Combining Antidepressant Medications: A Good Idea?
In this issue, Blier and colleagues report on an important 6-week double-blind randomized trial in outpatients with nonpsychotic major depressive disorder (1). Mirtazapine combined with venlafaxine, bupropion, or fluoxetine resulted in significantly greater sustained remission rates (58%, 46%, 52%, respectively) than did fluoxetine plus placebo (25%) (Figure 1).
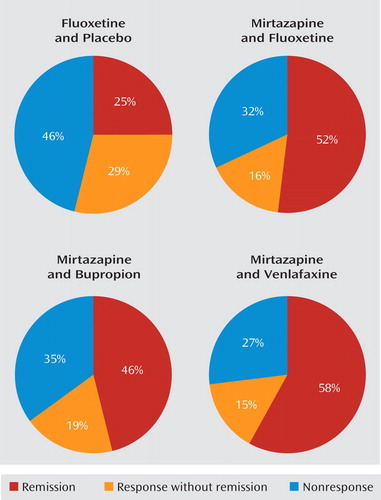
Similar significant results were reflected in the primary outcome measure (change in score on the 17-item Hamilton Depression Rating Scale from baseline to exit). Response rates, however, were not significantly different (fluoxetine, 54%; mirtazapine plus fluoxetine, 68%; mirtazapine plus bupropion, 65%; mirtazapine plus venlafaxine, 73%). Time to sustained response was not statistically different (22 days for fluoxetine alone compared with 14–15 days for the combination treatments). The mean time to sustained remission was 23–24 days (for all groups). Some measures did not reach statistical significance (the Montgomery-Åsberg Depression Rating Scale and the improvement and severity scales of the Clinical Global Impressions scale). Results of the 6-month double-blind prolongation/discontinuation trial that followed the 6-week acute trial suggested the value of combinations, especially mirtazapine plus fluoxetine and mirtazapine plus venlafaxine.
These results provide justification for combining antidepressant medications, especially mirtazapine, with other agents, and they are consistent with results from a previous study of paroxetine plus mirtazapine compared with either agent alone (2) and from a smaller placebo-controlled study of a selective serotonin reuptake inhibitor (SSRI) combined with mirtazapine (3).
The results are striking, and the message is encouraging. Could we actually double acute-phase treatment remission rates by combining two generic antidepressant medications? Does the finding apply only to mirtazapine, or is this a general principle applicable to any combination of two antidepressant medications? Some questions do remain, and firm conclusions may be premature.
What are the limitations of the study? First, patients were treated for only 6 weeks, which is well known to be insufficient time to realize the full remission rates (4). Second, as noted by the authors, the dose of fluoxetine monotherapy may have been too low for some patients. Third, the long half-lives of fluoxetine and norfluoxetine may result in a slower onset of action. The fluoxetine monotherapy might have been associated with higher remission rates had the trial been longer and had higher doses been used. Would a 12-week trial have resulted in equivalent outcomes? Perhaps each combination simply had a faster onset of action than fluoxetine plus placebo that was not detected in the analyses done in the study but that might have been had the trial been longer. This issue cannot be resolved with the available data.
How much of the 25% remission rate observed with fluoxetine might have been due to the nonspecific effects of treatment? There was no placebo group, so that estimate will remain unknown. Some patients were recruited through advertisements, while others had failed a prior treatment. One might expect a remission rate in such patients of perhaps 15%–20%. If so, the overall efficacy that could be specifically attributed to each combination might be a good deal less than the 50% remission rate that was observed. On the other hand, the prolongation/discontinuation phase results do suggest specific efficacy for two combination treatments: mirtazapine plus venlafaxine and mirtazapine plus fluoxetine, given the roughly 40% relapse rates noted when one of the two drugs was discontinued (compared with a 25% relapse rate for fluoxetine).
Assuming that there is a combination effect, is it limited to combining other antidepressant medications with mirtazapine, or is this a more general finding? That is, would any two antidepressant medications exceed the effect of any monotherapy? The study by Blier et al. cannot answer this question. But given the different presumed mechanisms of action, it could well be that the most robust and predictable benefits are limited to a reuptake blocking agent combined with mirtazapine.
For which patients might the combination be most or least valuable? While this question cannot be addressed in such a small trial, it is noteworthy that about half of the patients had not done well on at least one prior medication and most had melancholic features. These two sample characteristics may have worked against fluoxetine monotherapy, and these may be the features or clinical indicators for the combination of mirtazapine plus another antidepressant medication.
Finally, the patients in the Blier et al. study may not be entirely representative of depressed patients who are typically seen in practice. As recently noted (5), many patients in practice have substantial concurrent general medical and psychiatric comorbidity, which in turn seems to reduce the likelihood of response and remission (4). The concurrent burden from general medical and psychiatric conditions in the Blier study seems to have been modest. In addition, this was a single center study. Thus, replication and extension of this important work are needed to determine how generalizable the findings are.
Nevertheless, these results do provide a basis for considering the use of antidepressant medications in combination in practice—especially the use of mirtazapine plus a reuptake blocking agent, and particularly if the first treatment has been of sufficient dose and duration but has not been of sufficient therapeutic benefit. In my view, these combinations should not be routine first-step treatments yet, as we lack large-scale trials to establish safety and efficacy in a more generally representative population. On the other hand, the results of the Blier et al. study and work by others (2, 3) support the use of an SSRI or a selective norepinephrine reuptake inhibitor combined with mirtazapine as a second or third step. The decision to use such combinations must be tempered by and weighed in conjunction with the knowledge that weight gain and sedation are expected side effects. Further efforts to evaluate the safety, efficacy, and place for antidepressant medication combinations are called for.
1 : Combination of antidepressant medications from treatment initiation for major depressive disorder: a double-blind randomized study. Am J Psychiatry 2010; 167:281–288 Link, Google Scholar
2 : Mirtazapine and paroxetine in major depression: a comparison of monotherapy versus their combination from treatment initiation. Eur Neuropsychopharmacol 2009; 19:457–465 Crossref, Medline, Google Scholar
3 : A double-blind, placebo-controlled study of antidepressant augmentation with mirtazapine. Biol Psychiatry 2002; 51:183–188 Crossref, Medline, Google Scholar
4 ; : Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006; 163:28–40 Link, Google Scholar
5 : Can phase III trial results of antidepressant medications be generalized to clinical practice? a STAR*D report. Am J Psychiatry 2009; 166:599–607 Link, Google Scholar



