Depressive Symptoms, Health Behaviors, and Subsequent Inflammation in Patients With Coronary Heart Disease: Prospective Findings From the Heart and Soul Study
Abstract
Objective:
Depression has been associated with inflammation in patients with coronary heart disease. However, it is uncertain whether depressive symptoms lead to inflammation or vice versa.
Method:
The authors evaluated 667 outpatients with established coronary heart disease from the Heart and Soul Study. Depressive symptoms were assessed annually with the 9-item Patient Health Questionnaire. Participants were categorized as having no significant depressive symptoms (score below 10 at all interviews), depressive symptoms (score of 10 or higher) at one interview, or depressive symptoms at two or more interviews. At baseline and 5-year follow-up, fasting blood samples were collected to measure three inflammatory biomarkers: fibrinogen, interleukin-6 (IL-6), and high-sensitivity C-reactive protein (hsCRP).
Results:
Of the 667 participants, 443 had no depressive symptoms, 86 had depressive symptoms at one assessment, and 138 had depressive symptoms at two or more annual assessments. Across the three groups, greater depressive symptoms were associated with higher subsequent log-transformed levels of IL-6 and hsCRP, and the association with higher fibrinogen levels approached significance. Baseline inflammation did not predict subsequent depressive symptoms. The association of depressive symptoms with subsequent inflammation levels was eliminated after adjustment for health behaviors associated with depression—physical inactivity, smoking, and higher body mass index.
Conclusions:
Depressive symptoms predicted higher IL-6 and hsCRP levels among outpatients with coronary heart disease, but higher inflammation levels did not predict subsequent depressive symptoms. The association between depressive symptoms and inflammation was no longer significant after adjustment for health behaviors, which suggests these behaviors may mediate depressive effects.
Depression is common in patients with cardiac disease, with prevalence rates nearly three times as high as in the general population (1, 2). Depression is also associated with worse cardiac prognosis and greater mortality (3). However, there is still considerable debate regarding how depression might contribute to a worse cardiac prognosis or mortality (4). Inflammation is associated with both cardiac disease and depression and is a plausible physiological link between depression and coronary heart disease. Cross-sectional studies have demonstrated an association between depression and inflammation in healthy subjects (5–9) and in cardiac patients (10, 11), but relatively little is known about the directionality of the association (i.e., whether depression causes inflammation or vice versa).
Previous studies on the direction of the association between depression and inflammation have yielded conflicting results. Stewart and colleagues (12) examined the directionality of the relationship in otherwise healthy subjects. They found that depressive symptoms as measured with the Beck Depression Inventory-II significantly predicted interleukin-6 (IL-6) levels after 6 years of follow-up, whereas inflammation did not predict depressive symptom scores at follow-up, suggesting that depression leads to an up-regulation of IL-6 levels. However, the Whitehall study came to the opposite conclusion. In over 3,000 British civil servants followed for almost 12 years, Gimeno and colleagues found that inflammation preceded depressive symptoms, whereas depressive symptoms did not predict inflammation (13).
To our knowledge, whether depressive symptoms are the cause or result of inflammation has not been evaluated in patients with cardiovascular disease. We therefore set out to evaluate the prospective association between depressive symptoms and inflammation using repeated measurements of depressive symptoms and inflammation in patients with stable coronary heart disease. We evaluated whether depressive symptoms (assessed annually for 6 years) were associated with higher levels of subsequent inflammation and vice versa. In addition, we evaluated the role of health behaviors in mediating the association between depression and inflammation.
Method
Design and Participants
The Heart and Soul Study is an ongoing prospective cohort study of psychosocial factors and health outcomes in patients with coronary heart disease. Its methods have been described previously (14). Briefly, 1,024 outpatients with stable coronary heart disease were recruited and completed a baseline examination between September 2000 and December 2002. Following the baseline examination, patients received annual telephone calls for assessment of depressive symptoms. Between September 2005 and December 2007, 667 participants (80% of the 829 survivors) completed a 5-year follow-up examination that included measures of inflammation. The study protocol was approved by the appropriate institutional review boards, and all participants provided written informed consent.
Depressive Symptoms
Depressive symptoms were assessed annually for 6 years by using the 9-item Patient Health Questionnaire, a self-report instrument that measures the frequency of depressive symptoms corresponding to the nine DSM-IV criteria for depression (15). A paper-and-pencil version of the questionnaire was administered at the baseline examination (year 0), telephone versions were administered after 1, 2, 3, and 4 years of follow-up, and a paper-and-pencil version was again administered after 5 years of follow-up. Of the 667 participants who were examined at 5 years, 640 completed five or more interviews, 23 (3.4%) completed four interviews, three (0.4%) completed three interviews, and one (0.1%) completed two interviews.
At each assessment, participants were asked to indicate the frequency of experiencing each depressive symptom during the last 2 weeks. Each of the nine symptoms was scored as 0 (not at all), 1 (on several days), 2 (more than half the days), or 3 (nearly every day), with total scores ranging from 0 to 27 (16). The Patient Health Questionnaire has demonstrated excellent validity when compared with a mental health interview for depression in patients with coronary heart disease (17, 18). Telephone and in-person assessments yield similar results (19). As a summary measure of depressive symptoms, we calculated the mean score for each participant as the sum of the annual questionnaire scores divided by the number of interviews completed. We also created a categorical variable by defining “depressive symptoms” as a score of 10 or higher on the Patient Health Questionnaire. We used this to group the participants into three categories: depressive symptoms at two or more interviews, depressive symptoms at one interview, or no depressive symptoms. We chose these groups for analysis because further divisions would have yielded too few participants in each category.
Inflammatory Biomarkers
Fasting blood samples were obtained at baseline and after 5 years of follow-up. Levels of high-sensitivity C-reactive protein (hsCRP), IL-6, and fibrinogen were determined from plasma and serum samples. The laboratory technicians were blinded to the depression status of the participants.
A highly sensitive CRP assay was performed with a BN II nephelometer (Dade-Behring, Newark, Del.). Interassay coefficients of variation were 1.66%–5.32%. IL-6 was measured by using the Millipore Milliplex Map kit (Millipore, Billerica, Mass.), with interassay coefficients of variation from 6.3% to 11.6%. Concentrations of serum fibrinogen were determined by the Clauss assay with coefficients of variation less than 3%. We log-transformed the hsCRP and IL-6 levels because they were not normally distributed, and we verified that the transformation resulted in normal distributions. We also created three separate dichotomous variables for IL-6, hsCRP, and fibrinogen, defining a priori a high level as one in the highest quartile. For IL-6 a high level was defined as above 5.40 pg/ml, for hsCRP a high level was one above 3.48 mg/liter, and a high fibrinogen level was defined as above 420 mg/dl.
Other Assessments
We recorded self-reported age, gender, ethnicity, education, and medical history at baseline and at the 5-year examination. In addition, we assessed health behaviors known to be associated with inflammation, such as smoking status, physical activity, and body mass index (BMI) (20, 21). To assess physical activity, we asked, “Which of the following statements best describes how physically active you have been during the last month, that is, done activities such as 15–20 minutes of brisk walking, swimming, general conditioning, or recreational sports?” Participants responded by choosing one of the six following categories: not at all active, a little active (one or two times per month), fairly active (three or four times per month), quite active (one or two times per week), very active (three or four times per week), or extremely active (five or more times per week). Self-report of physical activity has been shown to be valid, accurate, and reliable (22–25). Physical inactivity was defined as “not at all active” or “a little active.” Height and weight were measured and used to calculate BMI (weight in kilograms divided by the square of height in meters). Alcohol consumption was measured with the AUDIT-C self-report questionnaire (26). Left ventricular ejection fraction was assessed by means of two-dimensional echocardiography. High-density lipoprotein (HDL) levels were measured from fasting venous blood samples, and non-HDL cholesterol was calculated as total cholesterol minus HDL cholesterol. Participants were asked to bring all of their medication bottles to the study appointment, and all current medications were recorded. Medications were categorized by using Epocrates Rx (Epocrates, San Mateo, Calif.).
Statistical Analyses
The goal of this study was to determine the directionality of the depression-inflammation relationship. We used general linear models to compare mean levels of each marker across three groups: participants who were not depressed at any interview (having a score below 10 on the Patient Health Questionnaire at all interviews), participants who reported depressive symptoms (a score of 10 or higher) at one interview, and those who reported significant depressive symptoms at two or more interviews.
To further evaluate the association between Patient Health Questionnaire score and levels of inflammation, we used multivariate analysis of variance adjusted for other patient characteristics associated with depressive symptoms (age, gender, education, race, aspirin use, history of diabetes, myocardial infarction, and congestive heart failure) and health behaviors (physical activity, smoking, and BMI). In addition, we evaluated change in inflammation over 5 years across the three subgroups. Finally, we used multivariate analysis of covariance (MANCOVA) to evaluate the effect of depressive symptoms on all three measures of inflammation as a single dependent variable, adjusted for the baseline level of inflammation. We also used MANCOVA to evaluate the effect of inflammation on subsequent depressive symptoms (as a single dependent variable), adjusted for the baseline level of depressive symptoms. Analyses were performed by using SAS 9.2 (SAS Institute, Cary, N.C.).
Results
Characteristics of Participants
As compared with the 162 patients who were alive and did not complete the 5-year follow-up examination, the 667 participants who completed the exam were older (mean age=66 years, SD=10, versus mean=64 years, SD=12; t=–2.09, df=827, p=0.04) and had fewer baseline depressive symptoms as indicated by scores on the Patient Health Questionnaire (mean=4.75, SD=5.26, versus mean=6.77, SD=5.84; t=4.29, df=827, p<0.01). Participants who completed the exam also had lower log-transformed baseline hsCRP levels (mg/liter) than those who did not complete the 5-year exam (mean=0.57, SD=1.29, versus mean=0.85, SD=1.32; t=2.48, df=795, p=0.01). Baseline levels of IL-6 and fibrinogen were similar in the patients who did and did not complete the 5-year examination (p>0.10 in both cases).
Of the 667 participants who completed the 5-year examination, 138 (21%) had depressive symptoms (i.e., a score of 10 or higher on the Patient Health Questionnaire) at two or more interviews, 86 (13%) had depressive symptoms at one interview, and 443 (66%) had no significant depressive symptoms (a score less than 10 on the Patient Health Questionnaire at all interviews). As compared to participants without significant depressive symptoms, those with depressive symptoms at any interview were younger and less likely to be male, to be white, or to have graduated from high school (Table 1). They were more likely to have a history of diabetes, myocardial infarction, or congestive heart failure and had higher BMI values. Participants with depressive symptoms were less likely to use aspirin and more likely to smoke and to be physically inactive. The three groups had similar levels of hypertension, cardiac disease severity, cardiac risk factors, and use of cardioprotective medications.
| Patients With Depressive Symptoms, Defined as Score ≥10 on Patient Health Questionnaire | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Patients With No Depressive Symptoms at Any Interview (N=443b) | Depressive Symptoms at One Interview (N=86b) | Depressive Symptoms at Two or More Interviews (N=138b) | Analysis | |||||
| Demographic characteristics | |||||||||
| Mean | SD | Mean | SD | Mean | SD | F | df | p | |
| Age (years) | 72.8 | 9.2 | 68.6 | 12.0 | 66.3 | 11.0 | 24.26 | 2, 661 | <0.01 |
| N | % | N | % | N | % | χ2 | df | p | |
| Male | 381 | 86 | 62 | 72 | 106 | 77 | 13.18 | 2 | <0.01 |
| White | 277 | 63 | 42 | 49 | 79 | 57 | 6.03 | 2 | 0.05 |
| High school graduate | 402 | 91 | 70 | 81 | 112 | 81 | 13.63 | 2 | <0.01 |
| Comorbid conditions | |||||||||
| Hypertension | 324 | 74 | 65 | 77 | 110 | 80 | 2.55 | 2 | 0.28 |
| Myocardial infarction | 205 | 47 | 53 | 63 | 70 | 51 | 7.66 | 2 | 0.02 |
| Congestive heart failure | 68 | 16 | 17 | 20 | 42 | 31 | 15.56 | 2 | <0.01 |
| Diabetes mellitus | 122 | 28 | 35 | 41 | 35 | 26 | 7.12 | 2 | 0.03 |
| Medication use | |||||||||
| Aspirin | 338 | 76 | 58 | 67 | 86 | 62 | 11.40 | 2 | <0.01 |
| Beta-blocker | 300 | 68 | 62 | 72 | 91 | 66 | 0.94 | 2 | 0.62 |
| Renin-angiotensin system inhibitor | 298 | 67 | 55 | 64 | 85 | 62 | 1.63 | 2 | 0.44 |
| Statin | 123 | 28 | 20 | 23 | 26 | 19 | 4.66 | 2 | 0.10 |
| Health behaviors | |||||||||
| Regular alcohol use | 132 | 30 | 19 | 23 | 32 | 24 | 3.24 | 2 | 0.20 |
| Current smoking | 42 | 10 | 13 | 16 | 37 | 27 | 25.81 | 2 | <0.01 |
| Physical inactivity | 140 | 32 | 41 | 49 | 82 | 60 | 38.43 | 2 | <0.01 |
| Mean | SD | Mean | SD | Mean | SD | F | df | p | |
| Body mass index (kg/m2) | 28.1 | 5.0 | 29.8 | 6.0 | 29.6 | 7.0 | 5.59 | 2, 663 | <0.01 |
| Cardiac disease severity and risk factors | |||||||||
| Left ventricular ejection fraction | 0.62 | 0.11 | 0.63 | 0.11 | 0.60 | 0.13 | 1.43 | 2, 654 | <0.01 |
| LDL cholesterol (mg/dl) | 93.7 | 34.2 | 98.0 | 28.6 | 95.0 | 32.9 | 0.62 | 2, 653 | 0.54 |
| HDL cholesterol (mg/dl) | 47.5 | 14.8 | 47.3 | 18.6 | 45.6 | 14.3 | 0.75 | 2, 663 | <0.01 |
| Non-HDL cholesterol | 115.7 | 38.9 | 124.0 | 34.1 | 120.8 | 37.0 | 2.28 | 2, 663 | 0.10 |
TABLE 1. Baseline Characteristics of 667 Patients With Coronary Heart Disease, by Depressive Symptoms Over 5 Yearsa
Presence of Depressive Symptoms as Predictor of Subsequent Inflammation
MANCOVAs evaluating the effect of depression on subsequent inflammation showed that depressive symptoms predicted subsequent inflammation in an unadjusted analysis (F=2.56, df=6, 1304, p=0.02) and after adjustment for age, gender, education, race, history of diabetes, myocardial infarction, congestive heart failure, aspirin use, and baseline levels of inflammatory markers (F=2.15, df=6, 1182, p<0.05). This association was no longer significant after adjustment for health behaviors (F=1.74, df=6, 1128, p=0.11).
Across the three groups, greater depressive symptoms were associated with higher subsequent log-transformed levels of IL-6 and hsCRP, and they were nonsignificantly associated with higher levels of fibrinogen (Table 2). Depressive symptoms remained associated with subsequent levels of IL-6 and were marginally related to levels of fibrinogen but were not associated with hsCRP, after adjustment for age, gender, education, race, history of diabetes, myocardial infarction, congestive heart failure, and aspirin use (Table 2). However, after further adjustment for health behaviors (physical activity, smoking, and BMI), depressive symptoms were no longer associated with any inflammation index. When we evaluated the multivariable-adjusted mean change in levels of inflammatory markers, depressive symptoms did not predict statistically significant changes in IL-6, hsCRP, or fibrinogen (Table 3).
| Level of Inflammatory Marker at 5-Year Follow-Up | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients With Depressive Symptoms, Defined as Score ≥10 on Patient Health Questionnaire | |||||||||
| Patients With No Depressive Symptoms at Any Interview (N=443) | Depressive Symptoms at One Interview (N=86) | Depressive Symptoms at Two or More Interviews (N=138) | Analysis | ||||||
| Inflammatory Marker and Modelb | Mean | SD/SEc | Mean | SD/SEc | Mean | SD/SEc | F | df | p |
| Interleukin-6, log-transformed (pg/ml) | |||||||||
| Unadjusted | 1.19 | 0.66 | 1.19 | 0.71 | 1.38 | 0.71 | 4.36 | 2, 658 | 0.01 |
| Model 1 | 1.13 | 0.06 | 1.34 | 0.08 | 1.51 | 0.07 | 4.46 | 2, 631 | 0.01 |
| Model 2 | 1.40 | 0.06 | 1.38 | 0.08 | 1.52 | 0.70 | 1.91 | 2, 602 | 0.15 |
| High-sensitivity C-reactive protein, log-transformed (mg/liter) | |||||||||
| Unadjusted | 0.34 | 1.14 | 0.58 | 1.30 | 0.70 | 1.30 | 5.12 | 2, 658 | <0.01 |
| Model 1 | 0.70 | 0.11 | 0.88 | 0.15 | 0.90 | 0.12 | 1.78 | 2, 631 | 0.17 |
| Model 2 | 0.80 | 0.12 | 0.90 | 0.16 | 0.91 | 0.13 | 0.45 | 2, 602 | 0.64 |
| Fibrinogen (mg/dl) | |||||||||
| Unadjusted | 371 | 79 | 370 | 87 | 391 | 102 | 2.87 | 2, 659 | 0.06 |
| Model 1 | 385 | 8 | 381 | 11 | 404 | 9 | 2.50 | 2, 632 | 0.08 |
| Model 2 | 393 | 9 | 386 | 12 | 408 | 10 | 1.80 | 2, 603 | 0.17 |
TABLE 2. Levels of Inflammatory Markers at 5-Year Follow-Up in Patients With Coronary Heart Disease, by Depressive Symptoms Over 5 Yearsa
| Change in Level of Inflammatory Marker From Baseline to Year 5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients With Depressive Symptoms, Defined as Score ≥10 on Patient Health Questionnaire | |||||||||
| Patients With No Depressive Symptoms at Any Interview (N=443) | Depressive Symptoms at One Interview (N=86) | Depressive Symptoms at Two or More Interviews (N=138) | Analysis | ||||||
| Inflammatory Marker and Modelb | Mean | SD/SEc | Mean | SD/SEc | Mean | SD/SEc | F | df | p |
| Interleukin-6, log-transformed (pg/ml) | |||||||||
| Unadjusted | 1.24 | 2.83 | 1.30 | 2.82 | 1.80 | 3.14 | 1.87 | 2, 628 | 0.16 |
| Model 1 | 1.45 | 0.26 | 1.60 | 0.35 | 1.95 | 0.30 | 1.33 | 2, 602 | 0.26 |
| Model 2 | 1.43 | 0.30 | 1.54 | 0.39 | 1.92 | 0.33 | 1.11 | 2, 574 | 0.33 |
| High-sensitivity C-reactive protein, log-transformed (mg/liter) | |||||||||
| Unadjusted | –0.54 | 7.74 | 0.98 | 12.38 | 0.26 | 13.28 | 1.00 | 2, 629 | 0.37 |
| Model 1 | –0.85 | 0.89 | 0.85 | 1.23 | 0.10 | 1.03 | 1.12 | 2, 603 | 0.33 |
| Model 2 | –1.48 | 1.04 | 0.57 | 1.36 | –0.18 | 1.14 | 1.54 | 2, 575 | 0.22 |
| Fibrinogen (mg/dl) | |||||||||
| Unadjusted | –13.36 | 81.72 | –16.68 | 80.52 | 7.06 | 102.64 | 3.10 | 2, 626 | 0.05 |
| Model 1 | –14.22 | 7.85 | –19.35 | 11.05 | 5.07 | 9.00 | 2.63 | 2, 600 | 0.07 |
| Model 2 | –13.67 | 9.04 | –17.13 | 12.07 | 7.21 | 9.85 | 2.66 | 2, 573 | 0.07 |
TABLE 3. Change in Inflammatory Marker Levels Over 5 Years in Patients With Coronary Heart Disease, by Depressive Symptoms Over 5 Yearsa
When we analyzed depressive symptoms as a continuous variable, the average score on the Patient Health Questionnaire across the annual assessments predicted subsequent log-transformed levels of IL-6 (β=0.102, p=0.009) and hsCRP (β=0.121, p=0.002) but not the level of fibrinogen (β=0.058, p=0.13). Again, this association was no longer significant after adjustment for health behaviors (log IL-6: β=0.038, p=0.36; hsCRP: β=0.018, p=0.67; fibrinogen: β=0.018, p=0.68).
When inflammation was evaluated as a dichotomous variable, the proportions of participants with levels of inflammatory markers in the highest quartile were 22% to 24% in those without depression at any interview, 23% to 28% in those with depressive symptoms at one interview, and 30% to 35% in patients with depressive symptoms at two or more interviews (Figure 1).
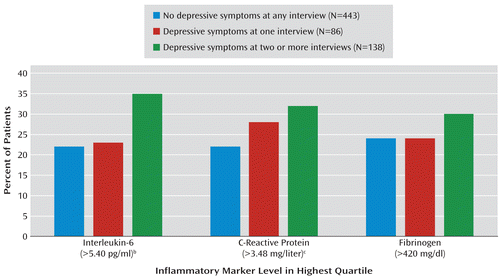
FIGURE 1. Proportion of Patients With Coronary Heart Disease Who Had High Levels of Inflammatory Markers at 5-Year Follow-Up, by Depressive Symptoms Over 5 Yearsa
a Inflammatory markers were measured at baseline and at the 5-year follow-up assessment. Depressive symptoms were assessed with the nine-item Patient Health Questionnaire at baseline and annually for the next 5 years.
b Significant difference among groups (χ2=8.45, df=2, p=0.01).
c Significant difference among groups (χ2=5.96, df=2, p=0.05).
Inflammation as Predictor of Subsequent Depressive Symptoms
MANCOVAs revealed no effect of inflammation on subsequent depression in unadjusted analyses (F=1.37, df=6, 1256, p=0.22) or after adjustment for age, gender, education, race, history of diabetes, myocardial infarction, congestive heart failure, aspirin use, and baseline depressive symptoms (F=0.65, df=6, 1224, p=0.69). In addition, baseline levels of inflammatory markers were not associated with subsequent depressive symptoms (Table 4). As compared with participants who had significant depressive symptoms at two or more annual assessments, those without depressive symptoms had similar baseline levels of log IL-6, log hsCRP, and fibrinogen.
| Baseline Level of Inflammatory Marker | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patients With Depressive Symptoms, Defined as Score ≥10 on Patient Health Questionnaire | |||||||||
| Patients With No Depressive Symptoms at Any Interview (N=443) | Depressive Symptoms at One Interview (N=86) | Depressive Symptoms at Two or More Interviews (N=138) | Analysis | ||||||
| Inflammatory Marker and Modelb | Mean | SD/SEc | Mean | SD/SEc | Mean | SD/SEc | F | df | p |
| Interleukin-6, log-transformed (pg/ml) | |||||||||
| Unadjusted | 0.84 | 0.66 | 0.85 | 0.66 | 0.92 | 0.73 | 0.66 | 2, 633 | 0.52 |
| Model 1 | 0.91 | 0.06 | 0.94 | 0.08 | 1.06 | 0.07 | 2.15 | 2, 618 | 0.12 |
| Model 2 | 1.05 | 0.07 | 1.04 | 0.08 | 1.05 | 0.07 | 0.02 | 2, 612 | 0.98 |
| High-sensitivity C-reactive protein, log-transformed (mg/liter) | |||||||||
| Unadjusted | 0.49 | 1.26 | 0.66 | 1.28 | 0.76 | 1.37 | 2.45 | 2, 634 | 0.09 |
| Model 1 | 0.94 | 0.12 | 1.00 | 0.16 | 1.13 | 0.14 | 0.98 | 2, 619 | 0.37 |
| Model 2 | 1.14 | 0.13 | 1.12 | 0.16 | 1.09 | 0.14 | 0.05 | 2, 613 | 0.95 |
| Fibrinogen (mg/dl) | |||||||||
| Unadjusted | 384 | 82 | 387 | 78 | 383 | 92 | 0.05 | 2, 630 | 0.95 |
| Model 1 | 400 | 8 | 399 | 11 | 401 | 9 | 0.02 | 2, 615 | 0.99 |
| Model 2 | 414 | 9 | 409 | 11 | 402 | 9 | 0.91 | 2, 609 | 0.40 |
TABLE 4. Baseline Levels of Inflammatory Markers in Patients With Coronary Heart Disease, by Depressive Symptoms Over 5 Yearsa
Discussion
The aim of this study was to examine the directionality of the depression-inflammation relationship in a group of patients with stable coronary heart disease. The rate of depressive symptoms was consistent with the rate in a previous study (27): 34% of the participants had a score of 10 or higher on the Patient Health Questionnaire at one or more time points. We found that depressive symptoms were associated with higher subsequent levels of inflammation, whereas baseline levels of inflammation did not predict subsequent depressive symptoms. The association between depressive symptoms and subsequent inflammation was eliminated after adjustment for health behaviors associated with inflammation—physical inactivity, smoking, and higher BMI. Taken together, these findings suggest that depression may lead to inflammation through poor health behaviors, but inflammation does not lead to depression in patients with coronary heart disease.
There have been only a few studies regarding the directionality of the depression-inflammation relationship, and to our knowledge, this is the first study on this subject in a cardiac population. We also believe this to be the first study evaluating the association of depressive symptoms over time with subsequent inflammation. Our results extend the findings of Stewart and colleagues (12), who found that depression predicted high levels of IL-6, whereas high levels of IL-6 did not predict depression. However, our results differ from those of Gimeno and colleagues, who reported that CRP and IL-6 levels were predictive of subsequent depressive symptoms, but not vice versa, in the Whitehall II study (13).
One possible explanation for this discrepancy is that the Whitehall II study used a different measure of depressive symptoms. Their assessment was based on four items from the General Health Questionnaire (thinking of yourself as a worthless person, feeling that life is entirely hopeless, feeling that life is not worth living, and finding times when you could not do anything because your nerves were too bad) and did not include measures of sleep, appetite, concentration, energy, suicidal thoughts, or depressed mood. In contrast, we administered the Patient Health Questionnaire, which was specifically designed to assess the nine symptoms of depression. Another possible explanation for the difference in findings is the difference between study populations. The association between depressive symptoms and inflammation in the general population may differ from the relationship in patients with established coronary heart disease.
It is interesting that our results show that depressive symptoms predicted high levels of hsCRP and IL-6 but not fibrinogen. A possible explanation could be that hsCRP and IL-6 are more specific for the underlying inflammation process in stable patients with coronary heart disease. In contrast to hsCRP and IL-6, which are both markers of inflammation, fibrinogen not only reflects inflammation but also is a blood coagulation factor (28), which could explain why we did not find a prospective association between depression and fibrinogen.
We also found that patients with depressive symptoms at two or more interviews had higher levels of inflammation at follow-up than patients with depressive symptoms at one interview. To the extent that depressive symptoms at two or more interviews represented chronic or recurrent depression, our findings suggest that persistent depression may have greater effects on inflammation than a single episode of depression. Consistent with these findings are the results of Hamer and colleagues (29), who found that in a group of 3,609 aging subjects, participants with depressive symptoms at two time points had higher levels of CRP and fibrinogen than those with depressive symptoms at one time point. In addition, Kaptein and colleagues (30) showed that patients with chronic depressive symptoms following myocardial infarction were at higher risk for having new cardiovascular events than those whose depressive symptoms resolved. Taken together, the findings suggest that the duration of depressive symptoms may influence cardiac health.
The prospective association of depression with inflammation was no longer significant after adjustment for health behaviors (physical inactivity, BMI, and smoking). Although our results cannot determine the direction of the association between depression and health behaviors, this raises the possibility that helping depressed cardiac patients improve these behaviors could potentially reduce inflammation. These findings are further supported by a longitudinal community-based study in which Matthews and colleagues (31) investigated the directionality of the depression-inflammation relationship among 1,781 premenopausal and early perimenopausal women who were free of cardiac disease at baseline. They found that depression, as measured with the Center for Epidemiologic Studies Depression Scale, was associated with higher CRP levels at follow-up. Similar to our results, this association was no longer significant after adjustment for a range of covariates including health behaviors. In addition, Hamer and colleagues (29) found that weight change, waist circumference, current smoking, alcohol use, and especially physical activity were significant mediators in the depression-inflammation relationship. Finally, Dod and colleagues (32) showed in a group of nonsmoking patients with stable coronary heart disease that a combination of intensive exercise and dietary changes significantly lowered levels of IL-6 and CRP, suggesting that physical inactivity and BMI may influence inflammation levels.
Several strengths can be attributed to this study. First, the annual assessments of depressive symptoms and medical health status presented us with the opportunity to assess the direction of this association. In addition, biological and behavioral mediators were carefully assessed at baseline and after 5 years of follow-up. However, there are also several limitations. This study focused on outpatients with stable coronary heart disease. Our results may therefore not apply to healthy participants or to patients with acute coronary syndromes. Furthermore, the study group mostly consisted of older men, so these results may not be generalizable to either women or younger men. Finally, 20% (162 of 829) of the surviving participants did not complete the 5-year follow-up examination. However, these participants were younger and had worse depression scores than those who completed the examination, so including them would probably have strengthened the association between depression and inflammation.
In conclusion, we found no evidence of a bidirectional relationship between depression and inflammation. Depression was prospectively associated with IL-6 and hsCRP, but not vice versa. This prospective association was no longer significant after adjustment for physical inactivity, BMI, and smoking. These findings raise the possibility that helping depressed cardiac patients improve health behaviors could reduce inflammation.
1. : Prevalence of depression in survivors of acute myocardial infarction. J Gen Intern Med 2006; 21:30–38Crossref, Medline, Google Scholar
2. : Course of depression in patients with hypertension, myocardial infarction, or insulin-dependent diabetes. Am J Psychiatry 1993; 150:632–638Link, Google Scholar
3. : Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med 2004; 66:814–822Crossref, Medline, Google Scholar
4. : Psychophysiological biomarkers explaining the association between depression and prognosis in coronary artery patients: a critical review of the literature. Neurosci Biobehav Rev 2010; 35:84–90Crossref, Medline, Google Scholar
5. : Association between depression and elevated C-reactive protein. Psychosom Med 2003; 65:347–356Crossref, Medline, Google Scholar
6. : A meta-analysis of cytokines in major depression. Biol Psychiatry 2010; 67:446–457Crossref, Medline, Google Scholar
7. : Depression and C-reactive protein: population-based Health 2000 Study. Psychosom Med 2009; 71:423–430Crossref, Medline, Google Scholar
8. : Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2004; 164:1010–1014Crossref, Medline, Google Scholar
9. : Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition Study. Biol Psychiatry 2003; 54:566–572Crossref, Medline, Google Scholar
10. : Association between major depressive disorder and C-reactive protein levels in stable coronary heart disease patients. J Psychosom Res 2009; 66:189–194Crossref, Medline, Google Scholar
11. : Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71:171–186Crossref, Medline, Google Scholar
12. : A prospective evaluation of the directionality of the depression-inflammation relationship. Brain Behav Immun 2009; 23:936–944Crossref, Medline, Google Scholar
13. : Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med 2009; 39:413–423Crossref, Medline, Google Scholar
14. : Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA 2008; 300:2379–2388Crossref, Medline, Google Scholar
15. : Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study: Primary Care Evaluation of Mental Disorders Patient Health Questionnaire. JAMA 1999; 282:1737–1744Crossref, Medline, Google Scholar
16. : The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Medline, Google Scholar
17. : Validity of the Hospital Anxiety and Depression Scale and Patient Health Questionnaire-9 to screen for depression in patients with coronary artery disease. Gen Hosp Psychiatry 2007; 29:417–424Crossref, Medline, Google Scholar
18. : Optimizing detection of major depression among patients with coronary artery disease using the Patient Health Questionnaire: data from the Heart and Soul Study. J Gen Intern Med 2008; 23:2014–2017Crossref, Medline, Google Scholar
19. : Assessing depression in primary care with the PHQ-9: can it be carried out over the telephone? J Gen Intern Med 2005; 20:738–742Crossref, Medline, Google Scholar
20. : To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav Immun 2009; 23:887–897Crossref, Medline, Google Scholar
21. : Biomarkers of potential harm among adult smokers and nonsmokers in the Total Exposure Study. Nicotine Tob Res 2011; 13:182–193Crossref, Medline, Google Scholar
22. : Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Cardiovasc Prev Rehabil 2007; 14:422–428Crossref, Medline, Google Scholar
23. : Validity and reliability of self-reported physical activity status: the Lipid Research Clinics questionnaire. Med Sci Sports Exerc 1993; 25:92–98Crossref, Medline, Google Scholar
24. : Construct validity of self-reported historical physical activity. Am J Epidemiol 2004; 160:279–286Crossref, Medline, Google Scholar
25. : Reliability and validity of self-reported physical activity in the Nord-Trondelag Health Study: HUNT 1. Scand J Public Health 2008; 36:52–61Crossref, Medline, Google Scholar
26. : The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking: Ambulatory Care Quality Improvement Project (ACQUIP) Alcohol Use Disorders Identification Test. Arch Intern Med 1998; 158:1789–1795Crossref, Medline, Google Scholar
27. : Depression and coronary heart disease: association and implications for treatment. Cleve Clin J Med 2008; 75(suppl 2):S48–S53Crossref, Medline, Google Scholar
28. : Risk factors for atherosclerotic disease, in Heart Disease: A Textbook of Cardiovascular Medicine, 6th ed. Edited by Braunwald EZipes DPLibby P. Philadelphia, WB Saunders, 2001, pp 1010–1039Google Scholar
29. : Persistent depressive symptomatology and inflammation: to what extent do health behaviours and weight control mediate this relationship? Brain Behav Immun 2009; 23:413–418Crossref, Medline, Google Scholar
30. : Course of depressive symptoms after myocardial infarction and cardiac prognosis: a latent class analysis. Psychosom Med 2006; 68:662–668Crossref, Medline, Google Scholar
31. : Are there bi-directional associations between depressive symptoms and C-reactive protein in mid-life women? Brain Behav Immun 2010; 24:96–101Crossref, Medline, Google Scholar
32. : Effect of intensive lifestyle changes on endothelial function and on inflammatory markers of atherosclerosis. Am J Cardiol 2010; 105:362–367Crossref, Medline, Google Scholar



