Effects of Pharmacologically Induced Hypogonadism on Mood and Behavior in Healthy Young Women
Abstract
Objective
The relationship between depression and estrogen withdrawal remains controversial. The authors examined the effects of gonadotropin-releasing hormone agonist-induced ovarian suppression on mood, sleep, sexual function, and nighttime hot flushes. They focused on whether participating women experienced clinically significant depressive symptoms and whether specific symptoms associated with hypogonadism (nighttime hot flushes and disturbed sleep) increased susceptibility to depression.
Method
Participants were 72 healthy premenopausal women, ages 19–52 years, with no current or past axis I psychiatric diagnosis or gynecological or other medical illness. After 2 months of baseline screening, women received monthly injections of leuprolide acetate (3.75 mg) for 2–3 months. Outcomes were measured using the Beck Depression Inventory (BDI) and a daily rating scale measuring the severity of several affective and behavioral symptoms. Data were analyzed by repeated-measures analysis of variance using PROC MIXED (for mixed models).
Results
BDI scores ≥10 were reported in four of the 72 women (5.6%). Relative to baseline, induced hypogonadism was associated with significantly decreased sexual interest, disturbed sleep, and more severe nighttime hot flushes, but no significant change in any mood-related symptom score. Hot flush severity was significantly correlated with disturbed sleep.
Conclusions
These data demonstrate that clinically significant depressive symptoms were rare accompaniments of short-term estradiol withdrawal and induced hypogonadism in healthy premenopausal women. Additionally, neither nighttime hot flushes nor disturbed sleep were sufficient to cause depressive symptoms in hypogonadal women.
Community-based studies (1–6) have demonstrated an association between the menopause transition or early postmenopause and a higher risk of both first-onset and recurrent depression. These studies have reported a 1.5- to threefold greater risk of first-onset and recurrent depression in women during the menopause transition compared with those who are premenopausal. At the same time, these studies have also demonstrated that most women experience no significant symptoms of depression during the menopause transition.
A history of depression, disturbing vasomotor symptoms, and negative life events have all been suggested as potential contributors to depression risk during the menopause transition. Yet none of these factors uniformly accompanies depression or has been shown to be a necessary antecedent for its development in menopausal women. Results from a small longitudinal observational study (7) and a clinic-based cross-sectional study (8), moreover, have suggested that depressions associated with menopause tend to cluster late in the transition and in early postmenopause, which are characterized by estradiol withdrawal and the recent onset of hypogonadism (9, 10). Clinic-based observational studies (7, 8) and some treatment studies (11, 12) have implicated changes in hormonal events during the menopause transition—in particular, estradiol withdrawal—in the onset of depression. Finally, in at least five studies, which together include over 70 premenopausal women, a greater-than-expected frequency of clinically significant mood symptoms was observed when a gonadotropin-releasing hormone (GnRH) agonist was used to suppress ovarian function (13–18).
While these results suggest an association between declining or suppressed ovarian estradiol secretion and the onset of depression, the contributions of age, preexisting mood symptoms, past psychiatric history, and current medical and gynecological conditions to this risk have yet to be systematically evaluated. In addition, most of these studies involved clinical samples in which the effects of GnRH agonists on mood could not be separated from symptoms of the conditions for which the GnRH agonists were prescribed (e.g., endometriosis and other gynecological conditions). Thus, it remains to be determined whether estradiol withdrawal and recent onset of hypogonadism are sufficient to trigger mood problems in otherwise healthy premenopausal women.
To further examine the effects of estradiol withdrawal and hypogonadism on mood and behavior, we administered leuprolide acetate to healthy premenopausal volunteers with no current or past psychiatric illness and no medical or gynecological illness with the aim of answering the following questions: 1) Does the acute induction of hypogonadism cause clinically significant depressive symptoms in healthy premenopausal women? 2) Are there individual symptoms associated with hypogonadism that increase susceptibility to depression? 3) Do changes in plasma levels of ovarian hormones correlate with changes in mood symptoms?
Method
Participants
Women were recruited through local advertisements or self-referred from the healthy volunteer program of the National Institutes of Health (NIH) from November 1995 to June 2010. All healthy individuals were medication free, were not pregnant, had no significant past or current medical illness, reported regular menstrual cycles (21–35 days in length), and had normal physical and gynecological examinations and laboratory results (including blood cell counts, chemistry panels, and thyroid function). The absence of current or past psychiatric illness was confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders (19). During a 2-month screening phase, potential participants completed a 100-mm visual analogue self-report scale measuring the severity of four affective symptoms (sadness, anxiety, irritability, and mood swings) to confirm the absence of symptoms across the menstrual cycle. Women were excluded from this study if they had a past or present psychiatric illness or showed evidence of significant menstrual cycle-related or other mood and behavioral symptoms on the daily visual analogue rating form. The protocol was reviewed and approved by the intramural research board of the National Institute of Mental Health, and all women provided oral and written informed consent. All women were paid for their participation in this protocol according to the guidelines of the NIH healthy volunteer program.
All participants served as healthy, asymptomatic comparison subjects in a larger program of research involving a series of separate studies examining the effects of ovarian hormone replacement during conditions of GnRH agonist-induced hypogonadism with and without ovarian steroid replacement on measures of brain activation and stress physiology in women with and without premenstrual dysphoria. The present study, however, considers only how GnRH agonist alone affected mood and behavior in the asymptomatic comparison group.
Procedures
After a 2-month screening phase, women received the first dose of depot leuprolide acetate (TAP Pharmaceuticals, Chicago), 3.75 mg i.m., between 3 and 6 days after the onset of menses (i.e., the early follicular phase). The subsequent leuprolide injections were then administered every 4 weeks for up to 3 months. One group (N=30) received leuprolide alone for 8 weeks. To match the duration of hypogonadism treatment with that used in the companion protocol (individuals with premenstrual dysphoria), a second group (N=42) received leuprolide alone for 12 weeks.
Participants were seen at the NIH clinic every 2 weeks for 8 or 12 weeks. Blood samples were obtained and symptom self-ratings were completed at each clinic visit.
Outcome Measures
To evaluate symptoms, the following assessments were obtained at baseline and during each clinic visit: 1) self-rated score from the Beck Depression Inventory (BDI; 20), used to measure severity of depression symptoms over the 24 hours before each visit, and 2) score from the daily rating form (21), a 6-point Likert-type scale to assess the symptoms measured in this study, completed nightly to obtain a composite rating for the previous 24 hours; daily rating scores ranged from 1 (symptoms not present) to 6 (symptoms present in the extreme). The symptoms evaluated by the daily rating form included sleep disturbance, decreased sexual interest, avoidance of social activity, loss of enjoyment or interest, irritability or anger, mood swings, feeling sad or depressed, feeling anxious or nervous, increased appetite, low energy, and nighttime hot flushes. For participants who had incomplete daily ratings for the baseline phase, we used the visual analogue scale scores, converted to a scale from 1 to 6 units, to complete the baseline ratings.
Hormone Assays
Blood samples were obtained at each clinic visit, then centrifuged, aliquoted, and stored at −70°C until the time of assay. For the first 27 women who participated in this study, plasma levels of estradiol were measured by radioimmunoassay as described previously (22, 23); levels for the remaining women were assayed by chemoluminescent analyzer (Ciba Corning Diagnostics, Cambridge, Mass., and the NIH Department of Laboratory Medicine). All samples obtained for each woman were assayed at the same location.
Statistical Analysis
Individuals were considered to have clinically significant depressive symptoms if their BDI scores met or exceeded the following threshold criteria: 1) BDI scores ≥10 consistent with mild or minor depression in general medical practice (24, 25), and 2) BDI scores ≥18 consistent with moderate to severe depression (24). The number of women meeting each of the cutoff scores was expressed as a percentage. In those women whose BDI scores were elevated on one visit, we also determined whether they had similarly elevated scores at the next visit—a proxy for the DSM-IV 2-week-duration criterion. We calculated 7-day averages from daily rating symptom scores for each week during one baseline month and for each month of leuprolide treatment. Both BDI and daily rating form data involved repeated measurements with the same woman during three hormone conditions (baseline, month 1 of leuprolide treatment, month 2 of leuprolide, and month 3 of leuprolide for those women receiving 3 months of treatment) at weekly intervals. We examined the pattern of change in symptom scores across three different hormone conditions: baseline prior to leuprolide treatment, the first month of leuprolide (characterized by an acute increase or “flare” as a result of the initial stimulation of the ovarian axis followed by a suppression of ovarian function and subsequent estradiol withdrawal to hypogonadal plasma levels), and the last month of leuprolide (or last 2 months in women receiving 3 months of treatment) associated with ovarian suppression and persistent hypogonadism. Additionally, we tested for differences in symptom severity scores and plasma estradiol levels between the second and third month of leuprolide in those women who received 3 months of treatment.
Repeated-measures analyses were conducted with SAS, version 9.2 (SAS Institute, Inc., Cary, N.C.). We used multivariate repeated-measures analysis of variance (ANOVA) for mixed models (PROC MIXED). Separately, for each of the 10 symptom ratings and plasma estradiol levels (both actual and log-transformed values), the predictor variable of interest, hormone condition, was modeled as a fixed effect. The covariance patterns were structured by two effects, condition (unstructured) and week (first-order autoregressive). We used the Kenward and Roger method for computing the degrees of freedom for tests of fixed effects. Because of the wide age range of participants, age was explored as a possible confounder for each symptom rating; models explored age as both a main effect and as an interaction term (i.e., age and hormone condition). Post hoc pairwise comparisons of least square means were compared among hormone conditions using t tests with the Bonferroni adjustment. A two-sided p value <0.05 was considered statistically significant.
We also calculated weekly (7-day) averages of daily rating form scores for those symptoms that changed significantly (compared with baseline) during hypogonadism. Weekly average daily rating form scores of 2 (indicating minimal severity of a particular symptom) or greater were used to identify women who experienced at least minimal levels of distress during leuprolide treatment.
Finally, we examined the relationships between plasma estradiol levels and symptom scores in the four ratings that increased significantly during leuprolide treatment compared with baseline (BDI scores and daily rating scores for decreased sexual interest, disturbed sleep, and nighttime hot flushes). First, we estimated the correlation coefficients between the log-transformed plasma estradiol levels and the symptom scores from weeks 2–12 of leuprolide treatment (or weeks 2–8 in those women receiving only 2 months of leuprolide). Because the raw estradiol data were skewed, we used log-transformed values. In this analysis, we calculated the partial correlation coefficients between two variables (e.g., log estradiol and BDI scores) with repeated observations on each variable by assuming that the repeated observations on each variable had an autoregressive structure as described by Roy (26). The partial correlation coefficient is a summary for all women of the correlations between the log estradiol level and symptom score in each woman. The coefficient summarizes whether an increase in log estradiol level in the women is associated with an increase in symptom scores. Additionally, in the four ratings that had a significant increase during leuprolide, separate Spearman correlations were computed to assess the following about the relationship between plasma estradiol levels and the average symptom severity during the final month of leuprolide treatment (presumed to reflect the effects of the longest exposure to hypogonadism in each woman): 1) the estradiol levels measured 2 weeks after the initial injection of leuprolide (i.e., during the flare), 2) the degree of estradiol withdrawal to which each woman was exposed from the high estradiol levels to the state of hypogonadism (i.e., the difference in estradiol levels between week 2 and week 4 of leuprolide treatment), and 3) the levels of estradiol during hypogonadism (the average level of estradiol during the final 4 weeks of leuprolide treatment).
Spearman correlation coefficients were also used to examine possible relationships between the severity of symptoms (i.e., statistically significant changes during leuprolide treatment compared with baseline) and the symptoms of sadness, anxiety, and irritability.
Results
Participant Characteristics
In total, 72 women ages 19–52 (mean=33 years, SD=8) participated in the study. Eight women were older than 45. Eighteen participants were African American (25%), and three (4%) were Asian. The mean body mass index of the participants was 24.5 (SD=4.8), and six women (8.3%) were smokers. Participants’ parity ranged from zero to four children.
Symptom Ratings
Table 1 and Figures 1 and 2 detail the results of our symptom measurements.
| Outcome Measure | Leuprolide Treatment | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Month 1 | Months 2–3 | Mixed Model | Analysis | ||||
| Mean | SE | Mean | SE | Mean | SE | F Testb | p | |
| Estradiol (pg/mL) | 67.2 | 32.2 | 254.8c | 32.2 | 26.8 | 32.1 | 14.4 | <0.0001 |
| BDI score | 0.8 | 0.2 | 1.3 | 0.2 | 1.5d | 0.2 | 3.5 | 0.03 |
| Nighttime hot flushes | 1.0 | 0.1 | 1.3 | 0.1 | 2.2d,e | 0.1 | 52.3 | <0.0001 |
| Disturbed sleep | 1.1 | 0.1 | 1.2 | 0.1 | 1.6d,e | 0.1 | 20.2 | <0.0001 |
| Decrease in sexual interest | 1.1 | 0.1 | 1.2 | 0.1 | 1.5d,e | 0.1 | 12.9 | <0.0001 |
| Depression | 1.1 | 0.02 | 1.1 | 0.02 | 1.1 | 0.02 | 0.7 | n.s. |
| Anxiety | 1.1 | 0.02 | 1.1 | 0.03 | 1.1 | 0.03 | 1.2 | n.s. |
| Mood swings | 1.1 | 0.03 | 1.1 | 0.03 | 1.1 | 0.03 | 0.1 | n.s. |
| Irritability | 1.1 | 0.03 | 1.2 | 0.03 | 1.2 | 0.03 | 1.4 | n.s. |
| Fatigue | 1.3 | 0.04 | 1.3 | 0.04 | 1.3 | 0.04 | 0.6 | n.s. |
| Increase in appetite | 1.2 | 0.03 | 1.1 | 0.03 | 1.1 | 0.03 | 1.2 | n.s. |
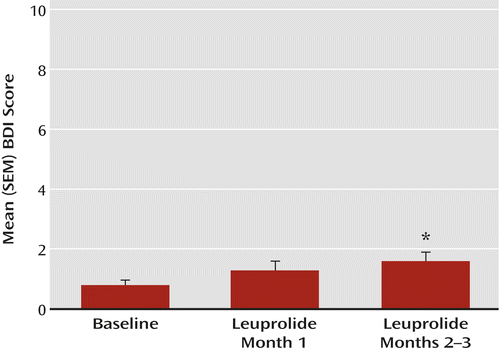
a Significant increases in BDI scores were observed during months 2–3 of leuprolide treatment compared with baseline scores (F=3.5, df=2, 154, p=0.03; Bonferroni-corrected t=2.6, df=155, p<0.03), but not when compared with month 1 of leuprolide treatment. One of the 72 women (1.3%) had a BDI score ≥18 after 6 weeks of leuprolide treatment; the symptoms did not persist for 2 consecutive weeks. Four women overall (5.6%) had BDI scores ≥10, and in only one woman did symptoms persist for 2 consecutive weeks (i.e., two visits).
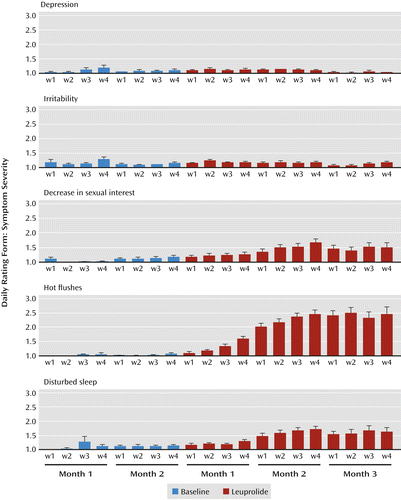
a Histograms represent the weekly 7-day means of the symptom rating scale scores for each month of baseline and leuprolide treatment (error bars indicate standard error of the mean). Higher scores reflect greater symptom severity. Leuprolide-induced ovarian suppression was accompanied by significantly decreased sexual interest (F=12.9, df=2, 155, p<0.0001), disturbed sleep (F=20.2, df=2, 162, p<0.0001) and increased nighttime hot flushes (F=52.3, df=2, 111, p<0.0001) compared with baseline. In contrast, no significant changes were observed in depression or any other mood or behavioral symptoms measured. We did not observe significant correlations between baseline plasma levels of estradiol (or the changes in plasma levels between week 2 and week 4 of leuprolide treatment [i.e., during flare]) and the severity of any symptom measured (depression, decreased sexual interest, nighttime hot flushes, and disturbed sleep).
BDI.
Only one woman (1.3%) had a BDI score ≥18 during leuprolide treatment. In total, four women (5.6%) had BDI scores ≥10 during leuprolide treatment, but symptoms persisted for more than 2 weeks in only one individual. Overall, the occurrence of BDI scores ≥10 was distributed across each of the months of leuprolide treatment, and therefore symptoms did not cluster during any specific hormonal state. During months 2–3 of leuprolide treatment, however, BDI scores were significantly higher than at baseline (least squares mean=1.5, SE=0.2, and least squares mean=0.8, SE=0.2, respectively) (main effect of hormone condition: F=3.5, df=2, 154, p<0.03; Bonferroni-corrected t=2.6, df=155, p<0.03). In contrast, no significant differences in BDI scores were observed between month 1 and months 2–3 of leuprolide treatment or between baseline and month 1. Finally, in those women who received 3 months of leuprolide treatment, no differences in BDI scores were observed between month 3 and month 2 of treatment.
Daily rating form.
Comparing measures during baseline with those taken during leuprolide-induced ovarian suppression, repeated-measures ANOVA identified significant main hormone condition effects for only three symptoms: decreased sexual interest (F=12.9, df=2, 155, p<0.0001), disturbed sleep (F=20.2, df=2, 162, p<0.0001), and nighttime hot flushes (F=52.3, df=2, 111, p<0.0001). Post hoc testing revealed that severity scores for all three symptoms rose significantly from baseline levels to the second and third months of leuprolide treatment. No differences were observed in the severity of nighttime hot flushes, disturbed sleep, and decreased sexual interest between baseline and the first month of leuprolide treatment. Finally, the severity of decreased sexual interest (but not nighttime hot flushes or disturbed sleep) was significantly greater during month 3 of leuprolide treatment compared with month 2 (Table 1). The proportion of women reporting weekly average severity scores ≥2 (minimal severity or greater) during leuprolide treatment was 75% (44/59 women) for nighttime hot flushes, 33% (24/72 women) for decreased sexual interest, and 47% (34/72 women) for disturbed sleep.
Additional analysis.
For BDI and daily symptom ratings, we explored models that included age as both a main effect and as an interaction term with hormone condition, and, if the interaction was not significant, age alone in a separate ANOVA. In all symptoms, both the main and interactive effects of age were not significant, and therefore age was not a confounder of BDI and daily rating form symptom scores.
Symptom-hormone correlations.
The partial correlation coefficients between log estradiol levels and symptom scores were as follows: −0.1 for BDI score, −0.2 for nighttime hot flushes, −0.08 for disturbed sleep, and −0.06 for decreased sexual interest. Therefore, as log estradiol levels decreased, we observed a slight increase in symptom scores. No p value was associated with these correlations; however, the magnitude of the correlations suggests only a slight association between serial plasma estradiol levels and corresponding symptom scores, which is consistent with the results of the overall study. The correlations with plasma estradiol levels should be interpreted with caution given the limitations of the estradiol assay to accurately measure plasma estradiol near the lower limits of quantitation for the assay. Spearman correlation coefficients indicated no significant relationships between BDI scores or severity of disturbed sleep, decreased sexual interest, or nighttime hot flushes and plasma levels of estradiol during the final month of leuprolide treatment, plasma levels at week 2 of leuprolide, or the change in plasma level between weeks 2 and 4 of leuprolide treatment (r values, −0.2 to 0.1).
Symptom-symptom correlations.
During the final month of leuprolide treatment, significant correlations were found between nighttime hot flushes and disturbed sleep (r=0.5, p<0.01) but not between severity scores for decreased sexual interest and disturbed sleep. Severity scores for sadness, anxiety, and irritability during leuprolide treatment were not correlated with any other symptom scores.
Discussion
It has been generally assumed that ovarian suppression and estrogen withdrawal adversely affect mood—a causal train of events that our findings in this study do not support, at least in healthy premenopausal women. Our results demonstrate that healthy premenopausal women rarely exhibit negative mood symptoms during medically induced hypogonadism or estrogen withdrawal. More importantly, these findings challenge the proposal that declining ovarian steroid secretion alone triggers the onset of depression during the menopause transition.
We induced hypogonadism in 72 healthy premenopausal women for 2–3 months. Yet, despite the definitive withdrawal of ovarian steroid secretion and the fact that most participants experienced accompanying nighttime hot flushes, disturbed sleep, and decreased libido, only four (5.6%) experienced significant alterations in mood. These findings are consistent with those of both Bloch et al. (27) and Owens et al. (28), who reported the absence of significant mood changes after GnRH agonist treatment. Against these findings, several other clinic-based studies reported that GnRH agonist-induced ovarian suppression was associated with depression that was sufficiently severe in some women to warrant antidepressant medication (13–17, 29). Depressive symptoms associated with GnRH agonist ovarian suppression could reflect the effects of ovarian suppression in women who are more vulnerable to the development of depression or who are currently depressed. Our study could not evaluate this possibility because women with past or current mood disorders were excluded.
During the menopause transition, up to 75% of women experience hot flushes and up to 60% experience insomnia; a smaller percentage experience a decrease in libido (30, 31). Our study revealed significant increases in severity of nighttime hot flushes, disturbed sleep, and decreased libido. These symptoms were reported to be of at least minimal severity and at frequencies similar to those reported by women undergoing menopause naturally (30–35). The fact that many of our participants complained of typical menopausal symptoms in the absence of depression during induced hypogonadism, moreover, would seem to rule these symptoms out, at least in the short term, as presumed triggers for the development of depressive symptoms.
Epidemiological studies have suggested that the menopause transition and early postmenopause are times of increased risk for the onset of depression in some women (1–6). However, these studies have also demonstrated that most women experience no significant symptoms of depression during the menopause transition. Our study recapitulates one component (loss of ovarian steroid secretion) but certainly not all of the multiple endocrine and somatic events that accompany aging and the normal menopause transition. Consistent with epidemiological data in perimenopausal women, our findings suggest that neither estradiol withdrawal nor menopausal symptoms are sufficient to induce depressive symptoms in healthy premenopausal women. Indeed, several studies have demonstrated the relative independence of hot flushes and mood disturbances (7, 11, 12, 36, 37). Despite limitations, our study challenges hypotheses suggesting that estrogen withdrawal, nighttime hot flushes, or disturbed sleep alone can trigger depression during the perimenopause. Nonetheless, the discrepancies between data collected from gynecological clinic-based samples and data from our study (and from Owens et al. [28]) emphasize, once again (38, 39), that the effects of ovarian steroids on the brain are not uniform across individuals.
Our study has several limitations that restrict the extension of our findings to older women transitioning through menopause and to those receiving GnRH agonists. First, we included healthy premenopausal women with no current or past medical, gynecological, or psychiatric illness. While these sample characteristics increase the internal validity of our study, they decrease the generalizability of our findings to many women in the menopause transition who, for example, could have a past history of depression. Second, the endocrine events of the menopause transition are far more complex, and in some women more prolonged, than simply suppressed ovarian steroid hormone secretion for 2–3 months. Thus, inasmuch as our sample consisted of premenopausal women, our study replicated neither the context of brain aging characteristics of most women during the natural transition to menopause nor the extent of hormonal changes at this stage of life. Third, in our study, too few women developed depression to examine whether or not depression occurs with nighttime hot flushes that are more severe. Moreover, since our study measured only nighttime hot flushes, we were limited to evaluating the impact of nighttime hot flushes on mood, and we therefore cannot speculate about the potential negative effects of daytime hot flushes on mood symptoms in our sample. Finally, a more accurate relationship between plasma estradiol levels and symptoms would have been possible had we employed more sensitive and accurate measures of estradiol using gas or liquid chromatography and mass spectroscopic techniques.
Our data suggest that normal medical, gynecological, and psychiatric health mitigates the risk of depression during hypogonadism. As other studies of reproductive-related mood disorders show, the precipitation of depression secondary to reproductive hormone manipulation requires a preexisting context of susceptibility as well as the endocrine trigger. The fact that our results stand in marked contrast to numerous case reports of clinically significant depression during GnRH agonist-induced hypogonadism suggests that other factors need to be studied as potential causes for developing depression in the context of estradiol withdrawal and the onset of hypogonadism. Trials that explore the impact of specific risk factors for depression in women receiving GnRH agonists to treat endometriosis or other gynecological illnesses, moreover, may shed new light on risk factors for depression accompanying ovarian failure or hypogonadism.
1 : Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry 2004; 61:62–70Crossref, Medline, Google Scholar
2 : Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry 2006; 63:385–390Crossref, Medline, Google Scholar
3 : Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006; 63:375–382Crossref, Medline, Google Scholar
4 : Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN). Arch Gen Psychiatry 2010; 67:598–607Crossref, Medline, Google Scholar
5 : Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN). J Affect Disord 2007; 103:267–272Crossref, Medline, Google Scholar
6 : Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med 2011; 41:1879–1888Crossref, Medline, Google Scholar
7 : A longitudinal evaluation of the relationship between reproductive status and mood in perimenopausal women. Am J Psychiatry 2004; 161:2238–2244Link, Google Scholar
8 : A cross-sectional evaluation of perimenopausal depression. J Clin Psychiatry 2008; 69:973–980Crossref, Medline, Google Scholar
9 : The menopausal transition. Am J Med 2005; 118(suppl 12B):8–13Crossref, Medline, Google Scholar
10 : Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab 1996; 81:1495–1501Medline, Google Scholar
11 : Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol 2000; 183:414–420Crossref, Medline, Google Scholar
12 : Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry 2001; 58:529–534Crossref, Medline, Google Scholar
13 : Effects of treatment with leuprolide acetate depot on working memory and executive functions in young premenopausal women. Psychoneuroendocrinology 2006; 31:935–947Crossref, Medline, Google Scholar
14 : Depression in women treated with a gonadotropin-releasing hormone agonist. Biol Psychiatry 1996; 39:378–382Crossref, Medline, Google Scholar
15 : Chronic GnRH agonist administration down-regulates platelet serotonin transporter in women undergoing assisted reproductive treatment. Psychopharmacology (Berl) 1996; 125:141–145Crossref, Medline, Google Scholar
16 : Sertraline in the treatment of depression associated with gonadotropin-releasing hormone agonist therapy. Biol Psychiatry 1998; 43:464–465Crossref, Medline, Google Scholar
17 : Treatment of endometriosis with a long-acting gonadotropin-releasing hormone agonist. Obstet Gynecol 1987; 69:403–411Medline, Google Scholar
18 : Anxiety and mood disorders associated with gonadotropin-releasing hormone agonist therapy. Psychopharmacol Bull 1997; 33:311–316Medline, Google Scholar
19 : Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCID-P), version 2.0. New York, New York State Psychiatric Institute, Biometrics Research, 1998Google Scholar
20 : An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
21 : Premenstrual changes: patterns and correlates of daily ratings. J Affect Disord 1986; 10:127–135Crossref, Medline, Google Scholar
22 : Radioimmunoassay for estrogens: a preliminary communication. Mayo Clin Proc 1969; 44:461–465Medline, Google Scholar
23 : Radioimmunoassay of plasma steroid hormones, in Modern Methods of Steroid Analysis. Edited by Heftman E. New York, Academic Press, 1973, pp 451–470Crossref, Google Scholar
24 : Beck depression inventory in general practice. J R Coll Gen Pract 1969; 18:267–271Medline, Google Scholar
25 : The Beck Depression Inventory with medical inpatients. Acta Psychiatr Scand 1967; 43:255–266Crossref, Medline, Google Scholar
26 : Estimating correlation coefficient between two variables with repeated observations using mixed-effects model. Biom J 2006; 48:286–301Crossref, Medline, Google Scholar
27 : GnRH-agonist induced depressive and anxiety symptoms during in vitro fertilization-embryo transfer cycles. Fertil Steril 2011; 95:307–309Crossref, Medline, Google Scholar
28 : Cognitive function effects of suppressing ovarian hormones in young women. Menopause 2002; 9:227–235Crossref, Medline, Google Scholar
29 : Depressive mood symptoms associated with ovarian suppression. Fertil Steril 2000; 74:984–986Crossref, Medline, Google Scholar
30 : Assessment and treatment of hot flushes and menopausal mood disturbance. Psychiatr Clin North Am 2003; 26:563–580Crossref, Medline, Google Scholar
31 : Sleep problems across the life cycle in women. Curr Treat Options Neurol 2004; 6:319–330Crossref, Medline, Google Scholar
32 : Hormones, mood, sexuality, and the menopausal transition. Fertil Steril 2002; 77(suppl 4):S42–S48Crossref, Medline, Google Scholar
33 : Sexual dysfunction among middle-aged women in the community. Br Med J (Clin Res Ed) 1988; 296:959–962Crossref, Medline, Google Scholar
34 : Pharmacologically induced hypogonadism and sexual function in healthy young women and men. Neuropsychopharmacology 2009; 34:565–576Crossref, Medline, Google Scholar
35 : Sleep difficulty in women at midlife: a community survey of sleep and the menopausal transition. Menopause 2003; 10:19–28Medline, Google Scholar
36 : Increased estradiol and improved sleep, but not hot flashes, predict enhanced mood during the menopausal transition. J Clin Endocrinol Metab 2011; 96:E1044–E1054Crossref, Medline, Google Scholar
37 : Temporal associations of hot flashes and depression in the transition to menopause. Menopause 2009; 16:728–734Crossref, Medline, Google Scholar
38 : Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998; 338:209–216Crossref, Medline, Google Scholar
39 : Effects of gonadal steroids in women with a history of postpartum depression. Am J Psychiatry 2000; 157:924–930Link, Google Scholar



