Suicidal Behavior and Severe Neuropsychiatric Disorders Following Glucocorticoid Therapy in Primary Care
Abstract
Objective:
The incidence and the risk of suicidal behaviors and severe neuropsychiatric disorders in people treated with systemic glucocorticoids are poorly known. The authors assessed the incidence rates of depression, mania, delirium, panic disorder, and suicidal behaviors in patients treated with glucocorticoids in primary care settings and the risk factors for developing these outcomes.
Method:
Data were obtained for all adult patients registered between 1990 and 2008 at U.K. general practices contributing to The Health Improvement Network (THIN) primary care database. The incidence rates for the outcomes of interest were assessed in patients who received prescriptions for oral glucocorticoids and compared with those in patients who did not receive such prescriptions. The predictors of these outcomes in exposed patients were ascertained using Cox proportional hazards models.
Results:
Overall, 786,868 courses of oral glucocorticoids were prescribed for 372,696 patients. The authors identified 109 incident cases of suicide or suicide attempt and 10,220 incident cases of severe neuropsychiatric disorders in these patients. The incidence of any of these outcomes was 22.2 per 100 person-years at risk for first-course treatments. Compared to people with the same underlying medical disease who were not treated with glucocorticoids, the hazard ratio for suicide or suicide attempt in exposed patients was 6.89 (95% CI=4.52–10.50); for depression, 1.83 (95% CI=1.72–1.94); for mania, 4.35 (95% CI=3.67–5.16); for delirium, confusion, or disorientation, 5.14 (95% CI=4.54–5.82); and for panic disorder, 1.45 (95% CI=1.15–1.85). Older men were at higher risk of delirium/confusion/disorientation and mania, while younger patients were at higher risk of suicide or suicide attempt. Patients with a previous history of neuropsychiatric disorders and those treated with higher dosages of glucocorticoids were at greater risk of neuropsychiatric outcomes.
Conclusions:
Glucocorticoids increase the risk of suicidal behavior and neuropsychiatric disorders. Educating patients and their families about these adverse events and increasing primary care physicians' awareness about their occurrence should facilitate early monitoring.
Natural glucocorticoids such as cortisol affect behavior, mood, and other CNS-related processes (1). Abnormalities of the hypothalamic-pituitary-adrenal axis, notably hypercortisolemia and specific dysfunction in glucocorticoid negative feedback, have been demonstrated in people with mood disorders (1–5). Additionally, some 50%–80% of patients with endogenous hypercortisolemia (Cushing's syndrome) meet DSM-IV criteria for major depression (6), and about 10% experience psychosis or mania (7). The association of exogenous glucocorticoids with depressive and manic syndromes is relatively well documented (8). In view of the frequency and severity of such disturbances in various clinical populations who receive prescriptions for glucocorticoids, there is a need for population-based prevalence studies. Data from retrospective studies indicate prevalence rates of glucocorticoid-induced neuropsychiatric disorders ranging from below 1% to 50% (9–12). While drug dosage is a known risk factor (13, 14), the effects of age, sex, and the underlying medical disease are unknown. Moreover, some authors have asserted that a single glucocorticoid-induced disturbance should not be regarded as a contraindication for future glucocorticoid treatment, but some case reports have described individuals who experienced recurrent psychiatric disturbances when challenged with glucocorticoids (15, 16). The evidence base for adverse events such as delirium, panic disorder, and suicidal behavior is poorly developed and limited to case reports (8).
Our aim in this study was to assess the incidence rates of depression, mania, delirium, panic disorder, and suicide or suicide attempt in people treated with glucocorticoids and the risk factors for developing these outcomes in U.K. primary care.
Method
Data Source
Approximately 98% of the population in the United Kingdom is registered with a general practitioner (17). The Health Improvement Network (THIN) is a database of anonymized electronic medical records from general practices across the country. Participating general practitioners systematically and prospectively retrieve and enter clinical information on patients, including demographic data, diagnoses, and prescriptions. The database thus provides a longitudinal medical record for each patient. The consultation and prescription data recorded in THIN compare favorably with national data (18). THIN has been validated through audits, comparisons with external statistics, and independent studies and has been shown to have a high level of completeness of clinical diagnostic and prescribing data (19–21). To minimize any bias on disease occurrence or prescriptions issued, we restricted our analyses to high-quality data by using quality indicators as defined elsewhere (18, 22), and we excluded entries made within 6 months after registration with the general practice as this may represent retrospective recording of a past history rather than a new episode of a problem (23). We used data from January 1, 1990, to December 31, 2008, from 424 general practices.
Identification of Glucocorticoid Prescriptions
In THIN, each drug is encoded using Multilex codes that are associated with data from the British National Formulary (http://bnf.org/bnf). Prescribing is particularly well recorded in THIN since physicians use the practice computers to issue prescriptions (18). We selected all oral glucocorticoids prescribed, which included prednisolone, prednisone, hydrocortisone, dexamethasone, triamcinolone, betamethasone, methylprednisolone, and deflazacort. Patients treated exclusively with topical, inhaled, or parenteral glucocorticoids were not included in the analyses.
Identification of Neuropsychiatric Disorders
All diagnoses and symptoms are recorded in THIN using the Read classification system (24). The Read classification and the Multilex codes were used to create medical and drug code lists that enabled us to identify cases of suicide or suicide attempt; depression; mania; delirium, confusion, or disorientation; and panic disorder in the database. We compiled lists for Read codes and for drug codes by conducting relevant word and code searches in the Read code and drug code dictionaries, as previously described (25). For instance, patients were defined as depressed if they had a Read code entry for unipolar depression, for symptoms of depression (e.g., low mood), or for a prescription for an antidepressant on a given consultation date. Diagnoses were considered first; prescriptions of antidepressants were used in defining the outcome only when there was no recorded diagnosis of a neuropsychiatric illness and no other recorded indication for the prescription. To exclude patients who may have received prescriptions for antidepressants for anxiety rather than for depression, we eliminated those who had an entry for anxiety or panic disorder but had no entry for depression in their entire computerized medical record. For Read code searches, depression was identified, with bipolar disorders and depression with psychosis excluded. We applied similar methods to define mania (diagnosis or symptoms of mania or prescription of antimanic medication), delirium/confusion/disorientation (diagnosis or symptoms of delirium, confusion, or disorientation or prescription of antipsychotic medication), panic disorder (diagnosis of panic disorder or panic attack but excluding codes for anxiety), and suicidal behavior (diagnosis of suicide or suicide attempt). Death certificates for people who died during the study period were also reviewed to identify those who died from suicide. A glucocorticoid-induced outcome was defined as one that was recorded after glucocorticoid initiation and with a preceding interval of at least 6 months with no similar entry (i.e., no coded entries for diagnosis or for symptoms or medications linked to the diagnosis). For long-term glucocorticoid therapies (i.e., lasting more than 3 months [26]), we arbitrarily defined a glucocorticoid-induced outcome as one that occurred within the first 3 months of the drug being prescribed.
Glucocorticoid-Exposed Group
We identified all patients age 18 and older who received at least one oral glucocorticoid prescription. For multiple consecutive prescriptions, the treatment duration was defined as the time from the first to the last prescription plus the duration of the last prescription. Patients who received a new prescription for an oral glucocorticoid after a period of 3 months or more without were considered to have started a new treatment course. We calculated the prescribed daily dose for each course by multiplying the number of pills prescribed by the dose per pill (calculated in prednisone equivalents) and dividing this result by the number of days for which the drug was prescribed. We took into account the daily dose recorded at the beginning of the course to analyze the effect of drug dosage on outcome. The medical diagnosis recorded on the date that glucocorticoids were started was used as the indication for the glucocorticoid prescription. If no medical diagnosis was recorded on that date, we searched for 12 relevant chronic conditions entered in the records 3 months before or after this prescription.
Unexposed Groups
Unexposed groups were patients who did not receive prescriptions for oral glucocorticoids. From the pool of eligible individuals, two comparison groups were identified. The first was a selection of a random sample of patients who did not receive glucocorticoid prescriptions, and the second was a random sample of patients who did not receive glucocorticoid prescriptions but who had diagnoses of the same underlying medical diseases as the exposed patients. We selected up to four unexposed individuals from each of these groups for every exposed individual. When selecting the unexposed groups, we stratified the sample to ensure that they had the same distribution of sex and age (within 10-year age bands) as the glucocorticoid-exposed group. We accounted for the clustering effect within general practices by selecting exposed and unexposed patients from within the same practice. For each unexposed patient, a randomly selected “index date” was defined at least 6 months after his or her registration.
Statistical Analysis
For each participant, follow-up time was accrued from glucocorticoid initiation or index date until the date of the outcome, the end date of glucocorticoid exposure (up to 3 months after glucocorticoid initiation), a date up to 3 months after the index date, the date of leaving the practice, the date of death, or the end of the study period. We calculated incidence rates by dividing the number of newly diagnosed cases of suicide or suicide attempt, depression, mania, delirium/confusion/disorientation, and panic disorder by the total follow-up time in the study cohort. This was first done as a total for all outcomes and then for each one in turn. Incidence rates were estimated for all glucocorticoid courses and then separately for the first, second, and third or later courses. We assessed risk factors for each outcome using Cox proportional hazards models. We initially compared the exposed and unexposed groups to assess hazard ratios associated with the first prescription issued for glucocorticoids, and we performed a sensitivity analysis that excluded prescriptions for antidepressants, antipsychotics, or antimanic agents as a definition of a neuropsychiatric disorder. The estimated hazard ratios were adjusted for age, sex, past history of neuropsychiatric disorders, and the underlying medical disease. We then ascertained the predictors of the outcomes in patients treated with glucocorticoids. In those who received multiple courses, only one course was randomly selected to avoid a clustering effect within patients. We selected a random mixture of first and later exposures to glucocorticoids to examine whether previous exposure to glucocorticoid therapy was associated with the risk of developing a neuropsychiatric illness.
Models were fitted sequentially, adjusting for each individual potential confounder—age (classified into four categories), sex, past history of glucocorticoid use (yes or no), past history of any neuropsychiatric disorder (yes or no), daily dose of glucocorticoids (classified into five categories), and underlying medical disease. The initial daily dose of glucocorticoids was missing for 11% of glucocorticoid courses and was then addressed through multiple imputation, which assumed that the missing covariate values were missing at random. The proportional hazard assumption was checked graphically and by analyzing Schoenfeld residuals. For continuous variables, we checked linearity by comparing two models, one with the linear term and the other with the categories using the log-likelihood ratio test. We examined for differences in the effect of glucocorticoid daily dose between men and women and according to age by testing the dose-by-sex and dose-by-age interaction terms. These were not significant and hence were excluded from the final model. Incidence rates are reported per 100 person-years at risk. All analyses were conducted using Stata, version 11.1 (StataCorp, College Station, Tex.). The study was approved by the University College London THIN steering committee and by the THIN scientific review committee.
Results
In total, 786,868 courses of oral glucocorticoids, representing 89,298 years of glucocorticoid exposure, were prescribed in 372,696 adult patients (age 18 or older). The clinical indication for the glucocorticoid prescription was identified for 560,472 courses prescribed in 261,272 patients. The characteristics of both the exposed and the unexposed patients are summarized in Table 1.
| Characteristic | All Exposed Patients (N=372,696) | First Unexposed Population, Stratified by Age and Sex (N=1,224,984) | Exposed Patients With an Identified Indication for Prescription (N=261,272) | Second Unexposed Population, Stratified by Age, Sex, and Underlying Disease (N=660,776) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years)a | 57.5 | 18.8 | 55.2 | 19.1 | 55.3 | 18.9 | 53.5 | 18.8 |
| N | % | N | % | N | % | N | % | |
| Female | 220,272 | 59.1 | 717,840 | 58.6 | 155,979 | 59.7 | 393,162 | 59.5 |
| Previous history of neuropsychiatric disorder | 89,396 | 24.0 | 296,777 | 24.2 | 65,862 | 25.2 | 174,386 | 26.4 |
| Underlying disease | ||||||||
| Lower respiratory tract infection | 100,720 | 38.5 | 309,177 | 46.8 | ||||
| Asthma | 78,924 | 30.2 | 229,746 | 34.8 | ||||
| Chronic obstructive pulmonary disease | 20,329 | 7.8 | 43,916 | 6.6 | ||||
| Polymyalgia rheumatica or giant cell arteritis | 19,250 | 7.4 | 1,206 | 0.2 | ||||
| Other | 42,049 | 16.1 | 76,731 | 11.6 | ||||
TABLE 1. Characteristics of Study Populations in an Analysis of Suicidal Behavior and Severe Neuropsychiatric Disorders Following Glucocorticoid Therapy in Primary Care
Incidence and Association With Glucocorticoid Exposure
In patients exposed to glucocorticoids, we identified 19 incident cases of completed suicide, 90 incident cases of suicide attempt, 6,918 incident cases of depression, 2,030 incident cases of delirium/confusion/disorientation, 898 incident cases of mania, 266 incident cases of panic disorder, and 108 incident cases of psychiatric referral without any details of the symptoms experienced. The incidence rate of all outcomes varied depending on the order of the glucocorticoid courses. It was 15.7 per 100 person-years at risk for all glucocorticoid courses, 22.2 per 100 person-years at risk for first courses, 14.0 per 100 person-years at risk for second glucocorticoid courses, and 11.7 per 100 person-years at risk for third and later courses.
Figure 1 presents the adjusted hazard ratios for all neuropsychiatric disorders (all exposed patients, hazard ratio=3.26, 95% CI=3.14–3.37; exposed patients with an indication for prescription, hazard ratio=2.26, 95% CI=2.15–2.37) and for each individual disorder associated with the first prescription of glucocorticoids issued. Compared with the unexposed populations, the risk of suicide or suicide attempt increased five- to sevenfold in people treated with glucocorticoids (all exposed patients, hazard ratio=5.27, 95% CI=3.82–7.29; exposed patients with an indication for prescription, hazard ratio=6.89, 95% CI=4.52–10.50), even though the overall risk was low. The increased risk was most marked for delirium/confusion/disorientation (all exposed patients, hazard ratio=6.35, 95% CI=5.92–6.81; exposed patients with an indication for prescription, hazard ratio=5.14, 95% CI=4.54–5.82) and mania (all exposed patients, hazard ratio=5.66, 95% CI=5.09–6.60; exposed patients with an indication for prescription, hazard ratio=4.35, 95% CI=3.67–5.16) when compared with depression (all exposed patients, hazard ratio=2.60, 95% CI=2.49–2.70; exposed patients with an indication for prescription, hazard ratio=1.83, 95% CI=1.72–1.94) and panic disorder (all exposed patients, hazard ratio=1.97, 95% CI=1.60–2.43; exposed patients with an indication for prescription, hazard ratio=1.45, 95% CI=1.15–1.85).
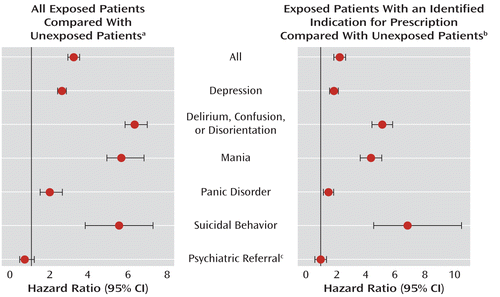
FIGURE 1. Association Between First Course of Glucocorticoids and Risk of Neuropsychiatric Outcomes in Exposed Compared With Unexposed Patients
a Model adjusted for sex, age, and past history of neuropsychiatric disorders. Unexposed patients (N=1,224,984) were a random sample of patients who did not receive glucocorticoid prescriptions, frequency-matched for age and sex with the exposed group (N=372,696).
b Model adjusted for sex, age, underlying medical disease, and past history of neuropsychiatric disorders. Unexposed patients (N=660,776) were a random sample of patients who did not receive glucocorticoid prescriptions but who had diagnoses of the same underlying medical diseases as the exposed patients, frequency-matched for age, sex, and underlying medical disorder with the exposed group (N=261,272).
c Record of psychiatric referral without any details of the symptoms experienced.
Sensitivity analysis excluding patients for whom medication was used to determine diagnoses of depression, mania, and delirium/confusion/disorientation revealed qualitatively similar findings. The adjusted hazard ratios were 1.39 (95% CI=1.29–1.51) for depression, 3.94 (95% CI=3.52–4.44) for delirium/confusion/disorientation, and 3.48 (95% CI=2.96–4.09) for mania when exposed patients with an identified indication for prescription were compared with unexposed patients with the same underlying medical disease.
Risk Factors
Larger daily doses of glucocorticoids and a prior history of neuropsychiatric disorders were associated with a greater risk of all incident outcomes, whereas prior treatment with glucocorticoids was associated with a lower risk (Table 2). Women were at higher risk of depression and at lower risk of mania and delirium/confusion/disorientation. The risk of depression, mania, and delirium/confusion/disorientation rose with age, but the reverse was observed for suicidal behavior and panic disorder. When considering only patients who received multiple glucocorticoid courses, a previous history of a glucocorticoid-induced neuropsychiatric disorder was associated with a greater risk of having a recurrence of the same disorder after a subsequent course (hazard ratio=1.32, 95% CI=1.00–1.74).
| Depressiona (N=3,424) | Delirium, Confusion, or Disorientationa (N=1,537) | Maniaa (N=599) | Panic Disorderb (N=109) | Suicidal Behaviorc (N=51) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk Factor | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI |
| Sex (women versus men) | 1.26 | 1.17–1.35 | 0.84 | 0.76–0.93 | 0.73 | 0.62–0.86 | 1.13 | 0.77–1.68 | 1.23 | 0.70–2.19 |
| Age (years) | ||||||||||
| 18–30 (reference) | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — |
| 31–50 | 1.14 | 0.97–1.35 | 2.06 | 1.20–3.56 | 1.47 | 0.85–2.53 | 0.58 | 0.30–1.09 | 0.89 | 0.41–1.91 |
| 51–70 | 1.27 | 1.09–1.49 | 6.39 | 3.82–10.68 | 2.55 | 1.53–4.24 | 0.53 | 0.29–0.97 | 0.22 | 0.08–0.54 |
| ≥71 | 1.24 | 1.06–1.46 | 10.30 | 6.17–17.20 | 2.93 | 1.76–4.88 | 0.39 | 0.21–0.74 | 0.27 | 0.11–0.65 |
| Initial daily dosed (mg) | ||||||||||
| ≤10 | 1.00 | — | 1.00 | — | 1.00 | — | 1.00 | — | ||
| 11–20 | 1.08 | 0.98–1.19 | 1.85 | 1.57–2.17 | 1.17 | 0.90–1.53 | 3.42 | 1.53–7.67 | ||
| 21–40 | 1.04 | 0.95–1.14 | 1.34 | 1.15–1.56 | 1.22 | 0.97–1.54 | 4.01 | 1.92–8.40 | ||
| 41–60 | 1.57 | 1.35–1.82 | 4.88 | 4.07–5.86 | 3.24 | 2.39–4.38 | 5.08 | 1.84–14.05 | ||
| ≥61 | 2.03 | 1.72–2.39 | 6.01 | 4.97–7.28 | 5.18 | 3.85–6.97 | 2.44 | 0.52–11.49 | ||
| Past history of glucocorticoid therapy | 0.67 | 0.63–0.73 | 0.34 | 0.29–0.39 | 0.47 | 0.38–0.57 | ||||
| Past medical history | ||||||||||
| Depression | 1.39 | 1.29–1.50 | 0.64 | 0.56–0.74 | 0.83 | 0.68–1.03 | ||||
| Delirium, confusion, or disorientation | 0.56 | 0.43–0.72 | 0.92 | 0.69–1.21 | 1.19 | 0.80–1.79 | ||||
| Mania | 0.80 | 0.63–1.01 | 0.59 | 0.37–0.94 | 1.82 | 1.19–2.77 | ||||
| Panic disorder | 0.99 | 0.83–1.19 | 0.84 | 0.58–1.20 | 0.68 | 0.38–1.21 | 4.64 | 2.75–7.83 | ||
| Suicide attempt | 1.30 | 1.02–1.65 | 1.09 | 0.62–1.94 | 0.88 | 0.39–1.98 | 9.87 | 4.74–20.53 | ||
| Underlying disease | ||||||||||
| Lower respiratory tract infection | 1.00 | — | 1.00 | — | 1.00 | — | ||||
| Asthma | 0.75 | 0.66–0.86 | 0.41 | 0.31–0.55 | 0.60 | 0.42–0.86 | ||||
| Chronic obstructive pulmonary disease | 1.06 | 0.91–1.23 | 0.59 | 0.45–0.79 | 0.77 | 0.52–1.14 | ||||
| Polymyalgia rheumatica/giant cell arteritis | 0.60 | 0.52–0.69 | 0.26 | 0.20–0.34 | 0.41 | 0.28–0.61 | ||||
| Other | 1.02 | 0.93–1.11 | 1.22 | 1.07–1.38 | 1.05 | 0.86–1.29 | ||||
TABLE 2. Risk Factors for Neuropsychiatric Disorders in the Glucocorticoid-Exposed Population
Discussion
To our knowledge, this is the largest study to date examining the effects of glucocorticoid treatment on adverse neuropsychiatric outcomes. We found a high incidence of neuropsychiatric adverse events in the first 3 months of treatment with glucocorticoids. Overall, the incidence was 15.7 per 100 person-years at risk, and for patients on their first course of glucocorticoids, it was 22.2 per 100 person-years at risk. Despite a low incidence, the risk of suicide or suicide attempt increased up to sevenfold in patients treated with glucocorticoids after adjustment for known confounders, including the underlying medical disease. Depression was more common than mania, delirium/confusion/disorientation, or panic disorder, although the increase in risk was most marked for delirium/confusion/disorientation and mania. The risk factors differed for each outcome except for higher daily doses of glucocorticoids, which consistently remained a risk factor.
At any given time in the United Kingdom, about 1% of the general adult population receives oral glucocorticoids (26–28). Psychiatric symptoms are among the most distressing adverse events experienced by this patient group (29), and yet only limited data are available on their incidence and predisposing risk factors (8, 30). A 1983 literature review (13) reported a weighted average incidence of 5.7% for severe neuropsychiatric adverse events in people treated with glucocorticoids, but the interval over which these occurred was not specified. We observed a twofold increase in risk of depression in people exposed to glucocorticoids, which is consistent with results reported a decade ago by Patten (31). The study, based on a general population sample, showed that the prevalence of major depression was approximately three times as high in patients treated with glucocorticoids as in those not exposed to glucocorticoids. Although the risk factors were different for the outcomes examined, some distinct patterns associated with age and sex were observed. First, women were more likely to develop depression after receiving a glucocorticoid prescription, and men were more likely to develop mania or delirium/confusion/disorientation. Second, the risk of depression, mania, and delirium/confusion/disorientation increased with age, while the risk of panic disorder and suicide or suicide attempt was more pronounced in younger people. We found that the initial daily dose of glucocorticoid was predictive of neuropsychiatric adverse disorders, which is consistent with previous research (13, 14). In the Boston Collaborative Drug Study, severe psychiatric symptoms were reported in 1.3% of patients treated with less than 40 mg/day of prednisone, compared with 18.4% of those receiving more than 80 mg/day (14). Contrary to findings from previous research (32–34), we found that a prior history of neuropsychiatric illness was associated with an increased risk of developing further neuropsychiatric disorders after glucocorticoid therapy. Our results indicate that a previous vulnerability to a specific neuropsychiatric disorder places a patient at risk of developing the same disorder when next exposed to glucocorticoids, hence reducing their chances of developing a new neuropsychiatric problem. This would explain why a past history of a given neuropsychiatric disorder may seem to protect against other neuropsychiatric disorders. We also found that previous treatment with glucocorticoids was associated with a lower risk of developing a neuropsychiatric outcome. The most likely explanation for this finding is that patients known to have had a psychiatric adverse event after starting treatment with a glucocorticoid may be less likely to receive a prescription for the drug again. Finally, we found that people with asthma and those with polymyalgia rheumatica or giant cell arteritis may be less likely to develop neuropsychiatric disorders and that the risk of panic attack decreased at the highest glucocorticoid dosage. There is no clear explanation for the lower risk of neuropsychiatric outcomes in people with polymyalgia rheumatica or giant cell arteritis. People with asthma are chronically exposed to low doses of glucocorticoids (e.g., inhaled glucocorticoids), and it is possible that this protects them when they are exposed to higher dosages. The most likely explanation for the decreasing risk of panic disorder in people exposed to the highest dosages of glucocorticoids is that our study was underpowered to examine this effect.
Our study has several strengths. One of them is its use of a very large population of patients with a wide range of conditions affecting both sexes and all age groups. The large number of incident neuropsychiatric outcomes allowed us to conduct a statistically powerful study. We were able to assess incidence risk for suicide or suicide attempt and to examine each subtype of severe neuropsychiatric disorder. The study had some limitations as well. For example, it is known that in the THIN database, symptoms (e.g., low mood) are frequently recorded instead of a definite illness for some common mental illnesses such as depression (35). Nevertheless, our findings are relevant to clinical practice, since we included diagnostic labels used in general practice rather than strict DSM-IV or ICD-10 classifications. Second, it is likely that the most severe forms of the underlying medical diseases may require treatment with glucocorticoids, and it can be argued that the observed neuropsychiatric outcomes may be associated with severity of the medical illness rather than the glucocorticoid treatment. However, the effects of glucocorticoids could be separated from those of disease severity only in a randomized controlled trial that included people with similar levels of disease severity receiving either glucocorticoids or placebo—an ethically unacceptable design. Third, we chose to use diagnoses and prescribed drugs (i.e., antidepressants, antipsychotics, and antimanic agents) to define cases of depression, delirium/confusion/disorientation, and mania. However, in some cases, medications may have been prescribed for other conditions (e.g., antidepressants for neuropathies). It is possible that this approach led to a slight overestimation of incidence rates of neuropsychiatric disorders. However, we also believe that these rates would have been underestimated if medication prescriptions had not been taken into account. Indeed, in our view, physicians are less likely to record a diagnosis of a neuropsychiatric illness they attribute to the glucocorticoid exposure and for which they know that the symptoms will improve once glucocorticoids are stopped or reduced. This hypothesis is supported by the fact that the hazard ratios were lower when we restricted our analysis to neuropsychiatric diagnoses only and excluded patients treated with relevant psychotropic drugs but without a relevant diagnosis. This could mean that patients exposed to glucocorticoids were more likely than unexposed patients to receive prescriptions for psychotropic medications without entry of a concomitant neuropsychiatric diagnosis in the record since the clinician would have taken for granted that concomitant neuropsychiatric dysfunction is a possible effect of glucocorticoid therapy. Lastly, suicide and suicide attempt were pooled because of the low number of completed suicides. However, it is noteworthy that they may represent two different phenomena that may or may not be related.
People treated with glucocorticoids have a twofold higher risk of developing depression, a four- to fivefold higher risk of developing mania or delirium/confusion/disorientation, and nearly a sevenfold higher risk of committing or attempting suicide compared with people unexposed to glucocorticoids. Physicians must exercise caution in administering these drugs, in particular when the reasons for prescribing are not in accordance with the consensual clinical recommendations (27). In instances where it is essential to prescribe a glucocorticoid, patients and their families should be informed about the possibility of these severe adverse events (36). Close monitoring of relevant neuropsychiatric adverse events must be undertaken by patients, their families, and their treating physicians so that cessation of the drug or reduction of the dosage is considered for those who develop such adverse reactions. The effectiveness of such monitoring in primary care requires evaluation.
1. : Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 2002; 96:23–43Crossref, Medline, Google Scholar
2. : Hypothalamic-pituitary-adrenal axis and bipolar disorder. Psychiatr Clin North Am 2005; 28:469–480Crossref, Medline, Google Scholar
3. : Glucocorticoid receptor polymorphisms in major depression. Ann NY Acad Sci 2009; 1179:216–228Crossref, Medline, Google Scholar
4. : Glucocorticoids: mood, memory, and mechanisms. Ann NY Acad Sci 2009; 1179:19–40Crossref, Medline, Google Scholar
5. : Effects of glucocorticoids on mood, memory, and the hippocampus: treatment and preventive therapy. Ann NY Acad Sci 2009; 1179:41–55Crossref, Medline, Google Scholar
6. : Diagnosis and complications of Cushing's syndrome: a consensus statement. J Clin Endocrinol Metab 2003; 88:5593–5602Crossref, Medline, Google Scholar
7. : Psychiatric aspects of Cushing's syndrome. QJM 1996; 89:543–551Crossref, Medline, Google Scholar
8. : Corticosteroid-induced adverse psychiatric effects: incidence, diagnosis, and management. Drug Saf 2000; 22:111–122Crossref, Medline, Google Scholar
9. : Depression, somatization, and steroid use in chronic obstructive pulmonary disease. Int J Nurs Stud 1989; 26:281–286Crossref, Medline, Google Scholar
10. : Assessment of mood states in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol 2004; 92:500–505Crossref, Medline, Google Scholar
11. : Corticosteroid-induced psychotic and mood disorders: diagnosis defined by DSM-IV and clinical pictures. Psychosomatics 2001; 42:461–466Crossref, Medline, Google Scholar
12. : Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum 2006; 55:420–426Crossref, Medline, Google Scholar
13. : Steroid-induced psychiatric syndromes: a report of 14 cases and a review of the literature. J Affect Disord 1983; 5:319–332Crossref, Medline, Google Scholar
14. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther 1972;13:694–698Crossref, Medline, Google Scholar
15. : Acute psychosis in systemic lupus erythematosus. Rheumatol Int 2008; 28:237–243Crossref, Medline, Google Scholar
16. : Recurrent cases of corticosteroid-induced mood disorder: clinical characteristics and treatment. J Clin Psychiatry 2000; 61:261–267Crossref, Medline, Google Scholar
17. : The VAMP research multi-purpose database in the UK. J Clin Epidemiol 1995; 48:431–443Crossref, Medline, Google Scholar
18.
19. : Feasibility study and methodology to create a quality-evaluated database of primary care data. Inform Prim Care 2004; 12:171–177Crossref, Medline, Google Scholar
20. : Validation studies of The Health Improvement Network (THIN) database for pharmacoepidemiology research. Pharmacoepidemiol Drug Saf 2007; 16:393–401Crossref, Medline, Google Scholar
21. : Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol 2010; 69:4–14Crossref, Medline, Google Scholar
22. : The importance of defining periods of complete mortality reporting for research using automated data from primary care. Pharmacoepidemiol Drug Saf 2009; 18:76–83Crossref, Medline, Google Scholar
23. : The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf 2005; 14:443–451Crossref, Medline, Google Scholar
24. : The Read clinical classification. BMJ 1990; 300:1092Crossref, Medline, Google Scholar
25. : Creating medical and drug code lists to identify cases in primary care databases. Pharmacoepidemiol Drug Saf 2009; 18:704–707Crossref, Medline, Google Scholar
26. : Use of oral corticosteroids in the community and the prevention of secondary osteoporosis: a cross sectional study. BMJ 1996; 313:344–346Crossref, Medline, Google Scholar
27. : Prevalence of long-term oral glucocorticoid prescriptions in the UK over the past 20 years. Rheumatology (Oxford) 2011; 50:1982–1990Crossref, Medline, Google Scholar
28. : Use of oral corticosteroids in the United Kingdom. QJM 2000; 93:105–111Crossref, Medline, Google Scholar
29. : Corticosteroid-induced clinical adverse events: frequency, risk factors, and patient's opinion. Br J Dermatol 2007; 157:142–148Crossref, Medline, Google Scholar
30. : Psychiatric adverse effects of corticosteroids. Mayo Clin Proc 2006; 81:1361–1367Crossref, Medline, Google Scholar
31. : Exogenous corticosteroids and major depression in the general population. J Psychosom Res 2000; 49:447–449Crossref, Medline, Google Scholar
32. : Mood changes during prednisone bursts in outpatients with asthma. J Clin Psychopharmacol 2002; 22:55–61Crossref, Medline, Google Scholar
33. : Psychic effects of ACTH and cortisone. Psychosom Med 1953; 15:589–612Crossref, Medline, Google Scholar
34. : Presentation of the steroid psychoses. J Nerv Ment Dis 1979; 167:229–236Crossref, Medline, Google Scholar
35. : Recent trends in the incidence of recorded depression in primary care. Br J Psychiatry 2009; 195:520–524Crossref, Medline, Google Scholar
36. : Exogenous corticosteroid effects on mood and cognition: case presentations. Int J Psychosom 1990; 37:57–61Medline, Google Scholar



