Effect of Acute Psychological Stress on Prefrontal GABA Concentration Determined by Proton Magnetic Resonance Spectroscopy
Abstract
Objective:
Impaired function of the central gamma-aminobutyric acid (GABA) system, which provides the brain's major inhibitory pathways, is thought to play an important role in the pathophysiology of anxiety disorders. The effect of acute psychological stress on the human GABA-ergic system is still unknown, however. The purpose of this study was to determine the effect of acute stress on prefrontal GABA levels.
Method:
A recently developed noninvasive magnetic resonance spectroscopy method was used to measure changes in the GABA concentration of the prefrontal cortex in 10 healthy human subjects during a threat-of-shock condition and during a safe condition (two sessions on different days). The main outcome measure was the mean GABA concentration within a 3×3×2-cm3 voxel selected from the medial prefrontal cortex.
Results:
Prefrontal GABA decreased by approximately 18% in the threat-of-shock condition relative to the safe condition. This reduction was specific to GABA, since the concentrations of N-acetyl-aspartate, choline-containing compounds, and glutamate/glutamine levels obtained in the same spectra did not change significantly.
Conclusions:
This result appeared compatible with evidence from preclinical studies in rodents, which showed rapid presynaptic down-regulation of GABA-ergic neurotransmission in response to acute psychological stress. The molecular mechanism and functional significance of this reduced inhibitory effect of acute psychological stress in relation to impaired GABA-ergic function in anxiety disorders merit further investigation.
Gamma-aminobutyric acid (GABA) is the main inhibitory neurotransmitter of the CNS. While this neurotransmitter plays a major role in the modulation of virtually all cognitive and behavioral processes, clinical studies have particularly highlighted the role of the GABA-ergic system in the central modulation of anxiety and stress responses (1, 2). To elucidate the mechanisms underlying the antianxiety effects of agents that enhance GABA-ergic neurotrans-mission, a substantial preclinical literature has accumulated, which characterizes the effects of environmental stress on GABA-ergic function in experimental animals. These effects have proven to be complex, since acute stressors can either increase or decrease brain GABA levels, depending on the type of stress, duration of stress, and brain region examined (3–8). In humans, only indirect evidence exists to support the involvement of GABA in stress response. Reduced GABA plasma concentrations, reduced brain GABA levels as determined by magnetic resonance spectroscopy (MRS), and reduced prefrontal GABA type A (GABAA)-benzodiazepine receptor complex have consistently been reported in pathological anxiety states and stress-related disorders (9–12). In both humans and rodents, GABA receptor antagonists generally exert anxiogenic effects, while GABA receptor agonists (e.g., benzodiazepines) and drugs that enhance GABA transmission reduce anxiety and stress responses during exposure to stressors or threats (13). However, due to the limited availability of noninvasive techniques for assessing GABA-ergic function, the direct effects of acute stress on the central GABA-ergic system have not been studied directly in humans.
The goal of this study was to examine the effects of acute psychological stress on prefrontal GABA concentrations. Subjects underwent two 32-minute GABA scan sessions on different days. One session included an 8-minute well-established threat-of-shock period to induce anxiety, and the other session did not include a threat-of-shock period (control condition). Prefrontal GABA concentrations were determined using a recently developed MRS method with a 3 Tesla General Electric magnetic resonance imaging (MRI) scanner (General Electric, Milwaukee) and a homogeneous resonator coil that allowed for reliable determination of GABA levels with a temporal resolution of 8 minutes. Since stress reduces GABA levels in the medial prefrontal cortex in rat models (6), we hypothesized that the prefrontal GABA concentration would decrease following acute psychological stress in humans.
Method
Participants
Ten right-handed medically and psychiatrically healthy volunteers (women: N=4; mean age=28.7 years [SD=9.0], range=19–49 years) participated in this study. Subjects were recruited through advertisements and evaluated during specific screening visits at the National Institutes of Health Clinical Center. The clinical evaluation included physical examination; electrocardiography; laboratory assessment of liver, kidney, and thyroid function; serum electrolytes analysis; hematological profile; urine analysis; and toxicology (drug screen). Mental health status was evaluated by an unstructured clinical interview with a psychiatrist and the Structured Clinical Interview for DSM–IV Axis I Disorders–Non-Patient Version (14). Exclusion criteria were having a lifetime history of a major psychiatric illness (including substance dependence), substance abuse within the preceding 1 year, exposure to psychotropic medications (including nicotine or any tobacco products) or other agents likely to influence cerebral metabolism within the 1 month prior to scanning, major medical or neurological disorders, or general MRI exclusions. Pregnant or nursing women also were excluded. Subjects provided written informed consent after receiving a full explanation of the study purpose, procedures, and risks as approved by the National Institute of Mental Health Institutional Review Board.
Experimental Design
Each subject was studied across two sessions scheduled on different days. One session was associated with a psychological stressor consisting of a threat-of-shock paradigm (threat-of-shock session). The other session served as the control and was associated with no threat-of-shock (control session). Session orders were counterbalanced across subjects. Each scan session lasted 32 minutes, with the MRS scan acquired as four 8-minute blocks (see below).
Threat-of-shock session.
Subject preparation included placement of disk electrodes on the right foot in order to deliver the electrical shocks. Prior to the threat-of-shock scan, subjects received a series of 200-msec duration shocks to the right foot, which began at a low intensity and then were increased until they reached a level the subjects found unpleasant but not painful. Shock intensity ranged from 3.4–5.0 mA. Previous studies have shown that the anticipation of an unpleasant shock is consistently anxiogenic (15). A minimum interval of 20 minutes elapsed between the sample shocks and MRS scanning in order to minimize residual effects of the shocks on the MRS measures.
The four 8-minute scan blocks were acquired during one of two sessions in a preset order: the first block was acquired during an initial “safe” condition, the second during the threat condition, and the third and fourth during the safe condition (safe, threat, safe, safe). During the safe condition, subjects were instructed that no shock could be administered. During the threat condition, subjects were warned that they would receive occasional shocks. They were asked to keep their eyes open and to watch a screen where the word “SHOCK” would be displayed during the threat condition and the word “SAFE” during the safe conditions. During the threat scan block, three electric shocks were administered unpredictably.
No-threat-of-shock session.
Because subjects were expected to show increased subjective anxiety even in the initial safe condition of the threat-of-shock session as they anticipated the upcoming threat condition, we also examined them in a no-threat-of-shock session during which they were instructed that no shock would be administered. During this control session, subjects did not receive sample shocks and no electrodes were placed on the foot to demonstrate that no risk of experiencing electrical shocks existed. Prior to scanning, volunteers were informed that they might experience nonpainful sensory stimuli on their right foot, applied by touching their right foot with a soft paint brush. We used this procedure to control for nonspecific effects of anticipated somatosensory stimulation (16). Volunteers were asked to keep their eyes open and watch a video screen where the word “SAFE” was displayed throughout the scan session.
Assessment of subjective anxiety.
At the end of each 8-minute scan block, subjects were asked to rate their anxiety level on an analogue scale that ranged from 0 (no anxiety) to 10 (extreme anxiety).
MRS
Volunteers were scanned in two sessions on the same 3 Tesla General Electric scanner using a body coil for radiofrequency pulse transmission, responsible for a homogeneous radiofrequency excitation field, and an eight-channel NOVA receive coil array (NOVA Medical, Inc., Wilmington, Mass.), providing a high signal-to-noise ratio for spectroscopic measurements from pre-frontal cortical tissue. Proton MRS spectra were acquired from one voxel (3×3×2 cm3) positioned within the medial prefrontal cortex by abutting the posterior border to the rostrum of the corpus callosum, centering the voxel on the midline in horizontal planes and on the bicommissural line in sagittal planes. Figures displaying the voxel position within the brain appear elsewhere (see reference 10) and in the data supplement accompanying the online version of this article. The echo time was 68 msec, the repetition time was 1.5 seconds, the number of excitations was two, and the number of acquisition points was 2,048, with a sample frequency/spectral width of 5,000 Hz. The scan time was 32 minutes for a total of 1,280 averages.
GABA was measured using an interleaved position resolved spectroscopy sequence-based J editing method (17, 18). The concentrations of GABA, choline, N-acetyl-aspartate, and coedited glutamate/glutamine levels were expressed in mmol/liter (mM), referenced to the concentration of creatine. To reference the spectra to a quantitative standard, the creatine concentration was set to 7.1 mmol/liter, a value consisting of the average concentration from literature reports of creatine in gray and white matter (19). This conventional creatine referencing method, which is well-suited for this study due to the extremely low turnover rate of total creatine in the brain (20), has been validated and detailed previously (21, 22). The coefficient of variation for GABA/creatine ratio reliability measurement at our site was 9% in a pilot study in nine healthy subjects.
Interleaved acquisition and careful in vivo and in vitro quality control procedures were employed to ensure the quality of the data. No subtraction errors were detected between the edited and nonedited scans. Macromolecule contribution was corrected after quantification (1, 10, 23, 24). Additional details on the methods for obtaining the MRS measures are presented in the data supplement.
Statistical Analysis
Full factorial linear-mixed models with restricted maximum likelihood estimation were used to examine the within-subject effects of session type (threat-of-shock versus no-threat-of-shock) and time (four 8-minute scan blocks) on the spectroscopic measures. The threat-of-shock session included the scan blocks acquired during the safe conditions, both before and after the 8-minute threat-of-shock scan block. Schwarz's Bayesian criterion was used to determine the best fitting covariance structure for each set of variables in cases where the typical compound symmetry approach used by analysis of variance did not provide the optimal structure for the extant data. Accordingly, the compound symmetry approach was used to assess condition and time effects on self-reported anxiety levels, and a Toeplitz covariance structure was used to determine best fit in the analyses of GABA and glutamate/glutamine levels. We used two-tailed paired t tests to compare N-acetyl-aspartate and choline concentrations measured over the entire duration (32 minutes) of each of the two scan sessions. The subjective ratings were analyzed according to session type (threat-of-shock versus no-threat-of-shock) and time (four 8-minute scan blocks).
Results
Figure 1 shows the course of self-reported anxiety during the two scan sessions. Subjective anxiety levels were significantly higher during the threat-of-shock session than in the control session (F=29.78, df=1, 67, p<0.0001). In the threat-of-shock session, anxiety levels increased from the first block (safe condition) to the second block (threat condition, p=0.0004) and then decreased from the second to the third block (safe condition, p=0.0003). In the control session, anxiety levels did not differ among blocks.
As shown in Figure 2, GABA levels were significantly lower in the threat-of-shock session than in the control session (F=19.02, df=1, 67, p<0.0001), and the interaction between session type and time was significant (F=9.57, df=1, 67, p=0.003). The difference in GABA concentration between sessions was significant for the first scan block (0–8 minutes, p=0.02), the second block (9–16 minutes, p=0.009), and the third block (17–24 minutes, p=0.004) but was not significant for the fourth block (25–32 minutes). Self-reported anxiety levels and GABA concentrations were inversely correlated (r=–0.31, p=0.005). When including session type (threat-of-shock versus no-threat-of-shock) as a covariate in a model regressing the GABA level on anxiety, the GABA level was no longer associated with anxiety (F=0.81, df=1, 68, p=0.37).
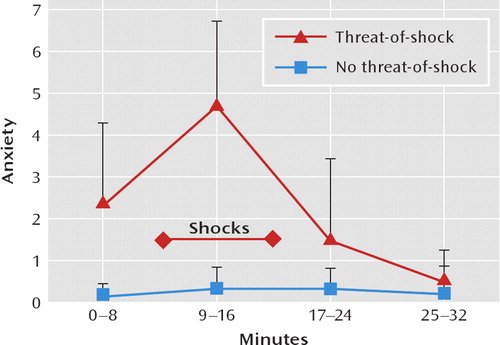
FIGURE 1. Course of Anxiety Levels Among 10 Healthy Volunteers During Threat-of-Shock and No-Threat-of-Shock (Safe) Sessionsa
aDuring the threat-of-shock session, subjects anticipated an unpleasant shock during minutes 9–16 only. Self-reported anxiety was indicated on a scale from 0 to 10 as follows: 0=absence of anxiety, 10=extreme anxiety. Anxiety levels are indicated as mean and standard deviation.
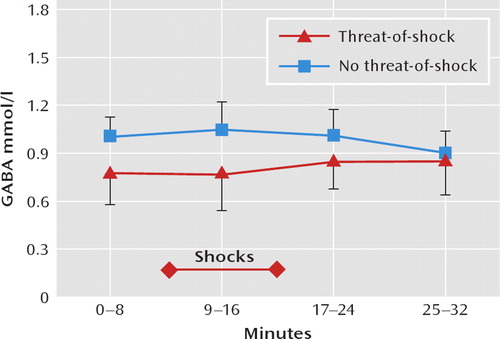
FIGURE 2. Course of Prefrontal Gamma-Aminobutyric Acid(GABA) Levels Among 10 Healthy Volunteers During Threat-of-Shock and No-Threat-of-Shock (Safe) Sessionsa
aGABA total concentration was expressed in mmol/liter (mM), referenced to the concentration of creatine; the creatine concentration is 7.1 mmol/liter. Metabolite concentrations are indicated as mean and standard deviation.
The coedited glutamate/glutamine levels, which are proportional to the total glutamate/glutamine level (Figure 3 [also see reference 1]), did not differ significantly between the threat-of-shock session and the control session, and there was no session type-by-time interaction. Similarly, N-acetyl-aspartate and choline levels measured over the entire scan session (32 minutes) did not differ between the threat-of-shock session and the control session (N-acetyl-aspartate: 9.92 mmol/liter [SD=0.60] versus 9.53 mmol/liter [SD=0.60]; choline: 1.55 mmol/liter [SD=0.16] versus 1.56 mmol/liter [SD=0.17]).
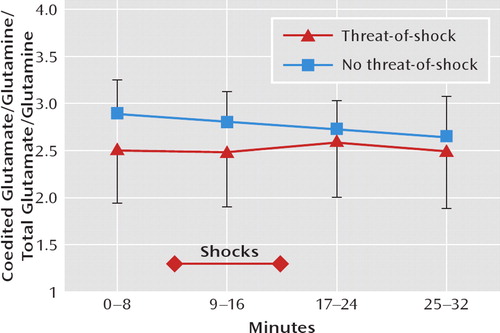
FIGURE 3. Course of Prefrontal Glutamate/Glutamine Levels Among 10 Healthy Volunteers During Threat-of-Shock and No-Threat-of-Shock (Safe) Sessionsa
aThe coedited glutamate/glutamine peak is proportional to the mean glutamate/glutamine concentration across the region of interest(see Methods in reference 10). The variation in glutamate/ glutamine editing effi ciency was estimated to be <17% (SD=4%) based on the magnitude of the residual N-acetyl-aspartate signal in the edited subspectra (1). Metabolite concentrations are indicated as mean and standard deviation.
Discussion
This study examined changes in prefrontal GABA concentration in 10 healthy volunteers during a threat-of-shock condition versus a nonthreatening control condition. As expected, the threat-of-shock condition induced robust elevations of subjective anxiety. This was associated with a concomitant reduction in prefrontal GABA concentration.
This change in GABA concentrations between the threat-of-shock session and the control session appeared relatively specific, insofar as they were not associated with significant alterations in the concentrations of N-acetylaspartate, choline-containing compounds, or coedited glutamate/glutamine levels. This observation suggests that the reduction in medial prefrontal cortex GABA concentrations during anticipatory anxiety was not attributable to nonspecific confounds such as head movement, which would cause subtraction errors in the edited spectra and reduce the coupling between the head and radiofrequency resonator coil. Such artifacts would have significantly altered signals of residual water and coedited glutamate/glutamine levels. The measured signals from N-acetyl-aspartate and choline-containing compounds would have also been affected. It is noteworthy that the GABA-editing pulse used in this study has a “top hat” frequency profile around GABA-H3 at 1.89 ppm, with the transition band in the metabolite spectrum from 2.77 ppm to 1.99 ppm. The flat portion of the spectrum reaches from 1.99 ppm to 0.43 ppm, rendering the edited detection of GABA insensitive to the effects of small head movements.
It also appears unlikely that the administration of electric shocks directly contributed to the reduction in GABA levels during the threat-of-shock session. At least 20 minutes elapsed between the sample shocks and the initiation of scanning in block one. In addition, reductions in the prefrontal cortical GABA concentration prior to the threat-of-shock session possibly reflected the effect of anticipatory anxiety. Finally, previous studies reported increased GABA concentrations following electroconvulsive therapy in depressed humans (22), and no change in GABA was found during electrical stimulation of the forepaws of anesthetized rats (25).
This study demonstrated a down-modulation of the pre-frontal cortical GABA concentration associated with acute psychological stress and a significant inverse correlation between self-reported anxiety levels and GABA concentrations. A striking feature of the results is that a substantial decrease (nearly 18% averaged over the entire 32-minute scan period) was observed using a relatively large voxel (3×3×2 cm3). These data are noteworthy, implying that a cortical region that encompasses areas that have been implicated in the brain mapping literature pertaining to anxiety-associated regional blood flow changes observed by positron emission tomography measurements in the pre-frontal cortex (26, 27) is involved. Brain GABA levels reflect the dynamic balance between GABA synthesis, reuptake, and degradation regulated at the level of glutamate decarboxylase and GABA-transaminase. A decrease in GABA concentration could result from decrease in GABA synthesis or through an increase in GABA degradation by GABA transaminase intracellularly within the mitochondria (28).
Studies in experimental animals showed consistently that stress modulates GABA release, although the direction of change has varied across brain regions and types of stress. In rats, acute restraint stress increased GABA efflux in the baso-lateral nuclei of the amygdala, whereas efflux remained unchanged in the central nucleus of the amygdala (29). In the hippocampus, in contrast, forced swimming in 25°C water resulted in a 70% decrease of extracellular GABA concentration, while forced swimming in 35°C water caused a transitory increase in hippocampal GABA (8). These data suggest that acute stress (i.e., forced swimming in cold water) exerted an effect on GABA concentrations in the rat hippocampus similar in direction to the effect of acute stress we observed in the human medial prefrontal cortex. Exposure to chronic stress has been associated with decreased GABA release (30, 31). Although we are not aware of preclinical studies that are directly comparable to our study in humans, because of the relatively slow time resolution of our measurements we cannot exclude the possibility that the acute psychological stress we used caused a transitory increase in extracellular GABA followed by more persistent reduction in total GABA concentration in the prefrontal cortex associated with GABA metabolism.
However, the extant data pertaining to the regulation of GABA suggest that the reduced GABA levels in the medial prefrontal cortex under acute stress may reflect a down-regulation of GABA synthesis. At the molecular level, it is well known from neurochemical studies that GABA-transaminase is likely to be saturated with respect to GABA levels under physiological conditions (32). Therefore, a possible change in GABA degradation is unlikely to account for the observed reduction in GABA concentration. GABA is synthesized in the presynaptic terminals and cell bodies of GABA-ergic interneurons from glutamic acid by the action of glutamate decarboxylase. Although a nearly signifi-cant decrease was observed for the coedited glutamate/ glutamine H2 peak at 3.8 ppm, a large body of evidence in the literature has shown that there is no definite correlation between alterations in cortical GABA and glutamate/ glutamine concentrations (e.g., reference 33). Taken together, it is suggested that the observed decrease in GABA concentration is likely related to a rapid down-regulation of glutamate decarboxylase associated with acute psychological stress. Glutamate decarboxylase activity and GABA concentrations are modulated during a variety of physiological and pathological conditions, including acute stress (6, 13). Previous kinetic studies performed on both animals and humans have also shown that GABA undergoes rapid turnover (34–36). Estimates based on these studies and enzyme kinetic parameters suggest that the rate of change in GABA synthesis is sufficiently rapid to account for the decrease in total GABA concentration observed in this study.
Studies using in vivo microdialysis (e.g., 37) have shown a strong positive correlation between GABA release and total GABA concentration, which appears attributable to a calcium-independent nonvesicular mechanism mediated by rapid reversal of GABA transporters (38). Thus, the reduction in total prefrontal cortical GABA concentrations as determined by MRS appears to indicate that extracelluar GABA decreases during acute psychological stress. The preclinical literature suggests that this change is most likely associated with a remarkably rapid pre-synaptic modulation of GABA-ergic input in response to acute psychological stress. It might be speculated that the net effect of this reduction in GABA concentrations would be to reduce the overall inhibitory influence of GABA on neural circuits involved in responding to stress or threat. This hypothesis would be compatible with human functional neuroimaging data, which demonstrate that neurophysiological activity normally increases in the medial prefrontal cortex during anxiety provocation or acute stress (39). The molecular mechanism and functional significance of this reduced inhibitory effect of acute psychological stress in relation to impaired GABA-ergic function in anxiety disorders (40) merit further investigation.
1. : Prefrontal cortical gamma-aminobutyric acid levels in panic disorder determined by proton magnetic resonance spectroscopy. Biol Psychiatry 2009; 65:273–275Crossref, Medline, Google Scholar
2. : Role of GABA and gluta-mate circuitry in hypothalamo-pituitary-adrenocortical stress integration. Ann N Y Acad Sci 2004; 1018:35–45Crossref, Medline, Google Scholar
3. : GABAergic modulation of learned helplessness. Pharmacol Biochem Behav 1981; 15:567–570Crossref, Medline, Google Scholar
4. : On the role of endogenous GABA in the forced swimming test in rats. Pharmacol Biochem Behav 1988; 29:275–279Crossref, Medline, Google Scholar
5. : GABAA receptors mediate the changes produced by stress on GABA function and locomotor activity. Neurosci Lett 1994; 176:29–31Crossref, Medline, Google Scholar
6. : Changes in central GABAergic function following acute and repeated stress. Br J Pharmacol 1988; 93:483–490Crossref, Medline, Google Scholar
7. : Regional levels of GABA and gluta-mate in mouse brain following exposure to pain. Neuropharmacology 1974; 13:673–675Crossref, Medline, Google Scholar
8. : Exposure to novelty and forced swimming evoke stressor-dependent changes in extracellular GABA in the rat hippocampus. Neuroscience 2007; 148:794–805Crossref, Medline, Google Scholar
9. : Magnetic resonance spectroscopy studies of GABA in neuropsychiatric disorders. J Clin Psychiatry 2003; 64(suppl 3):7–14Crossref, Medline, Google Scholar
10. : Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 2007; 64:193–200Crossref, Medline, Google Scholar
11. : Altered cerebral GABAA-benzodiazepine receptor binding in panic disorder determined by [11C]flumazenil PET. Arch Gen Psychiatry 2008; 65:1166–1175Crossref, Medline, Google Scholar
12. : Decreased brain GABA(A)-benzodiazepine receptor binding in panic disorder: preliminary results from a quantitative PET study. Arch Gen Psychiatry 1998; 55:715–720Crossref, Medline, Google Scholar
13. : Neurobiological mechanisms in generalized anxiety disorder. J Clin Psychiatry 2001; 62(suppl 11):22–27; discussion 28Medline, Google Scholar
14. : Structured Clinical Interview for DSM-IV Axis I Disorders–Nonpatient Version (SCID–I/NP). New York, New York State Psychiatric Institute, Biometrics Research, 1996Google Scholar
15. : Anxious responses to predictable and unpredictable aversive events. Behav Neurosci 2004; 118:916–924Crossref, Medline, Google Scholar
16. : Blood flow changes in human somatosensory cortex during anticipated stimulation. Nature 1995; 373:249–252Crossref, Medline, Google Scholar
17. : Detection of cerebral gamma-aminobutyric acid (GABA) in bipolar disorder patients and healthy volunteers at 3T. Proc Intl Soc Mag Reson Med 2001; 9:1011Google Scholar
18. : Normal prefrontal gamma-aminobutyric acid levels in remitted depressed subjects determined by proton magnetic resonance spectroscopy. Biol Psychiatry 2005; 58:969–973Crossref, Medline, Google Scholar
19. : Absolute quantification of water and metabolites in the human brain, II: metabolite concentrations. J Magn Reson B 1993; 102:9–19Crossref, Google Scholar
20. : Creatine and creatinine metabolism. Physiol Rev 2000; 80:1107–1213Crossref, Medline, Google Scholar
21. : In vivo GABA editing using a novel doubly selective multiple quantum filter. Magn Reson Med 2002; 47:447–454Crossref, Medline, Google Scholar
22. : Increased cortical GABA concentrations in depressed patients receiving ECT. Am J Psychiatry 2003; 160:577–579Link, Google Scholar
23. : Evaluation of anatomic variation in macromolecule contribution to the GABA signal using metabolite nulling and the J-editing technique at 3T. Proc Intl Soc Mag Reson Med 2007; 15:1391Google Scholar
24. : Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry 2009; 65:792–800Crossref, Medline, Google Scholar
25. : Increased oxygen consumption in the somato-sensory cortex of alpha-chloralose anesthetized rats during forepaw stimulation determined using MRS at 11.7. Tesla. Neuroimage 2006; 32:1317–1325Crossref, Medline, Google Scholar
26. : Regional cerebral blood flow during experimental phobic fear. Psychophysiology 1993; 30:126–130Crossref, Medline, Google Scholar
27. : A functional cerebral response to frightening visual stimulation. Psychiatry Res 1993; 50:15–24Crossref, Medline, Google Scholar
28. : 4-Aminobutyrate transaminase, in Neurotransmitter Enzymes. Edited by Boulton AABaker GBYu PHTotowa NJ Humana Press, 1986Google Scholar
29. : Effects of acute and repeated restraint stress on GABA efflux in the rat basolateral and central amygdala. Brain Res 2009; 1256:61–68Crossref, Medline, Google Scholar
30. : Extracellular levels of serotonin and GABA in the hippocampus after chronic mild stress in rats: a microdialysis study in an animal model of depression. Behav Brain Res 2007; 181:42–51Crossref, Medline, Google Scholar
31. : Reduced GABA neurotransmission underlies hyperalgesia induced by repeated forced swimming stress. Behav Brain Res 2008; 189:159–169Crossref, Medline, Google Scholar
32. : Purification and characterization of the 4-aminobutyrate: 2, ketoglutarate transaminase from mouse brain. Biochemistry 1973; 12:2868–2873Crossref, Medline, Google Scholar
33. : Elevated endogenous GABA concentration attenuates glutamate-glutamine cycling between neurons and astroglia. J Neural Transm 2009; 116:291–300Crossref, Medline, Google Scholar
34. : Amino acid precursors: their transport into brain and initial metabolism, in Amino Acids as Chemical Transmitters. Edited by Fonnum F. New York, Plenum Press, 1978, pp 669–689Crossref, Google Scholar
35. : The rate of turnover of cortical GABA from [1-13C]glucose is reduced in rats treated with the GABA-transaminase inhibitor vigabatrin (gamma-vinyl GABA). Neurochem Res 1996; 21:1031–1041Crossref, Medline, Google Scholar
36. : Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol 2006; 95:1639–1644Crossref, Medline, Google Scholar
37. : Effects of GABA-transaminase inhibition on brain metabolism and amino-acid compartmentation: an in vivo study by 2D 1HNMR spectroscopy coupled with microdialysis. Exp Brain Res 1999; 127:321–327Crossref, Medline, Google Scholar
38. : Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol 2003; 89:2021–2034Crossref, Medline, Google Scholar
39. : Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatry 1999; 156:575–584Abstract, Google Scholar
40. : Reductions in occipital cortex GABA levels in panic disorder detected with 1H-magnetic resonance spectroscopy. Arch Gen Psychiatry 2001; 58:556–561Crossref, Medline, Google Scholar



