A Systematic Review of Treatments for Refractory Depression in Older People
Abstract
Objective:
The authors systematically reviewed the management of treatment-refractory depression in older people (defined as age 55 or older).
Method:
The authors conducted an electronic database search and reviewed the 14 articles that fit predetermined criteria. Refractory depression was defined as failure to respond to at least one course of treatment for depression during the current illness episode. The authors rated the validity of studies using a standard checklist and calculated the pooled proportion of response to any treatment reported by at least three studies.
Results:
All the studies that met inclusion criteria investigated pharmacological treatment. Most were open-label studies, and the authors found no double-blind randomized placebo-controlled trials. The overall response rate for all active treatments investigated was 52% (95% CI=42–62; N=381). Only lithium augmentation was assessed in more than two trials, and the response rate was 42% (95% CI=21–65; N=57). Only two studies included comparison groups receiving no additional treatment, and none of the participants in these groups responded. In single randomized studies, extended-release venlafaxine was more efficacious than paroxetine, lithium augmentation more than phenelzine, and selegiline more than placebo.
Conclusions:
Half of the participants responded to pharmacological treatments, indicating the importance of managing treatment-refractory depression actively in older people. The only treatment for which there was replicated evidence was lithium augmentation. Double-blind randomized controlled trials for management of treatment-refractory depression in older people, encompassing pharmacological and nonpharmacological therapies and populations that reflect the levels of physical and cognitive impairment present in the general older population with depression, are needed.
By 2020, depression will be second only to heart disease as a cause of global disability and a major public health problem in older people (1). About 30% of people with depression do not respond to antidepressants at the recommended dosage and can be described as having treatment-refractory depression (2). The prevalence of treatment-refractory depression varies according to how it is defined and the population studied. Best estimates indicate that around 3% of the general population have depression that has failed to respond to one adequate trial of an antidepressant (3). The World Health Organization Primary Care Study found that 60% of primary care clinic attendees treated with antidepressant medication still met criteria for depression 1 year later (4).
Similar efficacy rates for antidepressant and psychological therapies have been reported in older adults and those under the age of 60 (5). Despite this, depression is often missed, ignored, or inadequately managed in older adults, sometimes because of the belief that depression is an inevitable part of aging or that treatment may be risky or ineffective (6). A recent systematic review found 17 randomized controlled trials of interventions for treatment-refractory depression in adults ages 18–75 years old, but they included few older patients, and none of the studies analyzed older participants separately (2). Similarly, a review of psychological treatments for resistant depression found 12 studies, all in younger adult populations (7). While the evidence base for treatment-refractory depression in general adult populations has been substantially improved by the publication of findings from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, and their incorporation into guidelines (8), there are no comparable evidence-based guidelines for older people. There are several reasons why the optimal treatment of depression in older people may differ from that for younger populations and thus requires specific guidelines. Higher rates of physical and cognitive comorbidity in older adults, their differing social circumstances, the greater likelihood of polypharmacy, and age-related pharmacodynamic and pharmacokinetic changes all suggest that this population should be considered separately. With age there is also increased susceptibility to side effects. In particular, changes reflected in MRI-identified hyperintensities may make older patients with depression more susceptible to the adverse cognitive effects of antidepressants (9).
The impact of polypharmacy, medical comorbidities, a greater vulnerability to side effects, and altered rates of metabolism must all be taken into account when considering treatment options for older people. In this study, our objective was to systematically review all trials evaluating pharmacological, physical, and psychological interventions for treatment-refractory depression in older people. This review was part of a series carried out by the Old Age Task Force of the World Federation of Biological Psychiatry to synthesize current evidence critical to the practice of old-age psychiatry (10, 11).
Method
We searched PubMed, Web of Science, and the Cochrane database of systematic reviews through August 2010. We used the following keywords: resistant depression treatment or trial, refractory depression treatment or trial, and sequential treatment or trial. We searched the references of all included articles.
We included primary research evaluating a treatment for refractory depression in older people (age 55 and up). We defined refractory depression as depression that failed to respond to at least one adequate treatment for depression during this illness episode in an entire cohort or a separately analyzed subgroup.
We excluded studies that also included people who had not previously completed an adequate trial of an antidepressant (e.g., because of side effects), studies that included people who had responded but had relapsed early, and studies whose data did not enable us to report results for nonresponders to an adequate trial separately. We excluded single case reports, dissertations, and meeting abstracts.
Data Extraction
Two of three of the authors (C.C., C.K., and G.L.) independently evaluated the validity of the studies using the Centre for Evidence Based Medicine (CEBM) randomized controlled trial evaluation criteria (http://www.cebm.net/index.aspx?o=1913):
1. Was the assignment of patients to treatments randomized?
2. Were the groups similar at the start of the trial?
3. Aside from the allocated treatment, were groups treated equally?
4. Were all patients who entered the trial accounted for?
5. Were they analyzed in the groups to which they were randomized?
6. Were measures objective or were the patients and clinicians kept “blind” to which treatment was being received?
Disagreements were resolved by consensus among the authors (C.C., C.K., and G.L.).
Data Analysis
We used the StatsDirect software package, version 2.6.6, to analyze data (12). We reported outcomes dichotomously as the proportion who responded to the treatment (or placebo or control where appropriate). Response can be defined as below a threshold (e.g., <10 on the Hamilton Depression Rating Scale [HAM-D]) or a reduction of at least 50% in depression score; in this review, response was defined differently by each trial. See definitions in Table 1.
| Validity Questionsb | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Main Inclusion Criteriaa | Trial Length | Treatment Groups | Response Definition | 1 | 2 | 3 | 4 | 5 | 6 | Total |
| Alexopoulos et al. (13) | Age 55+; DSM-IV criteria and HAM-D score 20+; MMSE score>23; at least one documented treatment failure and failure to achieve ≥50% reduction in HAM-D with 4–6 weeks of citalopram (20–40 mg) | 4–6 weeks | Risperidone (0.25–1 mg) and citalopram (20–40 mg) (N=93) | HAM-D≤7 or CGI score of 1 or 2 | N | X | X | Y | X | N | 1 |
| Dew et al. (14) | Age 70+; DSM-IV nonpsychotic depression and HAM-D score ≥15; MMSE score ≥15; failure to respond to 8 weeks of paroxetine and interpersonal psychotherapy | ≤55 weeks | Augmentation with bupropion (first line), nortriptyline, or lithium (N=48) | HAM-D≤10 for 3 weeks | N | X | X | Y | X | N | 1 |
| Whyte et al. (15) | Same as Dew et al. | 12 weeks | Extended-release venlafaxine (N=12) | HAM-D≤10 for 3 weeks | N | X | X | Y | X | N | 1 |
| Finch and Katona (16) | Age 65+; ICD-9 depression; CAPE I/O score ≥9; no previous specialist care for index episode; failure to respond to an adequate course of a tricyclic antidepressant | 3–20 months | Tricyclic antidepressant and lithium (N=9); tricyclic antidepressant (N=9) | Not defined | N | N | N | Y | X | N | 1 |
| Flint and Rifat (17) | Age 60+ without dementia; DSM-III-R nonpsychotic depression and HAM-D score ≥16; failure to respond or intolerance to 6 weeks of nortriptyline and 2 weeks of lithium augmentation | 6 weeks | Phenelzine (N=15) | HAM-D≤10 | N | X | X | Y | X | N | 1 |
| Flint and Rifat (18) | DSM-III-R psychotic depression and HAM-D score ≥16; refusal of ECT and failure to respond to 6 weeks of nortriptyline and perphenazine | 2 weeks | Lithium augmentation (N=6) | HAM-D≤10; no hallucinations or delusions | N | X | X | Y | X | N | 1 |
| Karp et al. (19) | Age 65+; MMSE score ≥18; DSM-IV nonpsychotic depression and HAM-D score ≥15; HAM-D score ≥11 after escitalopram, 10–20 mg for 8 weeks | 16 weeks | Duloxetine titrated to 60–120 mg (N=40) | HAM-D≤10 for 3 weeks | N | X | X | Y | X | N | 1 |
| Kok et al. (20) | Age 60+; MMSE score ≥15; DSM-IV unipolar depression and MADRS score≤20; failure to respond to adequate trial of a tricyclic antidepressant or extended-release venlafaxine | 6 weeks | Lithium augmentation (N=15); phenelzine, 30–60 mg (N=14) | ≥50% reduction in MADRS score | Y | Y | N | Y | Y | N | 4 |
| Kok et al. (21) | Age 60+; MMSE score ≥15; DSM-IV unipolar depression and MADRS score≤20; failure to respond to adequate trial of a tricyclic antidepressant or extended-release venlafaxine | 3 years | Lithium augmentation (N=22); tricyclic antidepressant (N=15); phenelzine (N=8); ECT (N=5); SSRI (N=1); overall (N=32) | ≥50% reduction in MADRS score | N | X | Y | Y | Y | N | 3 |
| Lafferman et al. (22) | Age 61+; clinical diagnosis of depression; failure to respond to at least a 4-week trial of an antidepressant at adequate dosage | Case series | Lithium augmentation (N=14) | Full reversal of symptoms | N | X | X | Y | X | N | 1 |
| Mazeh et al. (23) | Age 65+ without dementia; DSM-IV major depression and HAM-D-21 score ≥18; failure to respond to 8 weeks of fluoxetine, 20 mg, and 8 weeks of an antidepressant (tricyclic or heterocyclic) at adequate dosage | 8 weeks | 10–60 mg paroxetine (N=15); 75–300 mg venlafaxine (N=15) | ≥50% reduction in HAM-D score | Y | Y | Y | Y | Y | N | 5 |
| Sheffrin et al. (24) | Age 65+; MMSE score ≥18; DSM-IV nonpsychotic depression and HAM-D score ≥11 after trials of escitalopram, then duloxetine or venlafaxine | 12 weeks | Augmentation with aripiprazole, 2.5–15 mg(N=24) | HAM-D≤10 for 2 weeks | N | X | X | Y | X | N | 1 |
| Sunderland et al. (25) | Age 55+ without severe cognitive impairment; DSM-III-R major depression and HAM-D score ≥18; no response to two antidepressants | 3 weeks × 2 | Selegiline, 60 mg, and placebo sequentially (N=16) | HAM-D≤11 post-selegiline | Y | Y | Y | Y | Y | Y | 6 |
| Zimmer et al. (26) | Age 59+; DSM-III criteria and HAM-D score ≥18 after 4 weeks of nortriptyline | 3 weeks | Lithium and tricyclic antidepressant (N=13) | HAM-D≤10 | N | X | X | Y | X | X | 1 |
TABLE 1. Characteristics and Validity of Studies Included in Review
Where response and remission rates were reported, we included response rates. We also reported mean change in depression scores where this was the primary outcome. We performed a meta-analysis of all active treatments. We calculated the I2 statistic, which estimates the percentage of variation across studies that is due to heterogeneity rather than chance (27). We used this to decide whether to use fixed- or random-effects models. We performed a funnel plot to examine publication bias and computed the Egger statistic as an indicator of bias (Figure 1). This tests for asymmetry of the funnel plot, with a significant result indicating significant asymmetry (28). We calculated the pooled proportion (and 95% confidence intervals [95% CIs]) for any treatment reported by at least three studies separately.
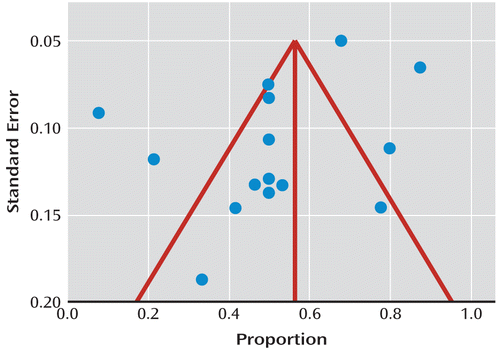
FIGURE 1. Funnel Plot of Included Studies Examining Publication Biasa
a There is no significant asymmetry, indicating that no publication bias was detected. Red lines indicate symmetrical distribution.
Results
Ten of the 198 studies identified by our electronic search met our criteria. We rejected 99 articles because findings for older people were not reported separately, 23 because the participants did not all meet our definition for having refractory depression, 44 that did not report primary research, and 22 that did not measure depression. We also included four additional articles identified as potentially relevant from the references of included articles. An updated search carried out in August 2010 found 63 additional studies, none of which was eventually included. We were finally able to include 14 articles (describing 13 studies) in our full analysis.
Description of Included Studies
The studies were supported by the governments of the United States (14, 15, 19, 24) and the Netherlands (20, 21), university endowments (19, 24), Forest Pharmaceuticals, Eli Lilly (19), Janssen Pharmaceutica (13), Wyeth (21), Glaxo-SmithKline (15), and Bristol-Myers Squibb (24). Seven studies did not name a funding source (16–18, 22, 23, 25, 26).
Mean ages of the participants included in the studies ranged from 65.6 years (25) to 77.1 years (14). Five studies reported the relationship between response to treatment and age; in four there was no association with the likelihood of responding (17, 19, 21) or time to recovery (14). In the trial comparing selegiline to placebo, responders were older on average than nonresponders (25). The studies variously defined resistant depression as failure to respond to at least one antidepressant (16, 19–22, 26), an antidepressant and a psychological therapy (14), an antidepressant with augmentation (17), or at least two antidepressants (13, 23–25). Many of the participants had also failed to respond to ECT, but none of the studies specified ECT treatment response in its inclusion criteria.
Three studies included only participants with nonpsychotic depression (14, 17, 24), one included only people with psychotic depression (18), and the remainder included people with psychotic or nonpsychotic depression. Among those studies that included people with psychotic depression, two included antipsychotic medication in the trial treatment regimen (13, 18), three were naturalistic studies of lithium augmentation in which participants did not receive antipsychotics during the study period (16, 22, 26), two permitted haloperidol and risperidone to be prescribed as judged necessary by clinicians (20, 21) (in one, 17/29 patients received antipsychotics [20], while the other did not specify), one explicitly excluded antipsychotics (25), and one did not specify a policy regarding use of antipsychotics and did not report that they were used (23). Table 1 and Figure 2 give details of the included studies.
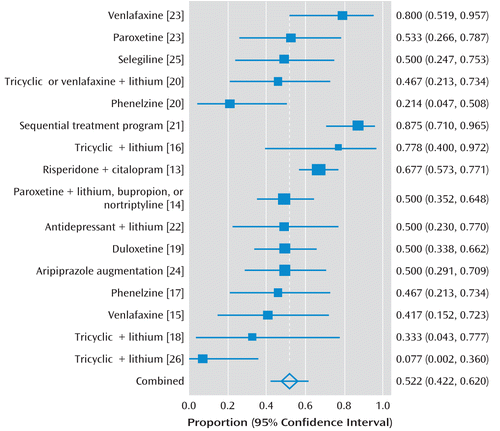
FIGURE 2. Proportion Meta-Analysis Plot for Included Studies With Weighted Response Rates and 95% Confidence Intervalsa
a Numbers in square brackets indicate the studies referenced.
Most studies recruited convenience samples not designed to be epidemiologically representative of an underlying population. Only two gave information about people who were referred but did not participate. In both of these, just over one-third of those potentially eligible participated (35.7% and 36.3% [17, 20]). A third study reported that 9/34 potentially eligible patients took part (16). All the studies excluded either people who had more severe cognitive impairment or those who were known to have a diagnosis of dementia. Therefore, while some of the participants would most likely have met criteria for mild or moderate dementia, those with more severe dementia are unlikely to have been included. Those with contraindications to the study medications were also excluded from the studies. Most of the studies excluded acute uncontrolled medical illness but allowed stable chronic conditions.
Validity
Only three studies were randomized, and we rated these as being of higher quality. One of these was not blinded (20), one was a single-blind study (23), and one was a double-blind study (25). One was placebo controlled, and the other two compared two active treatments (20, 23). The other 10 articles described open-label studies. Our definition of treatment-refractory depression was very inclusive, and many authorities would cite failure to respond to adequate trials of at least two antidepressants as a necessary criterion in any definition of treatment resistance. We therefore judged the two randomized controlled trials that used this definition as the most valid studies (23, 25).
Efficacy of Treatments
We did not find evidence of significant publication bias (Egger statistic=–2.6, p=0.11; Figure 2). The I2 statistic was 72% (95% CI=50–82). This indicated a moderately high degree of heterogeneity between the studies, so we used random-effects models for all meta-analyses. The overall response rate for the 16 active treatments investigated was 52% (95% CI=42–62). When we included only the randomized studies, the overall response rate was 50% (95% CI=33–68). None of the studies included a control group that only received a placebo, although in one study none of the nine participants receiving no additional treatment recovered (16), and in a crossover trial there was no significant response during the placebo phase of the trial (25).
Randomized Studies in Participants Failing to Respond to at Least Two Antidepressants
Sunderland et al. (25) examined the effects of high-dosage selegiline (60 mg/day) in 16 participants. Selegiline is a monoamine oxidase inhibitor (MAOI), which at low dosages is selective for MAO-B but at the study dosage also inhibits MAO-A (i.e., is nonselective). This was a crossover study in which participants received placebo and selegiline for 3 weeks each in random order. No serious side effects were reported, and no side effects were more common with selegiline compared with placebo. Dosing for the eight responders was switched to a clinically available MAOI, and informal follow-up for 6 months revealed no substantial relapses. Although one of the products of selegiline metabolism is amphetamine, significant decreases in activation, mania, anxiety, and self-rated irritability with selegiline suggested that amphetamine-like effects did not occur. The study authors questioned whether high-dosage selegiline offered potential benefits over clinically available (nonselective) MAOIs, given that at higher dosages it too acts as a nonselective MAOI. They argued that it does, since it was well tolerated, with little or no anticholinergic action.
Mazeh et al. (23) compared paroxetine (10–60 mg/day; mean=26 mg/day) to venlafaxine (75–300 mg/day; mean=165 mg/day) in a randomized controlled trial. Randomization was performed by alternating between paroxetine and venlafaxine depending on the order of admission. Clinicians were free to adjust the dosage based on response and tolerance. The ward psychologist completed the Clinical Global Impressions scale (CGI), the HAM-D, and the Geriatric Depression Scale. It was not stated whether the psychologist was blind to group allocation. There was statistically significant improvement between baseline and endpoints in both groups on all three outcome measures. The mean HAM-D score decreased more with venlafaxine than with paroxetine treatment (–19.5 compared with –12.5, respectively). Both treatments were well tolerated, with no patients discontinuing because of side effects.
Randomized Studies in Participants Failing to Respond to at Least One Antidepressant
Kok et al. (20) compared lithium augmentation to phenelzine in patients who had not responded to extended-release venlafaxine or a tricyclic antidepressant. They used computerized block randomization, with sealed opaque envelopes to conceal allocation. Patients received lithium augmentation (titrated to serum levels of 0.6–1.2 mmol/liter) in addition to the antidepressant to which they had not responded at the same dosage. This was either venlafaxine (N=3) or nortriptyline (N=12). Fourteen participants had physical illnesses rated as moderate or severe, and another 11 participants had at least some disability. Patients who received lithium augmentation were more likely to achieve remission (33.3%, N=5) compared with those receiving phenelzine (0%) based on scores from the Montgomery-Åsberg Depression Rating Scale and the HAM-D. Patients receiving lithium augmentation were also more likely to achieve response (46.7%, N=7) compared with those receiving phenelzine (7.1%, N=1). All but one patient experienced at least one adverse event, usually mild or moderate in intensity. The most common side effects were tremor (12/15 on lithium, 3/14 on phenelzine), dry mouth, insomnia, and weakness/fatigue. The only side effects for which there was a significant difference between the groups were tremor and memory impairment (present in no patients on lithium and 7/14 on phenelzine). There were no withdrawals due to side effects.
Nonrandomized Studies
The response rates reported by the 10 nonrandomized studies are listed in Figure 2.
Antidepressants.
Karp et al. (19) investigated the effectiveness of duloxetine, 60–120 mg/day (median=90 mg/day), in an open-label study. The authors noted that this dosage range is higher than that recommended by the manufacturer (60 mg/day). The median time to response was 12.0 weeks (95% CI=8.4–14.6). Five of the 40 participants (12.5%) stopped taking the drug because of adverse events such as dry mouth, bloating, sedation, elevated transaminase levels, sweating, diarrhea, and mania. The latter may have been due to concomitant administration of opioid analgesics. Whyte et al. (15) investigated venlafaxine in a single trial that was nested within a larger trial of augmenting agents (14). Some participants in the venlafaxine trial were transferred from the trial of lithium augmentation because of nonresponse.
Sequential treatment protocols.
Kok et al. (21) reported the highest response rates of all the studies we found in a trial of a sequential treatment strategy. The trial population overlapped with another randomized controlled trial (20). Patients received treatments in different orders, with most needing more than one. Collectively they received 51 treatments: lithium augmentation (N=22), phenelzine (N=8), nortriptyline or clomipramine (N=13, N=2), ECT (N=5), and a selective serotonin reuptake inhibitor (N=1). All treatment steps had a minimum duration of 6 weeks, if tolerated. Ten patients experienced side effects, and in four cases treatment was stopped. This included two hip fractures believed to have been caused by hypotension secondary to phenelzine and lithium, one case of side effects with ECT, and one case of side effects with phenelzine. The main analyses were a comparison of pre- and posttreatment Montgomery-Åsberg Depression Rating Scale scores. There were statistically significant reductions in the lithium augmentation and tricyclic groups.
Flint et al. (17) also conducted an open trial of a sequential treatment protocol. Fifteen patients were treated with phenelzine, titrated to a maximum dosage of 30 mg twice a day, and seven patients responded. We could not include data from subsequent treatment steps (ECT and fluoxetine) because noncompleters and nonresponders were not analyzed separately.
Augmentation.
Lithium augmentation was investigated in five nonrandomized trials (16, 18, 21, 22, 26). The overall response rate for lithium augmentation was 42% (95% CI=21–65; N=57). We included relevant data from the sequential treatment trial discussed above in this calculation (21). Two studies each reported two patients who discontinued lithium because of poor tolerability (16, 21).
Other augmentation strategies also appeared to be effective in nonrandomized trials. Alexopoulos et al. (13) reported on open-label addition of 0.25–1 mg/day of risperidone to 20–40 mg/day of citalopram. Psychotic features were present at baseline in only 4% of participants (N=4) in this trial. Two patients discontinued risperidone because of adverse events. Dew et al. (14) augmented antidepressant therapy with sustained-release bupropion (150–400 mg/day), or if this was contraindicated or ineffective, nortriptyline or lithium was selected according to a standard protocol. Most patients received more than one of the drugs consecutively. The dosages of nortriptyline and lithium were titrated as tolerated to maintain plasma levels of 80–120 ng/liter and 0.5–0.7 meq/liter, respectively. Sheffrin et al. (24) investigated the use of aripiprazole to augment antidepressant therapy, commenced at 2.5 mg/day and titrated to a maximum of 15 mg/day. The mean daily dosage was 9.0 mg (SD=4.5). Patients who had responded partially (defined as having a HAM-D score between 11 and 14) as well as those who had not responded (HAM-D score ≥15) were included. The mean HAM-D score at baseline was 17.9 (SD=5.7), and mean scores were significantly lower at the trial endpoint (mean=11.5, SD=3.9). Two patients dropped out of the study because of side effects (sedation and akathisia). Six patients reported akathisia, and 18 patients reported at least mild increases in restlessness that appeared to be dose dependent. Six patients gained more than 3 kg in weight.
Discussion
Given the high prevalence of treatment-refractory depression, the paucity of evidence—with only three randomized drug trials and one placebo-controlled trial—for its treatment in older people is disquieting. Most of the included studies were small open-label trials. The only placebo-controlled trial assessed an agent not currently licensed for depression. We found no studies of nonpharmacological somatic therapies (e.g., ECT, transcranial magnetic stimulation, vagal nerve stimulation) or psychological therapies that met our inclusion criteria. Treatment-refractory depression is clearly understudied, and clinicians have little to guide their treatment of individuals with this condition.
In the studies we examined, half of the older people with refractory depression responded to the active treatments, and most of the treatments were well tolerated. The only treatment for which there was evidence from more than one trial was lithium augmentation, which was an effective treatment for four out of 10 participants (although frequently with no comparator). This may be similar to a previous meta-analysis of placebo-controlled trials of lithium augmentation for treatment-refractory depression in adults of all ages, which reported a number needed to treat of 3.7 (29). In the STAR*D study, only 15.9% of participants with treatment-refractory depression responded to lithium augmentation. This was in part due to poor tolerability, which may have been related to the steep dosing regimen (450 mg for 1 week, then an increase to 900 mg) (30). The STAR*D study found that thyroid hormone augmentation was equally effective and better tolerated than lithium (30), but we did not find any trials evaluating this in older adults.
In single studies that we judged to be of higher quality, selegiline (not currently licensed for depression) and extended-release venlafaxine appeared efficacious, and both were well tolerated. Extended-release venlafaxine was more efficacious than paroxetine, and lithium augmentation was more efficacious than phenelzine in single higher-quality studies at the trial dosages.
Some limitations of this study should be mentioned. The summary response rates are mostly derived from open-label studies, so they should be considered preliminary. Definitions of response varied among studies but were almost always defined in terms of HAM-D or Montgomery-Åsberg Depression Rating Scale scores. While the trial populations probably enjoyed better health than the general population of depressed older people, rates of chronic disease were reasonably high in studies reporting it, suggesting that our results are valid. Our criteria were inclusive to ensure that we considered a broad range of evidence, but consequently there was a high degree of heterogeneity in the included studies. Trial durations ranged from 3 weeks to 3 years, and half (7/14) of the studies were less than 12 weeks in duration. This is a significant limitation as older people can take longer to respond to antidepressants, so efficacy may have been underestimated in the shorter trials. The amount of treatment previously received ranged from failure to respond to 4 weeks of antidepressant treatment to many years of documented treatment resistance, and some of the trials included people with psychotic and nonpsychotic depression. Some treatment resistance may be due to patients not taking medication rather than actual resistance to treatment, which was not measured, although failure to take medicine has ecological validity since this is what happens in clinical practice. Monitoring of drug levels and/or CYP450 genomics in future studies may clarify whether treatment-refractory depression is pharmacokinetically or pharmacodynamically mediated.
There are differing definitions of treatment resistance, and some studies suggest that it requires nonresponse to at least two treatments (31). To have used this criterion would clearly have reduced our trial base even further, and we therefore chose the more inclusive definition. Some participants may have been included in two separate studies run consecutively in the same center (e.g., Karp et al. [19] and Sheffrin et al. [24]), but the numbers of such patients were likely to be small, and as these studies included participants who did not respond to the initial therapy, any bias introduced would have been in favor of inefficacy.
Conclusions
Failure to respond to a single pharmacological treatment for late-life depression is common, but there is a paucity of data on which evidence-based treatment decisions can be made. In this meta-analysis of studies that included older individuals who had failed to respond to a single antidepressant trial, half responded to either the addition of a second agent or a change to another antidepressant. This indicates that failure to respond to a single antidepressant trial does not imply lack of response to other treatments.
The only treatment for which there was consistent evidence was lithium augmentation, and even this treatment was evaluated in nonrandomized studies and only in one small randomized study without a placebo control. All of the studies excluded people with more severe cognitive impairment, so there is currently no evidence base for the treatment of refractory depression in patients with dementia. This is worrying, given that a significant proportion of people with dementia are treated with antidepressants. High-quality randomized controlled trials comparing therapies for treatment-refractory depression in older people are urgently needed to inform the evidence base. Such studies should include populations that reflect the level of physical and cognitive impairment present in the general population of older people with depression, as well as trials of psychological therapies and ECT.
1. : Depression as a major component of public health for older adults. Prev Chronic Dis 2008; 5:A22Medline, Google Scholar
2. : Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression: systematic review. Br J Psychiatry 2002; 181:284–294Crossref, Medline, Google Scholar
3. : Prevalence and management of treatment-resistant depression. J Clin Psychiatry 2007; 68:17–25Crossref, Medline, Google Scholar
4. : The effects of detection and treatment on the outcome of major depression in primary care: a naturalistic study in 15 cities. Br J Gen Pract 1998; 48:1840–1844Medline, Google Scholar
5. : Depression in the elderly. Lancet 2005; 365:1961–1970Crossref, Medline, Google Scholar
6. : Narrowing the gap in treatment of depression. Int J Psychiatry Med 2001; 31:239–253Crossref, Medline, Google Scholar
7. : The effectiveness of psychological treatments for treatment-resistant depression: a systematic review. Acta Psychiatr Scand 2005; 111:331–340Crossref, Medline, Google Scholar
8. : Treatment of refractory depression, in The Maudsley Guidelines, 9th ed. Edited by Taylor DPaton CKerwin R. London, Informa Healthcare, 2007, pp 193–196Google Scholar
9. : Neuroanatomical substrates of depression in the elderly. J Geriatr Psychiatry Neurol 1993; 6:39–58Crossref, Medline, Google Scholar
10. ,
11. : Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affect Disord 2007; 101:75–89Crossref, Medline, Google Scholar
12.
13. : Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry 2008; 16:21–30Crossref, Medline, Google Scholar
14. : Recovery from major depression in older adults receiving augmentation of antidepressant pharmacotherapy. Am J Psychiatry 2007; 164:892–899Link, Google Scholar
15. : Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry 2004; 65:1634–1641Crossref, Medline, Google Scholar
16. : Lithium augmentation in the treatment of refractory depression in old age. Int J Geriatr Psychiatry 1989; 4:41–46Crossref, Google Scholar
17. : The effect of sequential antidepressant treatment on geriatric depression. J Affect Disord 1996; 36:95–105Crossref, Medline, Google Scholar
18. : The treatment of psychotic depression in later life: a comparison of pharmacotherapy and ECT. Int J Geriatr Psychiatry 1998; 13:23–28Crossref, Medline, Google Scholar
19. : Rescue pharmacotherapy with duloxetine for selective serotonin reuptake inhibitor nonresponders in late-life depression: outcome and tolerability. J Clin Psychiatry 2008; 69:457–463Crossref, Medline, Google Scholar
20. : Lithium augmentation compared with phenelzine in treatment-resistant depression in the elderly: an open, randomized, controlled trial. J Clin Psychiatry 2007; 68:1177–1185Crossref, Medline, Google Scholar
21. : Outcome of late-life depression after 3 years of sequential treatment. Acta Psychiatr Scand 2009; 119:274–281Crossref, Medline, Google Scholar
22. : Lithium augmentation for treatment-resistant depression in the elderly. J Geriatr Psychiatry Neurol 1988; 1:49–52Crossref, Medline, Google Scholar
23. : A randomized single-blind comparison of venlafaxine with paroxetine in elderly patients suffering from resistant depression. Int Clin Psychopharmacol 2007; 22:371–375Crossref, Medline, Google Scholar
24. : Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry 2009; 70:208–213Crossref, Medline, Google Scholar
25. : High-dose selegiline in treatment-resistant older depressive patients. Arch Gen Psychiatry 1994; 51:607–615Crossref, Medline, Google Scholar
26. : Adjunctive lithium carbonate in nortriptyline-resistant elderly depressed patients. J Clin Psychopharmacol 1991; 11:254–256Crossref, Medline, Google Scholar
27. : Measuring inconsistency in meta-analyses. Br Med J 2003; 327:557–560Crossref, Medline, Google Scholar
28. : Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315:629–634Crossref, Medline, Google Scholar
29. : Lithium augmentation in treatment-resistant depression: meta-analysis of placebo-controlled studies. J Clin Psychopharmacol 1999; 19:427–434Crossref, Medline, Google Scholar
30. ,
31. : Treatment-resistant depression: critique of current approaches. Aust N Z J Psychiatry 2008; 42:751–762Crossref, Medline, Google Scholar



