 | 72.1 |
| Divorced or widowed | 1,244,796 | 20.4 | 1,632 | 19.7 |
| Unknown | 48,804 | 0.8 | 7 | 0.1 |
| Education (years) | ||||
| ≤9 | 1,266,724 | 20.8 | 3,103 | 37.5 |
| 10–11 | 1,671,492 | 27.4 | 2,565 | 31.0 |
| ≥12 | 2,341,371 | 38.4 | 2,015 | 24.3 |
| Unknown | 818,247 | 13.4 | 594 | 7.2 |
| Employment status | ||||
| Employed | 3,660,931 | 60.0 | 589 | 7.1 |
| Not employed | 2,436,903 | 40.0 | 7,688 | 92.9 |
| Income | ||||
| Highest quartile | 1,512,614 | 24.8 | 328 | 4.0 |
| 2nd quartile | 1,512,137 | 24.8 | 999 | 12.1 |
| 3rd quartile | 1,512,588 | 24.8 | 3,850 | 46.5 |
| Lowest quartile | 1,511,701 | 24.8 | 3,093 | 37.4 |
| Unknown | 48,794 | 0.8 | 7 | 0.1 |
Comorbidities
Compared with the rest of the population, people with schizophrenia had more than twice as many outpatient clinic visits per year (mean=2.3, SD=5.5, median=1.4, compared with mean=1.1, SD=2.8, median=0.4; p<0.001 by Kruskal-Wallis test) and hospital admissions per year (mean=1.0, SD=4.5, median=0.4, compared with mean=0.3, SD=2.5, median=0; p<0.001 by Kruskal-Wallis test). The most commonly diagnosed specific chronic disease among schizophrenia patients was diabetes (12.5% of women and 11.0% of men during the 7-year follow-up period, compared with 5.1% and 6.4%, respectively, in the general population) (Table 2). After adjusting for age and other sociodemographic variables, the risk of having a diagnosis of diabetes was 2.2 times greater (95% CI=2.05–2.48) among women with schizophrenia and 1.8 times greater (95% CI=1.70–2.02) among men with schizophrenia relative to other women or men. Schizophrenia patients also had more than a twofold greater risk of diagnosis with COPD and influenza or pneumonia (Table 2).
| Total Population (N=6,097,834) | Schizophrenia (N=8,277) | Adjusted for Age | Adjusted for Age and Other Sociodemographic Variablesa | Adjusted for Age, Other Sociodemographic Variablesa, and Substance Use Disordersb | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Morbidity and Group | N | %c | N | %c | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI |
| Cardiovascular disease | ||||||||||
| Women | 764,340 | 24.4 | 764 | 21.9 | 1.07 | 1.00–1.15 | 0.95 | 0.89–1.02 | 0.90 | 0.84–0.97 |
| Men | 741,339 | 25.0 | 973 | 20.3 | 1.10 | 1.03–1.17 | 1.08 | 1.01–1.15 | 0.94 | 0.88–1.00 |
| Hypertension | ||||||||||
| Women | 373,060 | 11.9 | 260 | 7.4 | 0.78 | 0.69–0.89 | 0.74 | 0.66–0.84 | 0.71 | 0.63–0.80 |
| Men | 336,174 | 11.3 | 324 | 6.8 | 0.84 | 0.76–0.94 | 0.94 | 0.84–1.05 | 0.82 | 0.74–0.92 |
| Ischemic heart disease | ||||||||||
| Women | 188,319 | 6.0 | 183 | 5.2 | 1.20 | 1.03–1.38 | 1.08 | 0.93–1.25 | 1.04 | 0.90–1.20 |
| Men | 253,118 | 8.5 | 267 | 5.6 | 1.02 | 0.90–1.15 | 1.11 | 0.98–1.25 | 0.98 | 0.87–1.10 |
| Stroke | ||||||||||
| Women | 108,457 | 3.5 | 113 | 3.2 | 1.33 | 1.11–1.60 | 1.13 | 0.94–1.36 | 1.06 | 0.88–1.28 |
| Men | 111,779 | 3.8 | 138 | 2.9 | 1.28 | 1.08–1.51 | 1.19 | 1.01–1.41 | 1.03 | 0.87–1.22 |
| Cancer | ||||||||||
| Women | 282,434 | 9.0 | 278 | 8.0 | 1.04 | 0.92–1.17 | 1.09 | 0.97–1.23 | 1.08 | 0.96–1.21 |
| Men | 283,697 | 9.5 | 209 | 4.4 | 0.67 | 0.59–0.77 | 0.84 | 0.73–0.96 | 0.82 | 0.72–0.94 |
| Diabetes mellitus | ||||||||||
| Women | 158,144 | 5.1 | 436 | 12.5 | 3.12 | 2.84–3.42 | 2.25 | 2.05–2.48 | 2.15 | 1.96–2.36 |
| Men | 191,555 | 6.4 | 527 | 11.0 | 2.38 | 2.18–2.59 | 1.85 | 1.70–2.02 | 1.68 | 1.54–1.83 |
| Lipid disorders | ||||||||||
| Women | 80,734 | 2.6 | 66 | 1.9 | 0.87 | 0.68–1.11 | 0.86 | 0.67–1.09 | 0.79 | 0.62–1.01 |
| Men | 113,756 | 3.8 | 103 | 2.2 | 0.72 | 0.60–0.88 | 0.92 | 0.76–1.11 | 0.79 | 0.65–0.95 |
| Influenza or pneumonia | ||||||||||
| Women | 122,807 | 3.9 | 280 | 8.0 | 2.69 | 2.39–3.03 | 2.05 | 1.82–2.31 | 1.83 | 1.63–2.06 |
| Men | 132,396 | 4.5 | 394 | 8.2 | 3.00 | 2.71–3.31 | 2.10 | 1.90–2.32 | 1.68 | 1.52–1.86 |
| COPD | ||||||||||
| Women | 69,893 | 2.2 | 227 | 6.5 | 3.77 | 3.31–4.29 | 2.58 | 2.26–2.94 | 2.06 | 1.80–2.34 |
| Men | 67,824 | 2.3 | 186 | 3.9 | 2.83 | 2.45–3.26 | 2.12 | 1.84–2.45 | 1.53 | 1.32–1.76 |
| Liver disease | ||||||||||
| Women | 17,141 | 0.5 | 18 | 0.5 | 1.04 | 0.65–1.64 | 0.80 | 0.50–1.27 | 0.53 | 0.33–0.84 |
| Men | 19,802 | 0.7 | 51 | 1.1 | 1.89 | 1.44–2.49 | 1.12 | 0.85–1.48 | 0.66 | 0.50–0.87 |
| At least one of above | ||||||||||
| Women | 832,607 | 26.6 | 1,165 | 33.4 | 1.71 | 1.61–1.81 | 1.58 | 1.49–1.67 | 1.48 | 1.40–1.57 |
| Men | 819,937 | 27.6 | 1,362 | 28.5 | 1.60 | 1.52–1.69 | 1.59 | 1.50–1.67 | 1.38 | 1.31–1.46 |
In contrast, schizophrenia patients had no elevated risk of diagnoses of ischemic heart disease, hypertension, lipid disorders, cancer, or liver disease (Table 2). After adjusting for age and other sociodemographic variables, men but not women had a modestly elevated risk of stroke diagnosis (men: adjusted hazard ratio=1.19, 95% CI=1.01–1.41). Some risk estimates were lower among schizophrenia patients, including a hypertension diagnosis among women (adjusted hazard ratio=0.74, 95% CI=0.66–0.84) and a cancer diagnosis among men (adjusted hazard ratio=0.84, 95% CI=0.73–0.96). Additional adjustment for substance use disorders resulted in smaller risk estimates for influenza/pneumonia, COPD, and liver disease and had modest or negligible effects on all other outcomes (Table 2).
Mortality
In the entire study population, there were 634,276 deaths (10.4%) in 40.2 million person-years of follow-up. Crude mortality rates (per 1,000 person-years) were 32.5 and 27.7 for women and men with schizophrenia, respectively, compared with 15.7 and 15.3 for other women and men. Among women and men with schizophrenia, natural causes accounted for 90.9% and 82.3% of all deaths, respectively (compared with 96.5% and 94.1% in the general population), and suicide accounted for 3.5% and 7.6% of all deaths, respectively (compared with 0.6% and 1.6% in the general population) (Table 3).
| Deaths | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Population (N=6,097,834) | Schizophrenia (N=8,277) | Adjusted for Age | Adjusted for Age and Other Sociodemographic Variablesa | Adjusted for Age, Other Sociodemographic Variablesa, and Substance Use Disordersb | ||||||
| Cause of Death | N | %c | N | %c | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI |
| All causes | ||||||||||
| Women | 231,710 | 100.0 | 518 | 100.0 | 3.19 | 2.92–3.47 | 2.75 | 2.52–3.00 | 2.66 | 2.44–2.90 |
| Men | 214,678 | 100.0 | 603 | 100.0 | 3.35 | 3.09–3.63 | 2.44 | 2.25–2.64 | 2.21 | 2.04–2.39 |
| Natural causes | ||||||||||
| Women | 223,490 | 96.5 | 471 | 90.9 | 3.02 | 2.76–3.31 | 2.65 | 2.42–2.90 | 2.59 | 2.37–2.84 |
| Men | 201,916 | 94.1 | 496 | 82.3 | 3.00 | 2.75–3.28 | 2.36 | 2.16–2.58 | 2.20 | 2.01–2.40 |
| Cardiovascular disease | ||||||||||
| Women | 101,179 | 43.7 | 204 | 39.4 | 3.11 | 2.71–3.57 | 2.94 | 2.56–3.37 | 2.91 | 2.53–3.34 |
| Men | 91,678 | 42.7 | 216 | 35.8 | 3.03 | 2.65–3.46 | 2.42 | 2.12–2.77 | 2.32 | 2.03–2.65 |
| Ischemic heart disease | ||||||||||
| Women | 40,764 | 17.6 | 100 | 19.3 | 3.73 | 3.07–4.54 | 3.33 | 2.73–4.05 | 3.28 | 2.70–4.00 |
| Men | 47,405 | 22.1 | 113 | 18.7 | 2.96 | 2.46–3.56 | 2.20 | 1.83–2.65 | 2.11 | 1.75–2.54 |
| Stroke | ||||||||||
| Women | 17,520 | 7.6 | 25 | 4.8 | 2.12 | 1.43–3.13 | 1.91 | 1.29–2.83 | 1.89 | 1.27–2.79 |
| Men | 12,196 | 5.7 | 26 | 4.3 | 2.70 | 1.83–3.96 | 2.14 | 1.45–3.15 | 2.04 | 1.39–3.01 |
| Cancer | ||||||||||
| Women | 54,128 | 23.4 | 88 | 17.0 | 1.95 | 1.59–2.41 | 1.71 | 1.38–2.10 | 1.68 | 1.36–2.07 |
| Men | 57,707 | 26.9 | 77 | 12.8 | 1.47 | 1.18–1.84 | 1.44 | 1.15–1.80 | 1.39 | 1.11–1.74 |
| Lung cancer | ||||||||||
| Women | 7,444 | 3.2 | 18 | 3.5 | 2.70 | 1.70–4.29 | 2.24 | 1.41–3.56 | 2.05 | 1.29–3.27 |
| Men | 8,932 | 4.2 | 23 | 3.8 | 2.61 | 1.74–3.94 | 2.26 | 1.50–3.41 | 1.96 | 1.29–2.95 |
| Colon cancer | ||||||||||
| Women | 4,518 | 1.9 | 9 | 1.7 | 2.53 | 1.31–4.86 | 2.34 | 1.21–4.51 | 2.35 | 1.22–4.52 |
| Men | 4,216 | 2.0 | 7 | 1.2 | 1.84 | 0.87–3.86 | 1.84 | 0.87–3.88 | 1.87 | 0.89–3.95 |
| Breast cancer | 7,441 | 3.2 | 19 | 3.7 | 2.87 | 1.83–4.51 | 2.58 | 1.64–4.06 | 2.58 | 1.64–4.05 |
| Prostate cancer | 12,383 | 5.8 | 8 | 1.3 | 0.84 | 0.42–1.67 | 1.13 | 0.56–2.26 | 1.16 | 0.58–2.32 |
| Diabetes mellitus | ||||||||||
| Women | 4,835 | 2.1 | 21 | 4.1 | 6.21 | 4.05–9.54 | 4.20 | 2.73–6.46 | 4.08 | 2.65–6.27 |
| Men | 4,821 | 2.2 | 19 | 3.2 | 4.64 | 2.95–7.28 | 2.24 | 1.42–3.53 | 2.11 | 1.34–3.33 |
| Influenza or pneumonia | ||||||||||
| Women | 6,365 | 2.7 | 26 | 5.0 | 6.78 | 4.61–9.97 | 6.85 | 4.65–10.09 | 6.79 | 4.61–10.00 |
| Men | 5,412 | 2.5 | 28 | 4.6 | 7.73 | 5.33–11.22 | 7.00 | 4.81–10.19 | 6.62 | 4.54–9.64 |
| COPD | ||||||||||
| Women | 6,175 | 2.7 | 25 | 4.8 | 5.35 | 3.61–7.93 | 3.31 | 2.23–4.91 | 2.85 | 1.92–4.23 |
| Men | 6,343 | 3.0 | 45 | 7.5 | 9.17 | 6.84–12.30 | 6.28 | 4.66–8.46 | 5.23 | 3.87–7.07 |
| Liver disease | ||||||||||
| Women | 1,256 | 0.5 | 2 | 0.4 | 1.73 | 0.43–6.91 | 0.99 | 0.25–3.96 | 0.59 | 0.15–2.36 |
| Men | 2,286 | 1.1 | 5 | 0.8 | 1.80 | 0.75–4.32 | 0.75 | 0.31–1.82 | 0.51 | 0.21–1.23 |
| Unnatural causes | ||||||||||
| Women | 8,220 | 3.5 | 47 | 9.1 | 6.97 | 5.23–9.28 | 4.57 | 3.42–6.10 | 3.55 | 2.66–4.75 |
| Men | 12,762 | 5.9 | 107 | 17.7 | 6.98 | 5.77–8.44 | 3.12 | 2.57–3.78 | 2.12 | 1.75–2.57 |
| Suicide | ||||||||||
| Women | 1,425 | 0.6 | 18 | 3.5 | 11.92 | 7.49–18.98 | 6.02 | 3.77–9.64 | 3.82 | 2.38–6.12 |
| Men | 3,428 | 1.6 | 46 | 7.6 | 9.17 | 6.85–12.27 | 4.47 | 3.33–6.01 | 3.05 | 2.26–4.11 |
| Accidents or other | ||||||||||
| Women | 6,795 | 2.9 | 29 | 5.6 | 5.68 | 3.94–8.18 | 4.16 | 2.88–6.01 | 3.47 | 2.41–5.02 |
| Men | 9,334 | 4.3 | 61 | 10.1 | 5.92 | 4.60–7.62 | 2.57 | 1.99–3.31 | 1.75 | 1.35–2.26 |
On average, women with schizophrenia died 12.0 years earlier than other women (mean age, 70.5 years, compared with 82.5 years), and men with schizophrenia died 15.0 years earlier than other men (mean age, 62.6 years, compared with 77.6 years). This life expectancy difference was not explained by unnatural deaths. Among all persons who died from natural causes, women with schizophrenia died 10.5 years earlier than other women (mean age, 72.1 years, compared with 82.6 years), and men with schizophrenia died 13.1 years earlier than other men (mean age, 65.1 years, compared with 78.2 years). Among all people who died from ischemic heart disease, women with schizophrenia died 12.7 years earlier than other women (mean age, 72.2 years, compared with 84.9 years), and men with schizophrenia died 14.5 years earlier than other men (mean age, 64.2 years, compared with 78.7 years).
After adjusting for age and other sociodemographic variables, schizophrenia was strongly associated with elevated all-cause mortality (women: adjusted hazard ratio=2.75, 95% CI=2.52–3.00; men: adjusted hazard ratio=2.44, 95% CI=2.25–2.64). Both women and men with schizophrenia had an elevated risk of death from ischemic heart disease, stroke, diabetes, influenza/pneumonia, COPD, and cancer (Table 3). Among these causes, the largest hazard ratios were for influenza/pneumonia mortality in men and in women, COPD mortality in men, and diabetes mortality in women. However, the most common specific causes of death among schizophrenia patients were ischemic heart disease followed by cancer. Among specific cancers, both women and men with schizophrenia had more than a twofold greater risk of death from lung cancer (based on 41 deaths) relative to the rest of the population. Women with schizophrenia also had more than a twofold greater risk of death from breast cancer (based on 19 deaths) or colon cancer (based on nine deaths) (Table 3). Further adjustment for substance use disorders resulted in modest attenuation of risk estimates for COPD mortality and had negligible effects for all other natural causes (Table 3). Adjustment for other comorbidities in Table 2 also had a negligible effect on any of the risk estimates (data not shown).
Among people who died from ischemic heart disease or cancer, schizophrenia patients were less likely than other people to have been diagnosed previously with these conditions (proportion diagnosed >30 days before death: ischemic heart disease, 26.3% compared with 43.7%, p<0.001; cancer, 73.9% compared with 82.3%, p=0.005). There were no sociodemographic differences between schizophrenia patients who were previously diagnosed with these conditions and those who were not. After restricting the analysis to people who were previously diagnosed, schizophrenia was only modestly associated with ischemic heart disease mortality (adjusted hazard ratio=1.36, 95% CI=1.05–1.77) and was no longer associated with cancer mortality (adjusted hazard ratio=1.04, 95% CI=0.87–1.24).
Among unnatural causes, schizophrenia was strongly associated with an elevated mortality from both suicide and accidents, and the relative risks were higher among women (Table 3). After adjusting for age and sociodemographic variables, the risk of death from suicide was six times greater among women with schizophrenia and 4.4 times greater among men with schizophrenia relative to the rest of the population. Further adjustment for substance use disorders resulted in modest attenuation of these risk estimates, although they remained highly significant (Table 3).
Figure 1 presents hazard ratios for the association between schizophrenia and selected outcomes relative to people without schizophrenia, stratified by age at study entry in 10-year categories and by sex, and adjusted for age and other sociodemographic variables. These graphs show the gap between risk estimates for cause-specific mortality and the corresponding risk estimates for a previous diagnosis with the same condition. The gap suggests that these conditions may be substantially underdiagnosed among people with schizophrenia across most ages, especially in young adulthood.
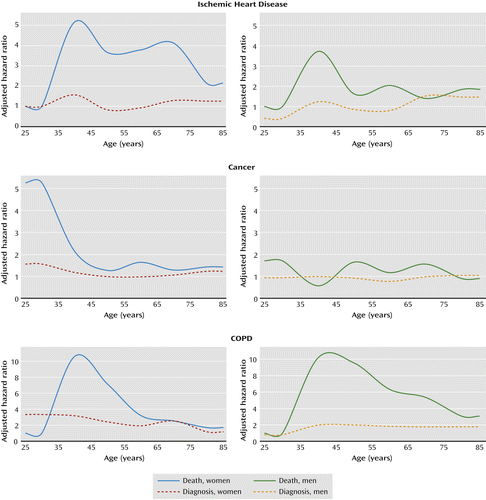
In analyses of potential interactions, the association between schizophrenia and all-cause mortality was stronger among women (pinteraction=0.005), currently employed individuals (pinteraction=0.007), and those without alcohol use disorder (pinteraction<0.001) or other substance use disorders (pinteraction<0.001). The association between schizophrenia and mortality was also slightly stronger among those age 65 years and older (pinteraction<0.001), although the stratified risk estimates did not have a consistent pattern across the full range of age categories. There were no significant interactions with marital status, education level, or income (for complete results, see Table S1 in the data supplement that accompanies the online edition of this article).
A sensitivity analysis to assess the potential mediating effect of smoking showed that adjustment for smoking, in addition to other substance use disorders, resulted in an attenuation of risk estimates of about 30% for lung cancer mortality and 10%–20% for mortality from ischemic heart disease, stroke, influenza/pneumonia, or COPD. However, all of these risk estimates remained significantly elevated, except for lung cancer mortality among women (fully adjusted standardized mortality ratio=1.54, 95% CI=0.97–2.45) (for complete results, see Table S2 in the online data supplement).
Antipsychotic Treatment
The association between specific antipsychotic medications and mortality was examined among persons who received any outpatient or inpatient diagnosis of schizophrenia between 2001 and 2009 (N=23,971), using “sole use of perphenazine” as the reference group (Table 4). After adjusting for age, other sociodemographic variables, and substance use disorders, lack of antipsychotic treatment was associated with a greater all-cause mortality (adjusted hazard ratio=1.45, 95% CI=1.20–1.76), and specifically a greater mortality from cancer (adjusted hazard ratio=1.94, 95% CI=1.13–3.32) and a nonsignificantly greater mortality from suicide (adjusted hazard ratio=2.07, 95% CI=0.73–5.87) (not shown in the table). Other statistically significant findings included a lower all-cause mortality among users of aripiprazole or olanzapine. Conflicting results were obtained for quetiapine: “Any use” was associated with a lower all-cause mortality, whereas “sole use” was associated with a greater all-cause mortality (based on 28 deaths, including nine from cardiovascular disease and five from medication overdose) and a nonsignificantly greater mortality from suicide (adjusted hazard ratio=3.45, 95% CI=0.78–15.37; based on three confirmed suicides; not shown in the table). “Other antipsychotics” that were not prescribed in sufficient numbers for separate analysis were also associated with a modestly greater mortality (adjusted hazard ratio=1.19, 95% CI=1.00–1.43).
| Antipsychotic | N | Deaths | Adjusted Hazard Ratioa | 95% CI |
|---|---|---|---|---|
| None | 1,691 | 315 | 1.45 | 1.20–1.76 |
| Perphenazine | ||||
| Sole use | 1,191 | 168 | Reference | |
| Any use | 3,773 | 426 | 0.87 | 0.71–1.06 |
| Aripiprazole | ||||
| Sole use | 196 | 5 | 0.57 | 0.23–1.41 |
| Any use | 3,268 | 108 | 0.60 | 0.46–0.79 |
| Ziprasidone | ||||
| Sole use | 137 | 6 | 0.71 | 0.31–1.61 |
| Any use | 939 | 51 | 0.89 | 0.63–1.24 |
| Olanzapine | ||||
| Sole use | 1,802 | 171 | 0.86 | 0.70–1.07 |
| Any use | 7,303 | 612 | 0.81 | 0.68–0.96 |
| Risperidone | ||||
| Sole use | 1,858 | 277 | 1.12 | 0.92–1.37 |
| Any use | 6,266 | 684 | 0.95 | 0.80–1.12 |
| Haloperidol | ||||
| Sole use | 828 | 158 | 1.13 | 0.90–1.41 |
| Any use | 3,481 | 487 | 0.97 | 0.81–1.16 |
| Clozapine | ||||
| Sole use | 1,420 | 119 | 1.29 | 0.99–1.68 |
| Any use | 3,672 | 285 | 1.20 | 0.97–1.49 |
| Quetiapine | ||||
| Sole use | 163 | 28 | 1.81 | 1.21–2.72 |
| Any use | 2,786 | 186 | 0.76 | 0.61–0.94 |
| Other antipsychotics | 2,714 | 483 | 1.19 | 1.00–1.43 |
Discussion
In this large national cohort study, schizophrenia was strongly associated with an elevated mortality that was not accounted for by unnatural deaths. The leading causes were ischemic heart disease and cancer. Despite having more than twice as many contacts with the health care system than other people, schizophrenia patients had no increased risk of having a diagnosis of nonfatal ischemic heart disease or cancer but had a far greater mortality from these conditions, suggesting substantial underdiagnosis and/or undertreatment. Schizophrenia patients also had markedly greater risks of having diagnoses of diabetes, influenza/pneumonia, and COPD, and even greater risks of death from these conditions, as well as greater mortality from stroke, suicide, and accidents.
To our knowledge, this is the first study to comprehensively examine the somatic health effects of schizophrenia using outpatient and inpatient diagnoses from all health care settings for a national population. Most previous studies have relied on hospital-based data (4, 7, 25–27), case-control data (28), or community-based samples (3). The availability of outpatient as well as inpatient diagnoses allows the inclusion of less severe cases of schizophrenia and comorbidities than studies limited to hospitalized cases, thereby avoiding potential bias and allowing the computation of more reliable risk estimates. It also enabled us to assess the extent to which important causes of mortality may be underdiagnosed in schizophrenia patients. Our findings suggest that the association between schizophrenia and ischemic heart disease or cancer mortality is largely related to underdetection rather than less effective treatment. Among people who died from ischemic heart disease or cancer, those with schizophrenia were significantly less likely than others to have been previously diagnosed with these conditions. However, among people who were previously diagnosed, those with schizophrenia had only a modestly greater mortality risk from ischemic heart disease and no increased cancer mortality risk compared with the rest of the population.
There are several potential reasons for underdetection of these conditions in schizophrenia patients. Some data have shown that schizophrenia patients are less likely to use general medical services than others with the same physical conditions (29). In addition, those who do access the health care system at a similar or more frequent rate may be less likely to receive appropriate health care than the general population (30, 31). Studies have reported that schizophrenia patients are less likely than others to receive coronary revascularization procedures (32, 33), antihypertensive or lipid-lowering medication treatment (34), standard diabetes care (35), and cancer screening (36). The large health risks we observed in schizophrenia patients highlight the need for more effective primary medical care tailored to this population and better adherence to standard clinical guidelines. Risk modification and screening for cardiovascular disease and cancer are particularly important given the elevated mortality from these conditions and the high prevalence of risk factors previously reported, including smoking, alcohol and other substance misuse, and poor nutrition and exercise (21, 37). Smoking cessation programs have been shown to be effective for schizophrenia patients (38) and need broader utilization to reduce the substantial health effects in this population.
Our overall mortality results are consistent with most previous estimates of a twofold to threefold greater mortality among persons with schizophrenia (6). Our cause-specific results are consistent with most earlier findings for cardiovascular disease (8), diabetes (25, 28), COPD (25, 28), pneumonia (39), suicide (6, 7), and accidents (7, 26) while also clarifying other important causes. Previous investigations of cancer in schizophrenia patients have been inconsistent (19, 40), with a recent meta-analysis reporting overall null results (41). Our findings suggest that people with schizophrenia have an elevated mortality from cancer, particularly lung, breast, and colon cancer, and that this is at least partly related to underdetection. Schizophrenia patients also had an elevated mortality from stroke, consistent with previous reports of an elevated risk of stroke and/or poststroke mortality (42, 43).
We found that the association between schizophrenia and all-cause mortality was stronger among women, the employed, and those without substance use disorders. Previous studies have reported inconsistent differences by sex, with a large meta-analysis reporting no overall difference (6). The stronger association we found among the employed is consistent with a previous report of higher all-cause and suicide mortality among schizophrenia patients who were currently employed relative to those on disability pension (44). Although this finding could be related to a better ability to cope with serious mental illness after retirement (44), noncausal explanations are also possible. Unemployment, as well as alcohol and other substance use disorders, are strong independent risk factors for increased mortality, and hence schizophrenia may have a smaller proportional effect on mortality among people with these factors than among those without. Several studies have reported that the association between schizophrenia and mortality weakens with age (4, 26, 45, 46), although this was not confirmed in our cohort. Additional studies with longitudinal sociodemographic and health data are needed to clarify potential modifying factors and the underlying mechanisms.
Lack of antipsychotic treatment was associated with a greater all-cause mortality in this cohort, as well as mortality from cancer and suicide. Second-generation antipsychotics have been hypothesized to increase mortality via metabolic pathways involving weight gain, diabetes, and dyslipidemia (47, 48) or by cardiac toxicity (49). The only evidence for such an effect in these data was limited to quetiapine, based on a small number of deaths. This was consistent with findings from a large study in Finland (15), although we did not confirm that study’s finding of decreased mortality with clozapine, which was associated with a nonsignificant, modestly greater mortality in our study. Other commonly used antipsychotics were not associated with an elevated mortality.
The most important strength of this study was its ability to examine the association between schizophrenia and mortality or comorbidities with more complete ascertainment than was possible in most previous studies, using outpatient and inpatient diagnoses for a national population. This enabled us to make more robust and generalizable inferences by including patients with less severe schizophrenia and comorbidities treated in outpatient settings, avoiding bias that may result from the sole use of hospital-based data. It also enabled us to assess the extent of underdiagnosis of important causes of mortality in schizophrenia patients and to provide insights into appropriate interventions.
As in most previous large studies, individual data on smoking, exercise, or other direct lifestyle measurements were unavailable. We assessed the potential mediating effects of smoking using previously reported population smoking rates, and other substance use disorders using outpatient and inpatient diagnoses, but incomplete measurement of these factors may have resulted in underestimation of their effects. Misclassification of mild schizophrenia cases is possible, but is likely to be reduced compared with most previous studies because of the inclusion of outpatient data. A Swedish registry-based diagnosis of schizophrenia is also highly accurate, with a reported positive predictive value of 94% (50). Finally, it is unclear to what extent our findings are generalizable to other health care systems. Underdetection of important causes of mortality in schizophrenia patients in Sweden, despite universal health care, raises the question of whether it may be an even larger problem in countries without universal health care.
1 : Schizophrenia. N Engl J Med 2003; 349:1738–1749Crossref, Medline, Google Scholar
2 : Life expectancy and cardiovascular mortality in persons with schizophrenia. Curr Opin Psychiatry 2012; 25:83–88Crossref, Medline, Google Scholar
3 : Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry 2010; 196:116–121Crossref, Medline, Google Scholar
4 : Mortality after hospital discharge for people with schizophrenia or bipolar disorder: retrospective study of linked English hospital episode statistics, 1999–2006. BMJ 2011; 343:d5422Crossref, Medline, Google Scholar
5 : Time trends in schizophrenia mortality in Stockholm County, Sweden: cohort study. BMJ 2000; 321:483–484Crossref, Medline, Google Scholar
6 : A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007; 64:1123–1131Crossref, Medline, Google Scholar
7 : Mortality and causes of death in older patients with schizophrenia. Int J Geriatr Psychiatry 2012; 27:1131–1137Crossref, Medline, Google Scholar
8 : Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005; 150:1115–1121Crossref, Medline, Google Scholar
9 : Excess mortality of mental disorder. Br J Psychiatry 1998; 173:11–53Crossref, Medline, Google Scholar
10 : Use of case registries in psychiatric epidemiology, in Textbook of Psychiatric Epidemiology, 3rd ed. Edited by Tsuang MTTohen MJones P. Chichester, UK, John Wiley & Sons, 2011Crossref, Google Scholar
11 : External review and validation of the Swedish national inpatient register. BMC Public Health 2011; 11:450Crossref, Medline, Google Scholar
12 : Causes of Death 2010. Stockholm, National Board of Health and Welfare, 2011Google Scholar
13 : Neighborhood deprivation and psychiatric medication prescription: a Swedish national multilevel study. Ann Epidemiol 2011; 21:231–237Crossref, Medline, Google Scholar
14 : Preterm birth and psychiatric medication prescription in young adulthood: a Swedish national cohort study. Int J Epidemiol 2010; 39:1522–1530Crossref, Medline, Google Scholar
15 : 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 2009; 374:620–627Crossref, Medline, Google Scholar
16 : Gestational age at birth and mortality in young adulthood. JAMA 2011; 306:1233–1240Crossref, Medline, Google Scholar
17 : Graphical assessment of the Cox model proportional hazards assumption. Stata Technical Bulletin 1997; 35:9–14Google Scholar
18 : Stata Statistical Software: Release 11.0. College Station, Tex, StataCorp, 2010Google Scholar
19 : Incidence of cancer in patients with schizophrenia and their first-degree relatives: a population-based study in Sweden. Schizophr Bull (Epub ahead of print, Apr 20, 2012)Google Scholar
20 : The occurrence of cancer in first admitted schizophrenic patients. Schizophr Res 1994; 12:185–194Crossref, Medline, Google Scholar
21 : A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 2005; 76:135–157Crossref, Medline, Google Scholar
22 : Cigarettes and oral snuff use in Sweden: prevalence and transitions. Addiction 2006; 101:1509–1515Crossref, Medline, Google Scholar
23 : Cancer, cigarette smoking, and premature death in Europe: a review including the Recommendations of European Cancer Experts Consensus Meeting, Helsinki, October 1996. Lung Cancer 1997; 17:1–60Crossref, Medline, Google Scholar
24 : Differences between studies in reported relative risks associated with smoking: an overview. Public Health Rep 1996; 111:420–426, discussion 427Medline, Google Scholar
25 : Chronic somatic comorbidity and excess mortality due to natural causes in persons with schizophrenia or bipolar affective disorder. PLoS ONE 2011; 6:e24597Crossref, Medline, Google Scholar
26 : Increased mortality among patients admitted with major psychiatric disorders: a register-based study comparing mortality in unipolar depressive disorder, bipolar affective disorder, schizoaffective disorder, and schizophrenia. J Clin Psychiatry 2007; 68:899–907Crossref, Medline, Google Scholar
27 : Mortality among patients with schizophrenia and reduced psychiatric hospital care. Psychol Med 2005; 35:725–732Crossref, Medline, Google Scholar
28 : Medical comorbidity in women and men with schizophrenia: a population-based controlled study. J Gen Intern Med 2006; 21:1133–1137Crossref, Medline, Google Scholar
29 : Use of general medical services by VA patients with psychiatric disorders. Psychiatr Serv 2002; 53:874–878Link, Google Scholar
30 : The schizophrenic patient in the somatic hospital. Acta Psychiatr Scand Suppl 2000; 407:96–99Crossref, Medline, Google Scholar
31 : Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry 2001; 58:565–572Crossref, Medline, Google Scholar
32 : Somatic hospital contacts, invasive cardiac procedures, and mortality from heart disease in patients with severe mental disorder. Arch Gen Psychiatry 2009; 66:713–720Crossref, Medline, Google Scholar
33 : Death rate from ischaemic heart disease in Western Australian psychiatric patients, 1980–1998. Br J Psychiatry 2003; 182:31–36Crossref, Medline, Google Scholar
34 : Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med (Epub ahead of print, Mar 12, 2012)Google Scholar
35 : Mental disorders and quality of diabetes care in the Veterans Health Administration. Am J Psychiatry 2002; 159:1584–1590Link, Google Scholar
36 : Identifying barriers and facilitating factors to improve screening mammography rates in women diagnosed with mental illness and substance use disorders. Women Health 2005; 42:111–126Crossref, Medline, Google Scholar
37 : The unhealthy lifestyle of people with schizophrenia. Psychol Med 1999; 29:697–701Crossref, Medline, Google Scholar
38 : Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev 2010; 6:CD007253Crossref, Medline, Google Scholar
39 : Poor clinical outcomes among pneumonia patients with schizophrenia. Schizophr Bull 2011; 37:1088–1094Crossref, Medline, Google Scholar
40 : Incidence of cancer among persons with schizophrenia and their relatives. Arch Gen Psychiatry 2001; 58:573–578Crossref, Medline, Google Scholar
41 : Cancer incidence in patients with schizophrenia and their first-degree relatives: a meta-analysis. Acta Psychiatr Scand 2008; 117:323–336Crossref, Medline, Google Scholar
42 : The incidence and relative risk of stroke in patients with schizophrenia: a five-year follow-up study. Schizophr Res 2012; 138:41–47Crossref, Medline, Google Scholar
43 : An increased risk of stroke among young schizophrenia patients. Schizophr Res 2008; 101:234–241Crossref, Medline, Google Scholar
44 : Five-year follow-up study of disability pension rates in first-onset schizophrenia with special focus on regional differences and mortality. Gen Hosp Psychiatry 2011; 33:509–517Crossref, Medline, Google Scholar
45 : The impact of psychiatric illness on suicide: differences by diagnosis of disorders and by sex and age of subjects. J Psychiatr Res 2011; 45:1445–1452Crossref, Medline, Google Scholar
46 : All-cause mortality among people with serious mental illness (SMI), substance use disorders, and depressive disorders in southeast London: a cohort study. BMC Psychiatry 2010; 10:77Crossref, Medline, Google Scholar
47 : Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156:1686–1696Link, Google Scholar
48 : The metabolic effects of antipsychotic medications. Can J Psychiatry 2006; 51:480–491Crossref, Medline, Google Scholar
49 : Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009; 360:225–235Crossref, Medline, Google Scholar
50 : Evaluation of diagnostic procedures in Swedish patients with schizophrenia and related psychoses. Nord J Psychiatry 2005; 59:457–464Crossref, Medline, Google Scholar



