Successful Rechallenge With Clozapine After Eosinophilia
Case Presentation
“Mr. B” was a 27-year-old, single, unemployed man who lived with and was financially supported by his mother. He had an 8-year history of treatment-resistant chronic paranoid schizophrenia. He had two previous psychiatric hospitalizations and no history of violence, substance abuse, or suicide attempts.
Mr. B was born to married parents. His parents divorced when he was in grade school, after which he continued to live with his mother and older sister and successfully completed high school at age 19. He was noted to be quiet and somewhat withdrawn, but he functioned well, got good grades, had friends, and developed several hobbies.
A few weeks before his high school graduation, Mr. B's mother noticed that he was more withdrawn and irritable, and she suspected that he was talking to himself. These symptoms worsened over several weeks, and Mr. B was hospitalized. During this first hospitalization, Mr. B was diagnosed with schizophrenia. He was treated with risperidone, which was not effective, and then with olanzapine. Although olanzapine controlled his positive symptoms relatively well, he never returned to his baseline functionality, and he continued to have intermittent auditory hallucinations and functional impairment, spending the next several years at home, taking classes and developing his hobbies.
One year before his admission to our hospital, Mr. B was hospitalized for a second time after he was switched from olanzapine to aripiprazole because of oversedation. Soon after this medication change, Mr. B became increasingly psychotic, with distressing auditory hallucinations and paranoia that ultimately prompted him to ask a friend to kill him. Despite treatment with concurrent high dosages of olanzapine and haloperidol during this second hospitalization, Mr. B's positive and negative symptoms progressed, and a trial of clozapine was started. He experienced notable improvement after several days on clozapine, with decreased auditory hallucinations, a greater ability to socialize, and a brighter, more engaged affect. Mr. B developed tachycardia, with a heart rate in the low 100s, and propranolol was started. Approximately 2 weeks after initiation of clozapine, Mr. B became febrile. At the time, his clozapine dosage was 100 mg/day. Clozapine was stopped and a fever workup was performed. No further evidence of acute inflammation was found, and multiple blood draws revealed normal total WBC and absolute neutrophil counts. Mr. B's psychotic symptoms worsened, and his clozapine dosage was titrated back to 100 mg/day after he had been afebrile for a few days.
Mr. B again developed tachycardia, which was treated this time with atenolol. His positive and negative symptoms also improved, but 1 week later he developed eosinophilia, with a count of 2.7×109/liter, 23% of the total WBC count. Eosinophilia persisted 2 days later, with the count at 2.8×109/liter, 27% of the total WBC count. Clozapine was again stopped, and a cardiac workup was performed, including cardiac enzymes, an ECG, and an echocardiogram, none of which showed evidence of myocarditis. At this point, the treating psychiatrist and consulting cardiologist recommended that Mr. B not be treated with clozapine again because of the risk of an acute inflammatory syndrome.
After discontinuation of clozapine, Mr. B's psychotic symptoms again worsened, and he was treated with relatively high dosages of haloperidol (15 mg/day), olanzapine (20 mg/day), and aripiprazole (20 mg/day). This combination improved his symptoms enough to allow him to be discharged. Over the next several months, Mr. B experienced intermittent and nondistressing auditory hallucinations. Haloperidol was eventually discontinued, and the dosage of aripiprazole was decreased. Mr. B was maintained on olanzapine (20 mg/day) and aripiprazole (2.5 mg/day), and he attended cognitive rehabilitation at a clinic for patients with psychotic disorders 3 days a week and saw a therapist once a week. He continued to be isolative and asocial, however, and was markedly impaired functionally, spending most of his time at home.
Given his persistent symptoms, functional impairment, and prior positive response to clozapine, Mr. B, his mother, and his psychiatrist decided to consider a clozapine rechallenge. A few days before the rechallenge, Mr. B abruptly stopped taking all of his medications and became more acutely psychotic. He became increasingly preoccupied with the idea that he had done something terrible, spoke of the need to go to a foreign country to escape his auditory hallucinations, and said he “[had] to die” because he had “ruined the world with [his] thoughts.” One day before admission, he asked his mother if drinking detergent would kill him, and then asked her for poison, prompting her to bring him to the emergency department.
On assessment in the emergency department, Mr. B's laboratory values were within normal limits, including liver function tests, a basic metabolic panel, and levels of amylase, lipase, thyroid-stimulating hormone, and blood alcohol, and his WBC count was 6.9×109/liter with 0% eosinophils. Urine toxicology results and a baseline ECG were normal. Mr. B was admitted to our psychiatric unit on an involuntary basis.
At the time of admission, Mr. B was disheveled, with extremely poor eye contact and a flat affect. During interviews he was guarded and his responses were curt. Although he denied having auditory hallucinations, he was frequently seen talking or laughing to himself. He endorsed recent suicidal ideation but declined to elaborate, saying it was “hard to talk about.” Because of Mr. B's deteriorating condition and several failed antipsychotic medication trials, the decision was made to rechallenge with clozapine using a slow dose titration and close monitoring of blood parameters. Mr. B's mother, who was his health care proxy, was involved in extensive discussions about the risks of clozapine, and Mr. B agreed to the trial.
When clozapine treatment was initiated, Mr. B's previous dosages of olanzapine (20 mg/day) and aripiprazole (2.5 mg/day) were initially continued for antipsychotic coverage. After 3 days of treatment with clozapine, aripiprazole was discontinued in order to minimize the risks of antipsychotic polypharmacy, and an olanzapine taper was started. Because of Mr. B's history of clozapine-induced eosinophilia, near-daily laboratory studies—including CBC with differential, basic metabolic panel, serum troponin level, liver function tests, amylase level, and lipase level—were performed to track his eosinophil count and monitor for signs of associated inflammation.
Figure 1 illustrates the course of Mr. B's clozapine dosage and eosinophil counts throughout the clozapine trial and beyond. On day 6 of the hospitalization, Mr. B's eosinophil count increased from 0% of the total WBC count to 6% (the upper limit of normal). Mr. B remained guarded and internally preoccupied, but he reported no distress and his other laboratory values were normal. Over the next 2 weeks, clozapine was titrated slowly (typically increased by 25 mg every few days, but at times more slowly), olanzapine was gradually decreased to 10 mg/day, and Mr. B's positive and negative symptoms improved. The voices were less bothersome, and Mr. B was more spontaneously conversant, made better eye contact, and voluntarily interacted more with the staff and patients. He also began exercising and playing the piano in the unit's lounge.
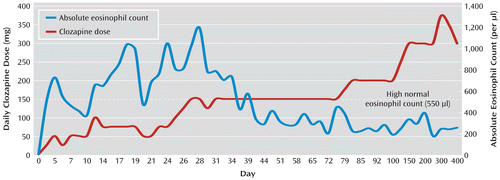
FIGURE 1. Changes in Absolute Eosinophil Count After Initiation of Clozapine Rechallenge
During the first 2 weeks of clozapine treatment, Mr. B's eosinophils increased gradually to 1.1×109/liter, 13% of the total WBC count. Lipase levels were transiently elevated, increasing over 2 days to 115 U/liter before returning to normal. At this time, even though Mr. B was physically asymptomatic and had not developed tachycardia or fever as he had during two previous trials with clozapine, we decided to hold his clozapine dose at 75 mg/day until consultations were obtained from the allergy and hematology services.
The consulting services gave conflicting recommendations. The allergy consultation service recommended stopping clozapine out of concern that the eosinophilia might reflect a “chronic inflammatory state” with a high likelihood of end organ damage (e.g., pulmonary fibrosis, renal insufficiency, diabetes, or myocarditis) if it persisted. However, a second consultant from the allergy department felt that continuing treatment with clozapine would be safe as long as the total number of eosinophils did not rise above 1.5×109/liter, the cutoff for a diagnosis of idiopathic eosinophilia, a condition not believed to lead to end organ damage (1). The recommendation of the hematology service was to continue clozapine treatment with close monitoring of the patient's erythrocyte sedimentation rate (ESR) and C-reactive protein level, even with the current level of eosinophilia, since the patient was not showing signs of end organ involvement.
Before deciding whether to continue the clozapine titration, we reviewed published treatment recommendations for idiopathic eosinophilia, defined as a total eosinophil count >1.5×109/liter for more than 6 months without an identifiable cause. In idiopathic eosinophilia, the standard of care is to monitor periodically for signs of end organ involvement with testing, including ECG, echocardiogram, serum troponin levels, and pulmonary function tests (2, 3). These considerations were discussed with the patient and his mother, who agreed with the treatment team that the known benefits of clozapine to the patient (i.e., his previous and current clinical improvement, including remission of command auditory hallucinations to kill himself) outweighed the potential risks (e.g., end organ damage) as long as close monitoring was continued. Mr. B was therefore continued on clozapine with frequent blood testing that included ESR and C-reactive protein level. We agreed to reevaluate this decision if Mr. B's total eosinophil count rose above 1.5×109/liter. Mr. B's cardiac and pulmonary functioning was assessed. An echocardiogram showed no change in cardiac function from 2 weeks before admission. Pulmonary function tests were consistent with poor patient effort but did not otherwise suggest compromised lung function.
Although there are no standard recommendations in the literature for monitoring renal or pancreatic function in patients with idiopathic eosinophilia, we monitored Mr. B's creatinine, BUN, amylase, and lipase levels closely. Throughout the clozapine titration, these levels were always within normal limits. Mr. B's lipase was elevated on four nonconsecutive days near the beginning of the titration but then normalized and remained within normal limits.
Mr. B's eosinophilia reached a peak of 1.2×109/liter (16% of the total WBC count) at a clozapine dosage of 150 mg/day approximately 1 week later. Although Mr. B continued to show no signs of end organ inflammation, the clozapine titration was slowed several times because of increased eosinophilia. Early in the course of treatment, it appeared that the degree of eosinophilia varied with the clozapine dosage (Figure 1). After 4 weeks, however, this relationship was less apparent. At the end of week 6 of hospitalization, Mr. B was discharged home on clozapine (150 mg/day), olanzapine (10 mg/day), and fluoxetine (20 mg/day) (the latter had been added for depressive symptoms during his hospitalization). His eosinophil count was 3.4×108/liter (5% of the total WBC count), and all other laboratory values were within normal limits. A follow-up echocardiogram showed no significant change. Mr. B was no longer suicidal, his auditory hallucinations were significantly improved, his mood and affect were brighter, and he continued to be more spontaneously conversant and to make better eye contact. He stated that he was especially pleased that he had been able to make friends while in the hospital. He was discharged with close follow-up, including cognitive rehabilitation, outpatient psychiatric appointments, and routine blood draws and repeat ECG, echocardiogram, and pulmonary function tests at 6-month intervals should the eosinophilia persist.
Mr. B's peripheral eosinophil levels decreased around the time of his discharge and remained within normal limits over the next year of clozapine treatment. The dosage was gradually raised as high as 400 mg/day but was then lowered to 300 mg/day because of persistent nocturnal enuresis. At the 1-year point, Mr. B was still taking clozapine and was not taking any other antipsychotic medications. He continued to show good symptom control and functional improvement.
Discussion
It is well established that the antipsychotic medication clozapine can cause blood dyscrasias, the most concerning of which is agranulocytosis (4). Clozapine-induced eosinophilia has also been reported, often in association with organ-specific inflammatory processes, with myocarditis being the most cited example (5–16). When there is evidence of organ-specific inflammation, clozapine is normally discontinued to prevent end organ damage (5–10, 12, 13, 15, 17). Less commonly reported are cases of clozapine-induced eosinophilia in which there is no evidence of specific organ inflammation (18–21). In these instances, it is less clear whether or not clozapine should be discontinued.
Since the discovery of agranulocytosis as a potentially fatal side effect of clozapine, close attention has been paid to all blood dyscrasias associated with the drug, including eosinophilia. Shortly after clozapine's introduction in the United States in 1989, Stricker and Tielens (18) reported the first case of clozapine-associated eosinophilia. In that case, clozapine was discontinued, although there were no signs of associated organ inflammation. Over the next few years, other reports were published describing “benign eosinophilia,” in which peripheral eosinophilia was noted shortly after clozapine administration, without other evidence of disease or inflammatory processes. Tiihonen and Paanila (19) reported the case of a 38-year-old woman who developed an eosinophil count of 1.5×109/liter without somatic symptoms. Banov et al. (20) described a retrospective study in which none of 17 patients who developed eosinophilia while taking clozapine had somatic complaints or an identifiable disease process to explain the eosinophilia. Schuepbach et al. (21) described two patients with elevated eosinophil counts without medical complaint. These reports concluded that eosinophilia was not necessarily a marker for medical illness, and no recommendations were made regarding discontinuation of clozapine treatment in the presence of eosinophilia without associated somatic symptoms. Banov et al. (20) and Hummer et al. (22) hypothesized that eosinophilia may be a herald sign for the development of neutropenia, but this hypothesis has not been borne out (23).
By the late 1990s, however, many cases of clozapine-associated inflammatory conditions had been described. Several cases of pancreatitis were reported (5–7), as well as hepatitis (8–10), colitis (11, 12, 16), and nephritis (13). Peripheral eosinophilia was reported in all of these cases, and when biopsies were performed, there was also evidence of tissue eosinophilic infiltrates (12–14, 16). Most worrisome were cases of myocarditis (14, 15). Kilian et al. (14) studied 8,000 patients started on clozapine in Australia over a 5-year period and documented a 1,000- to 2,000-fold increase in the rate of potentially fatal myocarditis following initiation of clozapine treatment. Subsequent studies placed the incidence of clozapine-induced myocarditis between 0.015% (24) and more than 3% (25), depending on the population under study and the diagnostic criteria used for myocarditis. In most cases where autopsy results were available, cardiac eosinophilic infiltrates were noted, suggesting an acute drug reaction. Several other reports of clozapine-induced myocarditis have been published (15, 26), and eosinophilia is now believed to be an important marker for potentially serious inflammatory conditions in patients treated with clozapine.
The pathophysiology of drug-induced blood dyscrasias is unclear. In recent years, several hypotheses for the development of drug-induced neutropenia and agranulocytosis have been posited (27, 28). Two potential explanations of clozapine-induced neutropenia involve reactive oxygen species. When clozapine is oxidized, a reactive nitrenium ion is produced. This ion reacts with sulfydryl groups in the glutathione cycle and may deplete the ATP supply of neutrophils, leading to apoptosis and eventual neutropenia (27). It may also be the case that when nitrenium ions react with sulfydryl groups, haptens are formed, inducing antibodies against neutrophils. An alternative, although not mutually exclusive, hypothesis is that the reactive metabolites formed when clozapine is oxidized affect the bone marrow stroma itself, limiting or prohibiting the maturation of progenitor cells, thereby leading to agranulocytosis (27). In addition to the haptenation-induced autoantibodies discussed above, other immunological explanations for drug-induced agranulocytosis have been proposed. For example, antibodies may form immune complexes with the drug, targeting membrane glycoproteins and leading to neutrophil destruction (27, 28). Several genetic hypotheses for drug-induced agranulocytosis have also been considered (27, 28). Single-nucleotide polymorphisms in genes that control drug metabolism may result in unusually high blood and tissue levels of the drug in some individuals, although this would not, in itself, account for the idiosyncratic nature of agranulocytosis (27). Single-nucleotide polymorphisms in genes encoding human leukocyte antigens have also been implicated in the development of drug-induced agranulocytosis (27).
The pathophysiology of clozapine-induced eosinophilia is similarly unclear, but it may also result from immunological processes. It has been suggested that a type I hypersensitivity reaction is involved, a hypothesis supported in some cases by an elevation of IgE levels (29), angioedema, and rash (9). Some investigators have hypothesized that clozapine may stimulate T-lymphocytes, with a subsequent increase in interleukin-5, which promotes the production of eosinophils (21).
Although immunological processes may underlie both clozapine-induced neutropenia/agranulocytosis and eosinophilia, they appear to have distinct pathophysiologies, as suggested by their different recurrence propensities. Patients who have discontinued clozapine as a result of confirmed agranulocytosis tend to experience a rapid and severe recurrence of neutropenia on clozapine rechallenge, which does not appear to be the case for many patients who develop eosinophilia when treated with clozapine. We found reports of eight such patients who were rechallenged with clozapine (5, 6, 11, 17–19, 21), and only two of them developed eosinophilia with signs of end organ damage (5, 6). In several cases in which “benign” eosinophilia (i.e., unaccompanied by other evidence of disease or inflammation) occurred, treatment with clozapine was continued (19–21). At least one of these cases was similar to ours: a patient developed “benign” eosinophilia that recurred, to a lesser extent, on rechallenge and then normalized (21). This resolution of eosinophilia despite ongoing clozapine treatment argues against a sustained immunologically mediated reaction. Rather, it suggests the possibility of an acute allergic reaction, as reflected by the “on/off” phenomenon noted in several other case reports (5, 6, 18, 21, 29).
Unlike clozapine-induced neutropenia, there are no standardized monitoring recommendations for eosinophilia that occurs in the context of clozapine treatment, and there is debate in the literature over how to manage it. In almost all case reports with clear evidence of end organ involvement (e.g., pancreatitis, hepatitis, nephritis, myocarditis), clozapine treatment was stopped (5–9, 12, 13, 15, 17). Despite the fact that in the majority of these cases, eosinophil counts and abnormal enzyme levels returned to normal, most patients were not rechallenged with clozapine (7–9, 12, 13, 15). Several well-tolerated rechallenge attempts (11, 17–19, 21) and cases of successful clozapine continuation despite eosinophilia (11, 19–21) suggest that when eosinophilia develops without signs or symptoms of organ involvement, it may be safe to continue clozapine treatment or to rechallenge if clozapine was stopped. We recommend that when a patient develops eosinophilia and signs or symptoms of organ inflammation, strong consideration be given to discontinuing clozapine. However, when such signs or symptoms are absent, the optimal course of action is less clear, and cases should be evaluated on an individual basis. If a patient has not responded to other antipsychotic treatment, it may be justified to continue or restart clozapine. The risks and benefits must be carefully considered. Our patient never developed evidence of significant organ involvement, and we decided to continue treatment with clozapine, as the clinical benefits he experienced were believed to outweigh the risks.
Conclusions
For many patients with refractory psychotic symptoms, clozapine remains the best therapeutic option. It is our recommendation that if eosinophilia develops without signs of end organ damage or inflammation, clozapine continuation or rechallenge with careful monitoring may be considered. The patient, family, and appropriate medical consultants should be involved in the decision-making process.
1. : Blood eosinophilia: a new paradigm in disease classification, diagnosis, and treatment. Mayo Clin Proc 2005; 80:75–83Crossref, Medline, Google Scholar
2. : Modern diagnosis and treatment of primary eosinophilia. Acta Haematol 2005; 114:52–60Crossref, Medline, Google Scholar
3. : The hypereosinophilic syndromes: current concepts and treatments. Br J Haematology 2009; 145:271–285Crossref, Medline, Google Scholar
4. : Clozapine-induced agranulocytosis: incidence and risk factors in the United States. N Engl J Med 1993; 329:162–167Crossref, Medline, Google Scholar
5. : Eosinophilia, clozapine, and pancreatitis. Lancet 1992; 340:251Crossref, Medline, Google Scholar
6. : Recurrent pancreatitis on clozapine re-challenge. J Psychopharmacol 1995; 9:381–382Crossref, Medline, Google Scholar
7. : The development of a clinical syndrome of asymptomatic pancreatitis and eosinophilia after treatment with clozapine in schizophrenia: implications for clinical care, recognition, and management. J Psychopharmacol 2002; 16:399–400Crossref, Medline, Google Scholar
8. : Toxic hepatitis by clozapine treatment. Am J Psychiatry 1993; 150:985–986Link, Google Scholar
9. : Clozapine-induced toxic hepatitis. Am J Psychiatry 1995; 152:296–297Link, Google Scholar
10. : Clozapine-induced toxic hepatitis with skin rash. J Psychopharmacol 2005; 19:107Crossref, Medline, Google Scholar
11. : Clozapine-caused eosinophilic colitis. Ann Clin Psychiatry 1995; 7:97–98Crossref, Medline, Google Scholar
12. : Clozapine-induced eosinophilic colitis. Am J Psychiatry 2005; 162:1386–1387Link, Google Scholar
13. : Clozapine-induced acute interstitial nephritis. Lancet 1999; 354:1180–1181Crossref, Medline, Google Scholar
14. : Myocarditis and cardiomyopathy associated with clozapine. Lancet 1999; 354:1841–1845Crossref, Medline, Google Scholar
15. : Myocarditis during clozapine treatment. Am J Psychiatry 2006; 163:204–208Link, Google Scholar
16. : Response to clozapine-induced microscopic colitis: a case report and review of the literature. J Clin Psychopharmacol 2008; 28:454–455Crossref, Medline, Google Scholar
17. : Successful re-challenge with clozapine following development of clozapine-induced cardiomyopathy. Aust NZ J Psychiatry 2008; 42:747–748Medline, Google Scholar
18. : Eosinophilia with clozapine. Lancet 1991; 338:1520–1521Crossref, Medline, Google Scholar
19. : Eosinophilia associated with clozapine. Lancet 1992; 339:488Crossref, Medline, Google Scholar
20. : High risk of eosinophilia in women treated with clozapine. J Clin Psychiatry 1993; 54:466–469Medline, Google Scholar
21. : Successful challenge with clozapine in a history of eosinophilia. Int Clin Psychopharmacol 1998; 13:33–37Crossref, Medline, Google Scholar
22. : Does eosinophilia predict clozapine induced neutropenia? Psychopharmacology (Berl) 1996; 124:201–204Crossref, Medline, Google Scholar
23. : Predictive value of eosinophilia for neutropenia during clozapine treatment. J Clin Psychiatry 1996; 57:579–581Crossref, Medline, Google Scholar
24. : Myocarditis and cardiomyopathy associated with clozapine use in the United States. N Engl J Med 2001; 345:224–225Crossref, Medline, Google Scholar
25. : Clozapine-related myocarditis and cardiomyopathy in an Australian metropolitan psychiatric service. Aust NZ J Psychiatry 2004; 38:915–922Crossref, Medline, Google Scholar
26. : Clozapine and myocarditis: a case series from the New Zealand Intensive Medicines Monitoring Programme. NZ Med J 2008; 121:68–74Medline, Google Scholar
27. : Idiosyncratic drug-induced agranulocytosis: possible mechanisms and management. Am J Hematol 2009; 84:428–434Crossref, Medline, Google Scholar
28. : Review: drug induced neutropenia: pathophysiology, clinical features, and management. Ann Clin Lab Sci 2004; 34:131–137Medline, Google Scholar
29. : Clozapine-induced eosinophilia: subsequent neutropenia and corresponding allergic mechanisms. J Clin Psychiatry 1998; 59:195–197Crossref, Medline, Google Scholar



