Interplay of Genetic Risk Factors (CHRNA5-CHRNA3-CHRNB4) and Cessation Treatments in Smoking Cessation Success
Abstract
Objective:
Smoking is highly intractable, and the genetic influences on cessation are unclear. Identifying the genetic factors affecting smoking cessation could elucidate the nature of tobacco dependence, enhance risk assessment, and support development of treatment algorithms. This study tested whether variants in the nicotinic receptor gene cluster CHRNA5-CHRNA3-CHRNB4 predict age at smoking cessation and relapse after an attempt to quit smoking.
Method:
In a community-based, cross-sectional study (N=5,216) and a randomized comparative effectiveness smoking cessation trial (N=1,073), the authors used Cox proportional hazard models and logistic regression to model the relationships of smoking cessation (self-reported quit age in the community study and point-prevalence abstinence at the end of treatment in the clinical trial) to three common haplotypes in the CHRNA5-CHRNA3-CHRNB4 region defined by rs16969968 and rs680244.
Results:
The genetic variants in the CHRNA5-CHRNA3-CHRNB4 region that predict nicotine dependence also predicted a later age at smoking cessation in the community sample. In the smoking cessation trial, haplotype predicted abstinence at end of treatment in individuals receiving placebo but not among individuals receiving active medication. Haplotype interacted with treatment in affecting cessation success.
Conclusions:
Smokers with the high-risk haplotype were three times as likely to respond to pharmacologic cessation treatments as were smokers with the low-risk haplotype. The high-risk haplotype increased the risk of cessation failure, and this increased risk was ameliorated by cessation pharmacotherapy. By identifying a high-risk genetic group with heightened response to smoking cessation pharmacotherapy, this work may support the development of personalized cessation treatments.
Tobacco smoking is a serious public health problem. Unfortunately, smoking is a quintessential dependence disorder, evidenced by a characteristic withdrawal syndrome and heavy, uncontrolled use (1). Cardinal manifestations of uncontrolled use are persistent use and an inability to quit successfully in a cessation attempt (1–3). Nicotine dependence is associated with both a reduced likelihood of quitting over time (4) and a rapid return to smoking following a quit attempt (3, 5–7). Therefore, identification of the factors that contribute to either sustained smoking or more rapid smoking relapse should help elucidate the causal basis of tobacco dependence, permit more accurate prediction of dependence and relapse risk, and support more effective application of treatment.
Recent meta-analyses (8–11) based on tens of thousands of subjects of European descent have confirmed the association of chromosome region 15q25.1 with smoking quantity, defined by cigarettes per day; the most robust associations have been reported for rs16969968 and rs1051730, two highly correlated variants (p<5.57×10–72) (10). In the CHRNA5-A3-B4 region, at least two independent signals have been identified (9, 12). The first signal, tagged by rs16969968, a variant that results in an amino acid change in the α5 nicotinic cholinergic receptor (CHRNA5), alters nicotinic receptor conductance in vitro (13, 14). A second, distinct signal, tagged by rs680244, is associated with variability in CHRNA5 mRNA levels (15). Resequencing of the CHRNA5-CHRNA3-CHRNB4 locus in subjects of European ancestry identified three common haplotypes in the region spanning CHRNA5 and the 3′ end of CHRNA3 (12), which can be defined by rs16969968 and rs680244 (15).
The CHRNA5-CHRNA3-CHRNB4 variants have been less consistently associated with cessation outcomes than with measures of smoking quantity. Five studies have shown an association between the CHRNA5-CHRNA3-CHRNB4 region and successful smoking cessation (16–20). All five found that the same genetic risk variants that contribute to smoking quantity and nicotine dependence also predicted smoking cessation. Other studies, however, failed to confirm this association (21–23). A genome-wide association study of three treatment cohorts did not identify any nicotinic receptor genes as predictors of prospectively measured smoking cessation (23). One large genome-wide association meta-analysis that strongly supported the association between 15q25.1 and smoking quantity failed to find an association with smoking cessation (current versus former smoking) at a genome-wide level of significance (10).
A logical case can be made for a relation between the CHRNA5-CHRNA3-CHRNB4 variants and smoking cessation. These variants are consistently related to measures of smoking quantity and nicotine dependence (12, 16), and there is copious evidence that measures of nicotine dependence predict cessation likelihood (4, 5, 24). These findings encourage examination of the involvement of the CHRNA5-CHRNA3-CHRNB4 variants in cessation and further suggest that this relation is mediated by dependence. If evidence for mediation is not found and yet the variants are related to cessation, it would suggest that the variants influence cessation through routes that are independent of their influence on dependence. Moreover, previous research suggests that the relation between the CHRNA5-CHRNA3-CHRNB4 variants and cessation should be examined with regard to pharmacotherapy. There is mounting evidence that pharmacotherapies work by mitigating the risks for cessation failure that are related to severity of nicotine dependence (25, 26). This suggests that the connection between the variants and cessation may be strongest among individuals who do not receive pharmacotherapy for smoking cessation.
Using data from a community-based project, the Atherosclerosis Risk in Communities (ARIC) study (27), and a smoking cessation clinical trial at the University of Wisconsin Transdisciplinary Tobacco Use Research Center, we extended the research on the CHRNA5-CHRNA3-CHRNB4 region and smoking cessation success. This research was predicated on hypotheses about the relations of CHRNA5-CHRNA3-CHRNB4 variants, tobacco dependence, and smoking cessation. The two types of studies differ in study duration, amount of experimental contact and monitoring, and type of participants. However, complementary hypotheses were developed for these two types of research designs. In the case of the ARIC community study, the assumption was that dependence would be manifest in the occurrence of cessation at a later age (28–30). In the case of the Wisconsin clinical trial, the assumption was that both heightened genetic risk and greater dependence would be demonstrated as failed abstinence at the end of treatment. Therefore, the difficulty of quitting was assessed in terms of both longer latency before quitting in a community sample and failed abstinence at the end of treatment in a treatment trial. Analyses addressed three major questions: 1) Does the natural history of smoking cessation vary by genetic variants in the chromosome 15q25 region? 2) Do these variants predict cessation in a treatment trial? 3) Does the relation between the genetic variants and cessation depend on treatment status? The results are relevant to understanding the nature of risk posed by the targeted variants for cessation and whether the variants have pharmacogenetic implications in treatment assignment or application.
Method
Community Study
We used the ARIC study to examine the genetic association with age at cessation in a nontreatment setting. It is a prospective epidemiologic study conducted in four U.S. communities to investigate atherosclerosis. In 1987, each ARIC field center recruited a cohort sample ages 45–64 from a defined population in its community, and almost 16,000 subjects participated. Self-reported age at cessation was assessed with the question “How old were you when you stopped smoking?” Genotyping was performed on the Affymetrix 6.0 chip at the Broad Institute of the Massachusetts Institute of Technology and Harvard University. Genetic and phenotype data were available for 12,771 subjects; data were obtained from the National Center for Biotechnology Information database of Genotypes and Phenotypes (http://www.ncbi.nlm.nih.gov/sites/entrez?db=gap; dbGaP accession number phs000090.v1.p1).
Smoking Cessation Clinical Trial
We used a randomized, placebo-controlled smoking cessation trial at the University of Wisconsin Center for Tobacco Research and Intervention (31) to examine the genetic association with time to relapse after quitting in a treatment trial setting. This study group has not been previously examined for the association of genetic risk with smoking cessation. The institutional review board at the University of Wisconsin–Madison approved this trial, and all subjects provided written informed consent.
Participants were eligible to participate if they were 18 years of age or older, smoked 10 or more cigarettes per day, and were motivated to quit smoking. Before randomization, the participants completed baseline assessments of demographic characteristics, smoking history (including cigarettes smoked per day), and tobacco dependence, which included the Fagerström Test for Nicotine Dependence (32). Each participant provided a breath sample for alveolar carbon monoxide analysis to verify smoking status and estimate the quantity of smoking.
The participants (N=1,073) were randomly assigned to six conditions: placebo (N=132), nicotine patch (N=187), nicotine lozenge (N=183), sustained-release bupropion (N=188), nicotine patch and nicotine lozenge (N=193), or bupropion and nicotine lozenge (N=190). All participants received six brief (10-minute) individual counseling sessions.
The point prevalence of biochemically confirmed 7-day abstinence was assessed at the end of treatment (8 weeks after quitting). All of the participants' self-reports of abstinence during study visits were confirmed by an expired carbon monoxide level of less than 10 ppm. Relapse was defined as any smoking on 7 consecutive days after the target quit date. Time of relapse was determined through timeline follow-up assessment (33, 34) and was available for 1,015 subjects.
Genotyping of the Wisconsin study group was performed by the Center for Inherited Disease Research at Johns Hopkins University and used the Illumina Omni2.5 microarray (www.illumina.com). Data cleaning was led by the Gene Environment Association Studies (GENEVA) Coordinating Center at the University of Washington.
Analysis
CHRNA5-CHRNA3-CHRNB4 haplotypes were analyzed. It is advantageous to model the genetic architecture by rational selection of haplotypes that maximizes information about the common variation at this locus and reflects potential underlying biological mechanisms. Three common haplotypes in the region spanning CHRNA5 and the 3′ end of CHRNA3 (12, 15) are defined by rs16969968 and rs680244. The rs16969968 allele associated with risk for smoking primarily occurs on the rs680244 allele with low mRNA expression of CHRNA5. Together, these variants identify three risk levels involving CHRNA5 and represent two distinct mechanisms for nicotine dependence. We used these three haplotypes as our standard of analysis: haplotype 1 (low smoking risk allele at rs16969968 and low mRNA expression allele at rs680244), haplotype 2 (low smoking risk allele at rs16969968 and high mRNA expression allele at rs680244), and haplotype 3 (high smoking risk allele at rs16969968 and low mRNA expression allele at rs680244). In the ARIC data set, these two variants were not genotyped, so two highly correlated single-nucleotide polymorphisms (SNPs) were used as proxies to estimate haplotypes: rs951266 as a proxy for rs16969968 and rs6495306 as a proxy for rs680244. In the Utah samples with northern and western Europe ancestry in the 1000 Genomes Project (http://www.1000genomes.org/), the correlations of these proxies with the target SNPs were r2=0.97 and r2=1.00, respectively.
We used PLINK (35) to estimate haplotype probabilities for each individual. We assumed that pairs of haplotypes in an individual are “additive.” The posterior probability for the haplotypes was 1 for more than 99% of the haplotypes. Dummy coding for different haplotypes was used.
We used a standard series of Cox proportional hazard models to analyze age at smoking cessation in the ARIC study, and we used logistic regression to analyze the point prevalence of biochemically confirmed end-of-treatment abstinence in the Wisconsin smoking cessation trial. In the cessation trial, days to relapse constituted a secondary outcome that was analyzed with Cox proportional hazard models. The primary predictor variables of interest were haplotypes and a haplotype-by-treatment interaction in the smoking cessation study. Covariates included gender, age (in quartiles), cigarettes per day (in four levels: ≤10, 11–20, 21–30, ≥31), and treatment (placebo versus active treatment) in the Wisconsin smoking cessation study.
Results
Community Study
Of the ARIC cohort members, 5,216 were of European descent, were identified as smokers (defined as smoking 400 cigarettes over a lifetime), and had genotype data. Demographic descriptions are given in Table S1, which accompanies the online version of this article. They smoked on average 23.6 cigarettes per day (SD=13.6) when smoking. Haplotype frequencies in the ARIC sample were as follows: G_C (24.4%), G_T (42.4%), and A_C (33.2%). The genotypes were labeled as haplotype 1, haplotype 2, and haplotype 3, respectively.
We found a robust association between the CHRNA5-CHRNA3-CHRNB4 haplotypes and smoking quantity, as defined by cigarettes per day, consistent with previous findings. These three haplotypes were strongly associated with the number of cigarettes per day (Wald statistic=56.43, df=2, omnibus p=5.8×10–13 for the overall haplotype effect) (see online Table S2).
The CHRNA5-CHRNA3-CHRNB4 haplotypes were also associated with age at self-reported smoking cessation in this community-based sample (Wald statistic=8.46, df=2, omnibus p=0.011). Compared to haplotype 1, the high-risk haplotype 3 was associated with a later quit age (Table 1). The median age at smoking cessation was 57 years for those with haplotype 3, and it was 55 years for those with haplotype 2 or 1. The strength of this association was more modest than the association with smoking quantity. To further understand this genetic association, we added cigarettes per day as a covariate in the model. Smoking a higher number of cigarettes per day was strongly associated with a later quit age (relative hazard=0.63, 95% confidence interval [CI]=0.61–0.65, p<1.0×10–8), and the haplotypes were no longer associated with age at smoking cessation (Wald statistic=1.72, df=2, omnibus p=0.42 for the overall haplotype effect). The analyses were repeated with smoking duration (from self-reported age at onset of smoking to age at smoking cessation) as the outcome variable, and similar results were seen.
| Effect on Age at Smoking Cessationc | |||
|---|---|---|---|
| Haplotypeb | Relative Hazard | 95% CI | p |
| Haplotype 1 (G_C) | Reference | ||
| Haplotype 2 (G_T) | 0.98 | 0.92–1.05 | 0.59 |
| Haplotype 3 (A_C) | 0.92 | 0.86–0.98 | 0.009 |
TABLE 1. Effect on Age at Smoking Cessation of Three Haplotypes in Nicotinic Receptor Gene Cluster CHRNA5-CHRNA3-CHRNB4 in a Community Samplea (N=5,216)
Smoking Cessation Clinical Trial
The analyses based on the trial at the University of Wisconsin Transdisciplinary Tobacco Use Research Center included 1,073 subjects of European ancestry with genotype and relapse data. Online Table S1 shows their demographic characteristics. The counts and frequencies of haplotypes 1, 2, and 3 in this group were as follows: G_C (20.8%), G_T (43.7%), and A_C (35.5%). In this treatment-seeking study group, the number of cigarettes per day was associated with the three haplotypes after adjustment for age and gender (Wald statistic=7.15, df=2, omnibus p=0.03 for the overall haplotype effect), but this effect was modest in this heavy-smoking cohort (online Table S2).
In this trial, 47.3% of the participants were abstinent at the end of treatment (8 weeks after the quit date). In a simple logistic regression model, abstinence was predicted by treatment assignment (placebo versus active treatment) after adjustment for age and gender. Having any pharmacologic treatment, compared to placebo, increased abstinence by over 85% (odds ratio=1.87, 95% CI=1.42–2.45, p=8.1×10–6). Smoking fewer cigarettes per day was the strongest predictor of abstinence (odds ratio=0.67, 95% CI=0.60–0.75, p=3.3×10–11).
The haplotypes did not predict abstinence in the entire study group, i.e., including both placebo and treatment groups. However, the association of the haplotypes with abstinence depended on treatment condition. In the placebo group, haplotype 3, which is associated with heavy smoking, predicted failed abstinence in comparison to haplotype 1 (odds ratio=0.37, 95% CI=0.19–0.78, p=0.009 for haplotype 3 compared to haplotype 1); overall haplotypes were associated with abstinence (χ2=7.02, df=2, omnibus p=0.03 for the overall haplotype effect in the placebo group). However, haplotype status was not associated with abstinence among individuals receiving active pharmacotherapy (χ2=1.45, df=2, omnibus p=0.48 for the overall haplotype effect). This is reflected by a significant interaction between treatment (placebo versus active treatment) and haplotype (χ2=8.97, df=2, omnibus p=0.02 for the interaction) (Table 2, Figure 1).
| Effect on Abstinence at End of Treatmentb | |||
|---|---|---|---|
| Predictor | Odds Ratio | 95% CI | p |
| Haplotypec | |||
| Haplotype 1 (G_C) | Reference | ||
| Haplotype 2 (G_T) | 0.62 | 0.33–1.17 | 0.14 |
| Haplotype 3 (A_C) | 0.37 | 0.19–0.75 | 0.006 |
| Treatment status | |||
| Placebo | Reference | ||
| Active treatment | 0.98 | 0.57–1.69 | 0.93 |
| Interaction of haplotype and interventiond | |||
| Haplotype 1 and active treatment | Reference | ||
| Haplotype 2 and active treatment | 1.83 | 0.93–3.63 | 0.09 |
| Haplotype 3 and active treatment | 3.12 | 1.48–6.55 | 0.003 |
TABLE 2. Effects on Endpoint Abstinence of Treatment and Haplotype in Nicotinic Receptor Gene Cluster CHRNA5-CHRNA3-CHRNB4 in an 8-Week Smoking Cessation Triala (N=1,073)
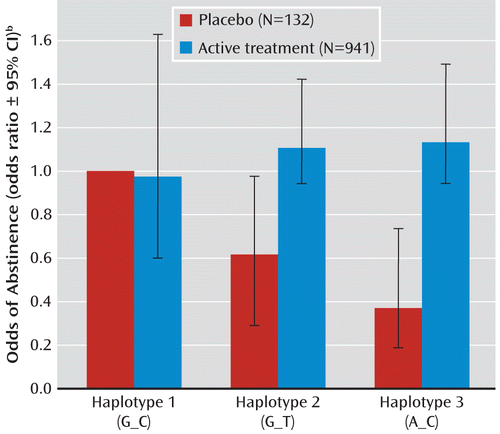
FIGURE 1. Effect on Endpoint Abstinence of Interaction Between Treatment and Haplotype in Nicotinic Receptor Gene Cluster CHRNA5-CHRNA3-CHRNB4 in an 8-Week Smoking Cessation Trial (N=1,073)a
a Haplotypes were defined by the rs16969968 to rs680244 single-nucleotide polymorphisms. The frequencies of haplotypes 1, 2, and 3 were 20.8%, 43.7%, and 35.5%, respectively. The interaction of haplotype and treatment was significant (χ2=8.97, df=2, p=0.011).
b Adjusted for age and gender.
To further understand the interplay between haplotype and treatment, we added the number of cigarettes per day and the interaction between cigarettes per day and treatment to determine whether the relationship between haplotype status and abstinence depended on smoking quantity (as a measure of dependence). Heavier smoking was associated with less abstinence (odds ratio=0.67, 95% CI=0.60–0.75, p=2.8×10–11), but this relationship did not differ by treatment status (χ2=0.98, df=1, omnibus p=0.32 for the interaction). The interaction between haplotype and treatment remained significant after adjustment for cigarettes per day (χ2=8.61, df=2, omnibus p=0.02 for the interaction) (see online supplemental Table S3). In addition, we found similar results when modeling the secondary cessation outcome: time to relapse after the quit date over 60 days. There was a consistent interaction between haplotype and treatment status (haplotype 3 versus 1: relative hazard=0.54, 95% CI=0.33–0.89, p=0.02; haplotype 2 versus 1: relative hazard=0.60, 95% CI=0.37–0.95, p=0.04; overall haplotype effect: χ2=6.46, df=2, omnibus p=0.04) (see online Table S4). Figure S1 in the data supplement accompanying the online version of this article illustrates the risk for relapse by haplotype in the entire study group and in the placebo and active treatment groups. The haplotypes were associated with the risk of relapse in the placebo group but not in the active treatment group. Online Figure S2 shows the risk for relapse by treatment status, stratified by the three haplotype groups. Active treatment was strongly associated with a lower risk of relapse in individuals with haplotype 3 (relative hazard=0.48, 95% CI=0.36–0.64, p=9.7×10-7) and haplotype 2 (relative hazard=0.48, 95% CI=0.37–0.62, p=2.7×10–8). Active treatment had no significant effect in those with haplotype 1 (relative hazard=0.83, 95% CI=0.56–1.24, p=0.36).
Furthermore, there was no significant difference in haplotypic effects on abstinence or relapse between different active treatment groups (bupropion only, nicotine replacement therapy only, and combined) (online Table S5) and no association between haplotype and abstinence or relapse in any specific active treatment group (online Figure S3 and Figure S4). Additional analyses using single SNPs showed consistent results (online Table S6 and Table S7).
Discussion
This study reveals an interaction between the genetic variants in CHRNA5-CHRNA3-CHRNB4 and smoking cessation pharmacotherapy in relation to smoking cessation. Smokers with the high-risk haplotype had a threefold increased likelihood of responding to pharmacologic cessation treatment, compared to smokers with the low-risk haplotype.
In addition to the interaction effect, this study showed that the genetic variants in the chromosome 15q25 region that predict heavy smoking and nicotine dependence also predicted a later age at smoking cessation in a large community-based sample. Individuals with the high-risk haplotype quit later than those at low genetic risk; this difference was manifested as a 2-year delay in median quit age. Recruitment for the ARIC study began in the late 1980s, when use of nicotine replacement therapy by the general population was quite low (36, 37), so most of ARIC subjects quit smoking without any pharmacologic treatment. Thus, they represent a “natural history” of smoking cessation. The observed association between haplotype status and quitting latency was no longer significant once smoking quantity was taken into account. This suggests that the targeted risk haplotypes confer heightened risk of heavy smoking and that this, in turn, constitutes an obstacle to successful quitting.
The large smoking cessation trial offers a distinct, complementary test of the association between haplotype status and cessation. In this study, the genetic associations with smoking cessation were manifested in the placebo group, and these results are consistent with those obtained in the ARIC “natural history” sample. In contrast, these genetic variants did not predict abstinence across active treatment conditions, and the smaller genetic effect in the context of active pharmacological treatments suggests that cessation treatments differ in effectiveness across the haplotypes and mitigate the genetic risks for cessation difficulty. Pharmacological cessation treatment significantly increased the likelihood of abstinence in individuals with the high-risk haplotype, haplotype 3, but exerted little effect in individuals with the low-risk haplotype, haplotype 1.
These findings may explain discrepancies in prior studies of these genetic variants and smoking cessation. Some previous studies indicated that the chromosome 15q25 region is associated with smoking cessation (16–20), whereas other studies did not (21–23). Given our findings, we believe that genetic risk varies by pharmacologic intervention and that a weaker genetic effect (or even none) will be seen in pharmacologic trials if the effect of an interaction with treatment (active treatment versus placebo) is not included. We believe that the effect of this genetic locus will be seen most clearly in placebo arms, or in a sample where pharmacotherapy use is rare. For example, Sarginson et al. reported very little association between CHRNA5-CHRNA3-CHRNB4 variants and cessation during the pharmacologic treatment phase of their trial, but the association increased at the 1-year follow-up after a maintenance phase with no medication (19). Our present findings suggest that an interaction of genetic risk with environment might account for such inconsistency, because the targeted genetic effects are most strongly expressed in environments that provide little support for cessation (e.g., no effective pharmacotherapy). To our knowledge, none of the previous studies has reported this interaction, which might explain the apparently inconsistent results in this area. Such interactions between genetic variants and treatment can serve as the basis for personalized medicine. The Tobacco and Genetics Consortium examined a different cessation phenotype (former versus current smoker) and reported a modest association with CHRNA5-CHRNA3-CHRNB4 (p<1×10–4) (10).
The results of this study should be interpreted in the context of several limitations. First, there was relatively little power to compare the magnitude of the targeted genetic effects among different active treatment conditions. The genetic risks for cessation were similar across the different pharmacotherapy conditions, indicating that multiple cessation interventions have the potential to be effective with such high-risk individuals. It is unclear whether pharmacotherapy mitigates particular biological processes that lead to cessation failure or whether the genetic effect is more likely expressed at high overall levels of quitting difficulty. Second, the placebo group in the cessation trial was fairly small, and this limitation adds to the importance of future replication. However, reported associations between these genetic variants and cessation in multiple groups suggest a valid relation between these variants and cessation in some environmental contexts. Third, the smoking reports in the ARIC sample were not supported by biochemical confirmation. However, such confirmation was obtained in the Wisconsin trial, and research shows that self-report is a valid indicator of current smoking, especially when there are no strong incentives to deceive (38). In addition, this work studied only one genetic locus, and it is clear that multiple genes contribute to smoking cessation success. Future research should explore whether greater accuracy in predicting treatment response can be attained by the addition of other genetic variants implicated in differential treatment effects (39). Finally, this study included only subjects of European descent, while the frequency and effects of these genetic variants differ by ancestry.
These results suggest that the biological effects of these haplotypes affect both smoking quantity and the ability to quit, and the interaction suggests that pharmacologic treatments are more effective for individuals who are biologically predisposed to have difficulty quitting. However, it is unclear whether the haplotypes are linked to both outcomes through the same mechanisms. In the ARIC sample, the effect of the targeted haplotypes on the number of cigarettes per day mediated the influence of the haplotypes on cessation. Such mediation was not found in the Wisconsin trial, indicating that more research is needed to clarify the causal paths from the haplotypes to cessation success. However, regardless of the specific causal paths that are ultimately determined, if the CHRNA5-CHRNA3-CHRNB4 haplotypes are indeed meaningfully related to both smoking quantity and cessation, they have an important role in the development and expression of nicotine dependence.
While acknowledging the limitations of our study, we note that this work complements and builds on previous research on treatment and genetic effects on smoking cessation. With diverse methods, this work underscores the relation between the targeted haplotypes and smoking cessation, shows a significant effect of the haplotype-treatment interaction on cessation success, and reveals that the effectiveness of cessation treatment is modulated by the haplotype.
In summary, our findings strengthen the case for the development and rigorous testing of treatments that target patients with different genetic risk profiles based on the chromosome 15q25 region that includes CHRNA5-CHRNA3-CHRNB4. Smokers with the high- and intermediate-risk haplotypes appear more biologically predisposed to have difficulty in quitting without treatment, but this risk may be ameliorated by effective pharmacological treatment. Further research that identifies genes associated with responsiveness to treatment for nicotine addiction may lead to treatment algorithms that further the promise of personalized medicine (40).
1. : Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Arch Gen Psychiatry 2001; 58:810–816Crossref, Medline, Google Scholar
2. : Applicability of DSM criteria to nicotine dependence. Addiction 2011; 106:894–895; discussion 895–897Crossref, Medline, Google Scholar
3. : Defining and assessing nicotine dependence in humans, in Understanding Nicotine and Tobacco Addiction: Novartis Foundation Symposium 275. Edited by Bock GGoode J. Chichester, UK, John Wiley & Sons, 2005, pp 36–58Google Scholar
4. : Predicting smoking cessation and major depression in nicotine-dependent smokers. Am J Public Health 2000; 90:1122–1127Crossref, Medline, Google Scholar
5. : Time to first cigarette in the morning as an index of ability to quit smoking: implications for nicotine dependence. Nicotine Tob Res 2007; 9(suppl 4):S555–S570Medline, Google Scholar
6. : Evaluating the validities of different DSM-IV-based conceptual constructs of tobacco dependence. Addiction 2008; 103:1215–1223Crossref, Medline, Google Scholar
7. : Predicting smoking cessation with self-reported measures of nicotine dependence: FTQ, FTND, and HSI. Drug Alcohol Depend 1994; 34:211–216Crossref, Medline, Google Scholar
8.
9. : Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta-analysis and comparison with lung cancer and COPD. PLoS Genet 2010; 6(8):e1001053Crossref, Medline, Google Scholar
10.
11. : Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 2010; 42:448–453Crossref, Medline, Google Scholar
12. : A candidate gene approach identifies the CHRNA5-A3-B4 region as a risk factor for age-dependent nicotine addiction. PLoS Genet 2008; 4(7):e1000125Crossref, Medline, Google Scholar
13. : Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 2008; 165:1163–1171Link, Google Scholar
14. : Acetylcholine receptor (AChR) alpha5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (alpha4beta2)alpha5 AChR function. Mol Pharmacol 2011; 79:119–125Crossref, Medline, Google Scholar
15. : Risk for nicotine dependence and lung cancer is conferred by mRNA expression levels and amino acid change in CHRNA5. Hum Mol Genet 2009; 18:3125–3135Crossref, Medline, Google Scholar
16. : Human neuronal acetylcholine receptor A5-A3-B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine Tob Res 2009; 11:785–796Crossref, Medline, Google Scholar
17. : A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5-CHRNA3-CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Hum Mol Genet 2009; 18:2922–2927Crossref, Medline, Google Scholar
18. : CHRNA3 rs1051730 genotype and short-term smoking cessation. Nicotine Tob Res 2011; 13:982–988Crossref, Medline, Google Scholar
19. : Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5-A3-B4) predict severity of nicotine addiction and response to smoking cessation therapy. Am J Med Genet B Neuropsychiatr Genet 2011; 156B:275–284Crossref, Medline, Google Scholar
20. : Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo-controlled clinical trials. Neuropsychopharmacology 2012; 37:641–650Crossref, Medline, Google Scholar
21. : Prospective association of dopamine-related polymorphisms with smoking cessation in general care. Pharmacogenomics 2010; 11:527–536Crossref, Medline, Google Scholar
22. : Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems-based candidate gene study of smoking cessation. Hum Mol Genet 2008; 17:2834–2848Crossref, Medline, Google Scholar
23. : Molecular genetics of successful smoking cessation: convergent genome-wide association study results. Arch Gen Psychiatry 2008; 65:683–693Crossref, Medline, Google Scholar
24. : Assessing tobacco dependence: a guide to measure evaluation and selection. Nicotine Tob Res 2006; 8:339–351Crossref, Medline, Google Scholar
25. : Should all smokers use combination smoking cessation pharmacotherapy? using novel analytic methods to detect differential treatment effects over 8 weeks of pharmacotherapy. Nicotine Tob Res 2012; 14:131–141Crossref, Medline, Google Scholar
26. : Treating heavy smokers in primary care with the nicotine nasal spray: randomized placebo-controlled trial. Addiction 2011; 106:824–832Crossref, Medline, Google Scholar
27. : Cigarette smoking and progression of atherosclerosis: the Atherosclerosis Risk in Communities (ARIC) Study. JAMA 1998; 279:119–124Crossref, Medline, Google Scholar
28. : Racial differences in smoking abstinence rates in a multicenter, randomized, open-label trial in the United States. Z Gesundh Wiss 2010; 18:59–68Crossref, Medline, Google Scholar
29. : Smoking trajectories, health, and mortality across the adult lifespan. Addict Behav 2009; 34:701–704Crossref, Medline, Google Scholar
30. : The relationship of smoking cessation to sociodemographic characteristics, smoking intensity, and tobacco control policies. Nicotine Tob Res 2005; 7:387–396Crossref, Medline, Google Scholar
31. : A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry 2009; 66:1253–1262Crossref, Medline, Google Scholar
32. : The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addiction 1991; 86:1119–1127Crossref, Medline, Google Scholar
33. : Efficacy of bupropion alone and in combination with nicotine gum. Nicotine Tob Res 2007; 9:947–954Crossref, Medline, Google Scholar
34. : Timeline follow-back: a technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods. Edited by Litten RZAllen J. Totowa, NJ, Humana Press, 1992, pp 41–72Crossref, Google Scholar
35. : PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007; 81:559–575Crossref, Medline, Google Scholar
36. : Nicotine replacement therapy use among a cohort of smokers. J Addict Dis 2005; 24:101–113Crossref, Medline, Google Scholar
37.
38.
39. : Catechol-O-methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biol Psychiatry 2007; 61:111–118Crossref, Medline, Google Scholar
40. : Symbiotic relationship of pharmacogenetics and drugs of abuse. AAPS J 2006; 8(1):E174–E184Crossref, Medline, Google Scholar



