An fMRI Study of the Effects of Psychostimulants on Default-Mode Processing During Stroop Task Performance in Youths With ADHD
Abstract
Objective: The authors examined the effect of psychostimulants on brain activity in children and adolescents with ADHD performing the Stroop Color and Word Test. Method: The authors acquired 52 functional MRI scans in 16 youths with ADHD who were known responders to stimulant medication and 20 healthy comparison youths. Participants with ADHD were scanned on and off medication in a counterbalanced design, and comparison subjects were scanned once without medication. Results: Stimulant medication significantly improved suppression of default-mode activity in the ventral anterior cingulate cortex in the ADHD group. When off medication, youths with ADHD were unable to suppress default-mode activity to the same degree as comparison subjects, whereas when on medication, they suppressed this activity to comparison group levels. Greater activation of the lateral prefrontal cortex when off medication predicted a greater reduction in ADHD symptoms when on medication. Granger causality analyses demonstrated that activity in the lateral prefrontal and ventral anterior cingulate cortices mutually influenced one another but that the influence of the ventral anterior cingulate cortex on the lateral prefrontal cortex was significantly reduced in youths with ADHD off medication relative to comparison subjects and increased significantly to normal levels when ADHD youths were on medication. Conclusions: Psychostimulants in youths with ADHD improved suppression of default-mode activity in the ventral anterior cingulate and posterior cingulate cortices, components of a circuit in which activity has been shown to correlate with the degree of mind-wandering during attentional tasks. Stimulants seem to improve symptoms in youths with ADHD by normalizing activity within this circuit and improving its functional interactions with the lateral prefrontal cortex.
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common psychiatric disorders in children. Although psychostimulant medications have robust clinical efficacy in treating ADHD, knowledge of the neural basis for this efficacy is limited. The knowledge we have derives mainly from animal models and positron emission tomography studies in adults, both of which are of limited relevance for children with ADHD. Brain regions that have been shown with fair consistency to activate less in children with ADHD compared with healthy comparison subjects during performance of tasks that require attention and inhibitory control include the striatum (1 – 3) , the anterior cingulate cortex (4) , the prefrontal cortex (3 , 5 , 6) , and the inferior frontal gyrus (5 , 7) .
Several studies have examined changes in brain activity associated with improved performance on attentional and inhibitory tasks following administration of stimulant medication in both children (1 , 8 – 12) and adults (13 – 15) with ADHD. These studies have varied considerably in design, sample characteristics, and behavioral paradigm used during scanning. They have typically studied small numbers of children with ADHD, and their findings have been highly variable, involving multiple regions across the brain, including the cerebellar vermis (11) , the basal ganglia (1 , 8) , and the cerebral cortex (1 , 11) .
A better understanding of the neural mechanisms underlying the therapeutic actions of these medications may provide important insight into the pathogenesis of ADHD and promote the development of improved treatments. Clarifying the effects of stimulants on brain functioning in ADHD requires identifying the brain regions that function abnormally without medication and change in response to the medication. The degree to which brain functioning changes in response to medication, along with the degree to which activation correlates with medication-induced improvements in symptom severity, should help in identifying the neural systems that mediate the therapeutic effects of stimulants.
ADHD symptoms involve primarily disturbances in attention and impulse control. We used functional MRI (fMRI) to examine the therapeutic effects of stimulants in stimulant-responsive youths with ADHD as they performed a task requiring selective attention and inhibitory control, both when they were taking and not taking stimulant medication. We hypothesized that brain activity in youths with ADHD off stimulants would differ from activity in healthy comparison subjects (3 , 6 , 12 , 15) and that stimulants would induce changes in activation levels in youths with ADHD in the direction of those observed in comparison subjects (1 , 15) .
Method
Study Design
We studied youths with ADHD who were documented robust responders to stimulant medication so as to enhance our ability to detect changes in brain activation that likely mediated the therapeutic effects of stimulants. We scanned these youths twice, once each when they were medicated and unmedicated, randomizing and counterbalancing the order of scanning in the medicated and unmedicated states to ensure the absence of practice or habituation effects on our findings. Healthy comparison subjects were scanned once, enabling us to determine whether the directions of medication-induced changes in brain functioning in youths with ADHD were toward or away from normal levels of activity.
Several interrelated considerations led to selection of the Stroop Color and Word Test (16) as the preferred paradigm to probe changes in neural activity associated with stimulant use in this study. First, ADHD has been regarded by some as a disorder of self-regulatory control (17) in which the individual has difficulty inhibiting more automatic behavioral tendencies in order to perform the less automatic behaviors that are required of them in a social or an experimental setting. These are in fact the requirements of the Stroop task—to inhibit the more automatic tendency to read a written word while performing the less automatic task of naming the color of ink in which the word is written (18) . Second, the color and word stimuli are perfectly counterbalanced across the active and control conditions (see the data supplement that accompanies the online edition of this article), thereby isolating neural activity associated with self-regulating response tendencies during the task. Third, youths with ADHD have been shown in meta-analyses to perform more poorly on the Stroop task than do comparison subjects (19 , 20) especially in their speed of response, indicating the possible presence of impaired self-regulatory control on this task. Fourth, the neural basis of ADHD is generally believed to involve disturbances in the cortical-subcortical circuits that support the capacity for self-regulatory control (17 , 19) , and the Stroop task produces robust activation of both the cortical and subcortical portions of these circuits (18) . Finally, stimulant medications are believed to alter activity in these circuits (21) , making use of the Stroop task a logical choice to study the effects of stimulant medications in ADHD.
Details of the behavioral assessments, MRI pulse sequence, image processing, and Stroop paradigm are described in the online data supplement.
Participants
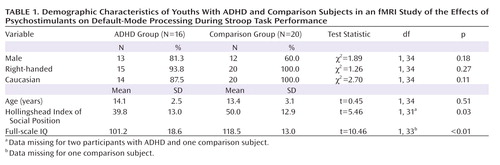
Participants were 16 children and adolescents with ADHD, ages 7–18 years, and 20 healthy comparison subjects in the same age group ( Table 1 ). Assessments included the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (22) , the DuPaul-Barkley ADHD Rating Scale (23) , Conners’ Parent Rating Scale (24) , the Hollingshead Index of Social Position, and the Wechsler Abbreviated Scale of Intelligence. All participants in the ADHD group met criteria for combined-type ADHD, tolerated stimulant medication well, and were documented responders to stimulant medication (which we defined as having had a statistically significant change in score on the Conners’ Parent Rating Scale before and after medication use). We tested this change by dividing the change score associated with stimulant use (pretreatment score minus posttreatment score) by the standard error of the change scores (estimated by the standard error of test-retest differences identified during instrument validation). A statistically significant change on this measure was defined as >1.96 standard deviations above the mean (i.e., p<0.05).
Exclusion criteria for all participants included history of seizure, head trauma, substance abuse, or medication other than stimulants. Comparison subjects had no current or lifetime psychiatric disorders and took no medications; they were recruited through phone calls to randomly selected households on a telemarketing list. The institutional review boards of Yale and Columbia Universities approved the study. After a complete description of the study was provided to the participants and their parents, written informed consent and assent were obtained.
fMRI Paradigm
Participants performed two runs, each lasting 3 minutes and 44 seconds, and each consisting of four blocks of 16 congruent or incongruent colored word stimuli. Stimulus and interstimulus durations were 1,300 and 350 msec, respectively. Participants named the color of each stimulus, regardless of the meaning of the written word, quietly and with minimal mouth movement. The on- and off-medication scanning sessions for participants in the ADHD group were conducted an average of 24.5 days apart (range=7–63 days, SD=17.3 days). For the on-medication scans, the most recent clinically effective dose of medication was given 45–60 minutes before scanning to generate peak blood levels during the scan. For off-stimulant scans, participants in the ADHD group were medication-free for at least 3 days, during medication holidays over the weekend or during school vacations. Given their short half-life, all stimulants should have cleared before the scans were conducted.
Hypothesis Testing
fMRI data were analyzed statistically using SPM99 (Wellcome Trust Centre for Neuroimaging, London). Stimulus blocks were entered into the design matrix and convolved with the canonical hemodynamic response function. Individual contrast images were created for each participant within each condition. Those contrast images were intensity-normalized using the respective last beta images in each run of the fMRI time series. A conjunction mask was applied to these individual contrast images to ensure that only voxels without signal voids were analyzed. These spatially smoothed images were entered into a random-effects analysis to eliminate highly discrepant variances between and within participants in constructing appropriate error terms for hypothesis testing and to enhance population generalizability. The multisubject group analysis required input of one scan per subject for each condition within a mixed-model analysis to account for both random effects (of scan) and fixed effects (of task condition). Centered age (the mean age of the group subtracted from the age of each participant) was entered as a covariate in all second-level analyses. The statistical contrast of interest was incongruent versus congruent colored word stimuli. Paired t-statistics compared this contrast across medication conditions within the ADHD group. We report voxels identified using a p threshold of 0.05 together with the requirement that the activation occurred in a spatial cluster of at least 25 adjacent pixels, a conjoint requirement which, based on an approximation formula (25) , yielded an effective p threshold of 0.000005, which reduced substantially the false positive identification of activated pixels.
Exploratory Analyses
A post hoc analysis using unpaired t-statistics compared brain activity in the incongruent versus congruent contrast across the ADHD and comparison groups to determine whether the stimulant-induced changes in brain activity in the children with ADHD represented a change either toward or away from activity in healthy children. A second post hoc analysis assessed whether activation in the ADHD group when off medication correlated with the change in total symptom severity following medication (medicated minus unmedicated severity). Finally, we used Granger causality analysis (26) to assess whether activity in the inferolateral prefrontal cortex and ventral anterior cingulate cortex significantly influenced one another and whether these causal influences changed significantly in response to stimulant medication (see the online data supplement).
Results
Participants’ demographic characteristics are summarized in Table 1 . Five of the youths with ADHD also had diagnoses of current comorbid disorders (one with depression and oppositional defiant disorder, two with specific phobias, and one each with separation or generalized anxiety disorders). Lifetime disorders were diagnosed in 11 youths with ADHD (four with depression, one with separation anxiety disorder, three with specific phobia, one with conduct disorder, and five with learning disorder, with all except the learning disorders resolving by the time of the study). The clinically effective stimulants taken most recently by participants in the ADHD group included methylphenidate (N=11; dosage: mean=24.8 mg/day [SD=12.1, range=10–50]), dextroamphetamine (N=4; dosage: mean=21.3 [SD=7.5, range=15–30]), and dextroamphetamine/amphetamine (N=1; dosage, 30 mg/day), all standard, immediate-release, oral preparations.
Behavioral Effects
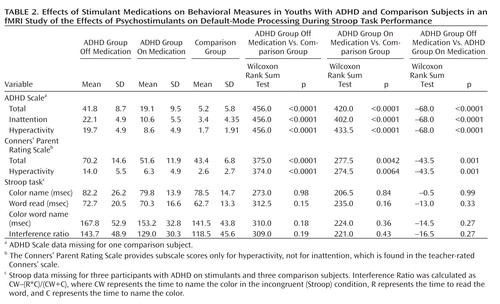
Youths in the ADHD group on and off stimulants scored significantly higher than comparison subjects on measures of inattention and hyperactivity, and when on stimulants their scores ranged between their own scores off stimulants and the scores of the comparison group. A similar pattern was observed for performance on the Stroop task, including a nonsignificant lessening of interference scores in youths with ADHD on medication ( Table 2 ).
Stimulant Effects on Brain Activation
Participants in the ADHD and comparison groups both activated prefrontal, anterior cingulate, and parietal cortices, basal ganglia, and thalamus ( Figure 1 ). All analyses were unaffected when covarying for IQ or lifetime presence of depression or learning disorder (not shown).

a These are transaxial views, with Talairach z-coordinates shown to the left of the corresponding slices. The left side of the brain is displayed on the right side of the image. Voxels represent a p value threshold of 0.05 together and a spatial cluster of >25 adjacent pixels, a conjoint requirement that yields a conservative uncorrected p threshold of 0.000005. The color bars depict p values for the threshold of the respective statistical contrast, without adjustment for the conjoint requirement of the spatial cluster. Section A shows activations in children with ADHD compared across the medicated and unmedicated states. The red box indicates images that are testing the a priori hypothesis that medication would produce changes in brain activation within regions that subserve performance of this task, which requires attention and impulse control. Section B shows activations in the unmedicated children with ADHD compared with healthy comparison subjects, and section C shows activations in medicated children with ADHD compared with the comparison group. Column D shows the correlation of activation in the ADHD group off stimulant medication with the change in ADHD symptoms produced by administration of stimulant medication (calculated as unmedicated minus medicated scores on the Conners’ Parent Rating Scale) while covarying for age. For column D, blue and purple voxels indicate an inverse correlation of activation when off medication with the medication-induced changes in symptom severity in youths with ADHD, in which greater activation when off medication predicts a greater medication-induced reduction in symptoms. Red and yellow voxels indicate a positive correlation, with less activation when off medication predicting a greater medication-induced reduction in symptoms. Activation of the left lateral prefrontal cortex at baseline strongly predicted medication responsiveness (r=–0.73, df=16, p<0.001). Solid arrow: ventral anterior cingulate (BA 25). Open block arrow: anterior cingulate (BA 24). Solid block arrow: posterior cingulate cortex (BA 23 and 31). Rounded arrow: left lateral prefrontal cortex (BA 46).
A priori hypothesis testing
When the ADHD group was on stimulant medications, more prominent deactivations were observed in the ventral anterior cingulate cortex (Brodmann’s area [BA] 24: Talairach coordinates x, y, z=5.95, 25.38, 20; z-statistic=–2.98; BA 25: x, y, z=0.85, 7.88, 0; z-statistic=–2.70) and the posterior cingulate cortex (BA 23 and 31: x, y, z=7.65, –39.79, 50; z-statistic=–3.71) than when not on stimulants ( Figure 1 A). Comparing activations in the first and second scans within the ADHD group revealed no significant effects of scan order (not shown).
Post hoc analyses
Comparing brain activation in unmedicated youths with ADHD and comparison subjects revealed significantly less prominent deactivation in the ADHD group than in the comparison group in the ventral anterior cingulate cortex (BA 25: x, y, z=–7.65, 42.88, 0; z-statistic=2.50). These abnormalities were not detected in the ADHD group when they were on stimulants ( Figure 1 ).
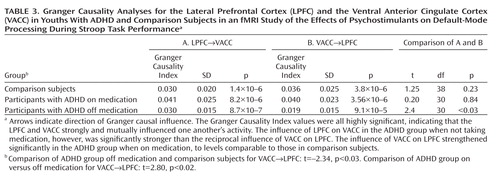
Activation of the left lateral prefrontal cortex (BA 46: x, y, z=–23.75, –0.43, 10; z-statistic=2.73) also tended to normalize in the ADHD group relative to the comparison group when medication was administered, although this increased activation in the medicated compared with the unmedicated condition did not reach statistical significance ( Figure 1 A). Greater activation of the same left lateral prefrontal cortex in the ADHD group when off medication predicted a larger reduction in symptom severity when on medication (r=–0.73, df=16, p<0.001) ( Figure 1 D). Granger causality analyses indicated that the lateral prefrontal cortex and ventral anterior cingulate cortex each significantly influenced activity in the other, but with the influence from ventral anterior cingulate cortex to lateral prefrontal cortex weaker in the ADHD group when off medication compared with when they were on medication and weaker than in the comparison group, and then increasing significantly to comparison group levels when taking medication ( Table 3 ).
Discussion
Administration of psychostimulants in youths with ADHD significantly improved the symptoms of hyperactivity and inattention. A similar but less pronounced effect was observed for measures of Stroop task performance. Stimulants also made task-related deactivations in the ventral anterior cingulate cortex and posterior cingulate cortex significantly more prominent in youths with ADHD, producing levels similar to those in healthy comparison subjects, and increased activation of the lateral prefrontal cortex to comparison group levels, although the change did not reach statistical significance. Greater activation of the lateral prefrontal cortex when off stimulants predicted a greater reduction in ADHD symptom severity when on stimulants. Causality analyses indicated that functional interactions between the ventral anterior cingulate cortex and the lateral prefrontal cortex were reduced in the ADHD group when off medication compared with interactions in comparison subjects and that these interactions in the ADHD group increased to normal levels when on stimulant medication. These findings suggest that stimulants improve symptoms in youths with ADHD by normalizing activity within a distributed network of brain regions in the anterior cingulate cortex and posterior cingulate cortex and by improving the functional interactions of that circuit with the lateral prefrontal cortex.
Numerous studies have reported deactivations in the ventral anterior cingulate cortex and posterior cingulate cortex across a wide range of tasks in both children and adults (27 , 28) . Deactivation reflects either increased activity during the less challenging control blocks of stimuli or the suppression of this activity during the more challenging active task condition. Which of these alternatives produces deactivation cannot be determined without absolute measures of brain activity in the resting condition, and our study, similar to most studies reporting deactivations, did not include these measures. Nevertheless, greater neural activity in these regions during an easier baseline condition is thought to represent a greater degree of non-task-related mental activity (termed “default-mode” activity) during the baseline condition, activity that must be suppressed during the more challenging active task for optimal performance. Indeed, the magnitude of deactivation in these regions correlates inversely with the frequency of interruptions by task-unrelated thoughts (29 , 30) , which suggests that mind-wandering during the easier baseline task and its suppression during the active task produces more prominent deactivation. Deactivations are more prominent in healthy adults than in healthy children performing the Stroop task (28) , which suggests that adults may more effectively suppress default-mode activity during the more difficult task condition. The absence of deactivations in children with ADHD when not taking stimulant medication suggests either that they did not generate default-mode activity during the easier baseline condition or that they did not successfully suppress it during the attentionally more challenging active task. Greater deactivation in default-mode circuits during the administration of stimulant medications, when ADHD symptoms were improved, suggests that the most parsimonious interpretation of the absence of deactivation when not taking medication is a failure to suppress default-mode processing during performance of the more difficult active task.
The ventral anterior cingulate cortex interconnects the orbitofrontal cortex, temporal pole, amygdala, ventral striatum, and hypothalamus. It is an anatomical crossroads that contributes to the motivational processes required for goal-directed behaviors. Lesions of this area produce premature, or “impulsive,” responding in rats, impaired social valuation in macaques, and distractibility, inattention, and emotional unconcern in humans (17) . Thus, default-mode activity in the ventral anterior cingulate cortex may reflect the motivational, affective, and rewarding properties of mind-wandering during the easier baseline task (31) . Activity in the ventral anterior cingulate cortex, and therefore these associated subjective experiences, presumably must be suppressed to reduce distraction and improve performance during the more difficult attention-demanding task.
Previous anatomical and functional imaging studies support involvement of the default-mode circuit or related, anatomically connected brain regions in the pathogenesis of ADHD. Reduced volumes and cortical thinning in the ventral anterior cingulate cortex have been demonstrated in both cross-sectional and longitudinal imaging studies of ADHD (32 , 33) . Reduced gray matter volumes (34) and reduced activation in proportion to symptom severity during inhibitory tasks (3 , 5 , 6) have also been detected in the posterior cingulate cortex. The sizes of the lateral prefrontal cortex and anterior temporal cortices bilaterally are reduced in children with ADHD (35) , both of which are densely connected with the ventral anterior cingulate cortex (36) . Disturbances in resting-state activity of the ventral anterior cingulate cortex and posterior cingulate cortex have been reported in adults with ADHD (37) . Finally, methylphenidate decreases blood flow to the ventral anterior cingulate cortex, posterior cingulate cortex, and inferior parietal cortex in adults with ADHD in direct proportion to improved performance on cognitive tasks, which has been attributed to an improved efficiency of neural processing in these regions and the reduced mind-wandering induced by stimulants (38) .
Several considerations suggest that stimulants may have produced greater suppression of default-mode activity in the ventral anterior cingulate cortex in our study by increasing activation of the lateral prefrontal cortex and strengthening the interactions of the lateral prefrontal cortex with the ventral anterior cingulate cortex. First, the lateral prefrontal cortex is known to regulate activity in multiple brain regions and neural systems (39) , and its anatomical connections with the ventral anterior cingulate cortex and posterior cingulate cortex (36) suggest that the lateral prefrontal cortex may regulate activity in the default-mode system as well. Second, a stronger inverse coupling of activity in the lateral prefrontal cortex with default-mode activity in the ventral anterior cingulate cortex predicts improved behavioral performance on Stroop-like tasks in healthy persons (40) . Moreover, causality analyses have provided compelling evidence that activity in the lateral prefrontal cortex plays a crucial role in controlling default-mode activity in the ventral anterior cingulate cortex across a wide range of tasks (41) . Consistent with these prior findings, our Granger causality analyses indicated that activity in the lateral prefrontal cortex and ventral anterior cingulate cortex mutually influence one another but that the influence of the ventral anterior cingulate cortex on the lateral prefrontal cortex is significantly reduced in youths with ADHD off medication relative to comparison subjects and that it increases significantly to normal levels when youths with ADHD are on stimulants. Third, previous studies have reported that stimulant medications increase activation of the lateral prefrontal cortex in children with ADHD during performance of cognitive control tasks (1 , 11) . Consistent with this prior finding, activation of the lateral prefrontal cortex increased to normal levels in our ADHD participants when they were on stimulants. Finally, greater lateral prefrontal cortex activation in our ADHD group off medication predicted a greater reduction in symptoms following medication. Together with our finding of increased functional interactions with the ventral anterior cingulate cortex while on stimulants and the prior evidence that activity in the anterior cingulate cortex indexes mind-wandering (29 , 30) , this finding suggests that greater activation of the lateral prefrontal cortex off medication may indicate a greater capacity to respond to stimulants by increasing functional interactions with the ventral anterior cingulate cortex and that this greater functional interaction is required to suppress default-mode activity and the mind-wandering that it indexes.
The findings and interpretations of this study should be considered in light of its limitations. First, our findings of differing brain activation across diagnostic groups could reflect the use of differing strategies to perform the task, rather than differences in the degree of suppression of default-mode activity. Normalization of this activity in response to stimulant medication, however, speaks against this possibility, as stimulants would be unlikely to alter task strategies. Second, subvocalization was selected as the response modality because it reduces fMRI artifacts caused by overt speech, it produces more robust Stroop interference than does a manual response, and use of a button press instead of a vocal response introduces a complex mapping of color names to finger response that fundamentally changes the nature of the task. Subvocalization, however, required measurement of task performance after the scan rather than during it, and therefore differential practice or habituation effects may have contributed to group differences in behavioral performance. Weighing against this possibility is that performance did not change with time during repeated behavioral testing in the ADHD group. Moreover, previous studies have shown similar performance and brain activation using either overt or covert verbal responses during the Stroop task (42) . Third, including both correct and incorrect responses in our block-design fMRI analyses may have allowed the effects of error processing to contaminate activation maps. Previous event-related Stroop studies, however, have demonstrated error rates of 3% in the scanner (43) , which is consistent with error rates noted outside of the scanner in this study, and inclusion of erroneous trials at these low rates would have a negligible effect on activation maps. Fourth, differences in reading proficiency could cause group differences in brain activation, but given comparable performance across groups on the word-reading subtest of the Stroop task, this potential confounder is unlikely. Fifth, the presence of comorbid illnesses and lower IQs in the ADHD group could have contributed to our results, although statistical covariation for these factors did not change any findings. Moreover, comorbidities would not invalidate within-subject analyses comparing brain activation on and off stimulants. Sixth, our findings cannot be generalized to youths with ADHD who are medication-naive, as our study included by design only children who were known robust responders to stimulant medication. Nevertheless, our findings do generalize to the vast number of children who take and benefit from stimulant medications. Seventh, we did not rescan youths in the comparison group, which would have helped to estimate the effects of habituation across all youths in the study, but at a substantial financial cost. The counterbalanced randomization adequately controlled for habituation effects in the ADHD group, however, and permitted us to address the central scientific question of the study, which was whether stimulants alter brain functioning during a selective attention task in youths with ADHD, and not whether rescanning produces habituation during the task in healthy youths. Finally, this study did not include a placebo arm in the ADHD group, and therefore we cannot exclude the possibility that the findings may also reflect placebo effects. The ADHD youths, however, were highly robust and enduring responders to stimulant medications, and stimulant effects in general are highly robust compared to placebo in this population, which suggests that a placebo response is an unlikely cause of their symptomatic improvement and changes in brain activity.
Despite its limitations, this study is, to our knowledge, the first to demonstrate explicitly that stimulant medications improve suppression of default-mode processing during an attentional task in youths with ADHD. Our findings support the previously stated hypothesis that the failure to suppress mental processes associated with default-mode neural processing in the ventral anterior cingulate cortex and posterior cingulate cortex may contribute to the symptoms of ADHD (37 , 44) . Stimulants improved suppression of this activity in known robust responders to stimulant medication. With improvement in this suppressive activity, brain activation generally normalized and parent-reported symptoms of ADHD improved, as did objective measures of performance during attentional tasks.
1. Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, Gabrieli JD: Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 1998; 95:14494–14499Google Scholar
2. Durston S, Tottenham NT, Thomas KM, Davidson MC, Eigsti IM, Yang Y, Ulug AM, Casey BJ: Differential patterns of striatal activation in young children with and without ADHD. Biol Psychiatry 2003; 53:871–878Google Scholar
3. Vaidya CJ, Bunge SA, Dudukovic NM, Zalecki CA, Elliott GR, Gabrieli JDE: Altered neural substrates of cognitive control in childhood ADHD: evidence from functional magnetic resonance imaging. Am J Psychiatry 2005; 162:1605–1613Google Scholar
4. Konrad K, Neufang S, Hanisch C, Fink GR, Herpertz-Dahlmann B: Dysfunctional attentional networks in children with attention deficit/hyperactivity disorder: evidence from an event-related functional magnetic resonance imaging study. Biol Psychiatry 2006; 59:643–651Google Scholar
5. Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E: Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry 2005; 162:1067–1075Google Scholar
6. Smith AB, Taylor E, Brammer M, Toone B, Rubia K: Task-specific hypoactivation in prefrontal and temporoparietal brain regions during motor inhibition and task switching in medication-naive children and adolescents with attention deficit hyperactivity disorder. Am J Psychiatry 2006; 163:1044–1051Google Scholar
7. Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H: Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol Psychiatry 2006; 60:1062–1070Google Scholar
8. Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA: The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am J Psychiatry 2004; 161:1990–1997Google Scholar
9. Lee JS, Kim BN, Kang E, Lee DS, Kim YK, Chung JK, Lee MC, Cho SC: Regional cerebral blood flow in children with attention deficit hyperactivity disorder: comparison before and after methylphenidate treatment. Hum Brain Mapp 2005; 24:157–164Google Scholar
10. Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, Gjedde A: Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. Neuroimage 2005; 25:868–876Google Scholar
11. Epstein JN, Casey BJ, Tonev ST, Davidson MC, Reiss AL, Garrett A, Hinshaw SP, Greenhill LL, Glover G, Shafritz KM, Vitolo A, Kotler LA, Jarrett MA, Spicer J: ADHD- and medication-related brain activation effects in concordantly affected parent-child dyads with ADHD. J Child Psychol Psychiatry 2007; 48:899–913Google Scholar
12. Konrad K, Neufang S, Fink GR, Herpertz-Dahlmann B: Long-term effects of methylphenidate on neural networks associated with executive attention in children with ADHD: results from a longitudinal functional MRI study. J Am Acad Child Adolesc Psychiatry 2007; 46:1633–1641Google Scholar
13. Aron AR, Dowson JH, Sahakian BJ, Robbins TW: Methylphenidate improves response inhibition in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry 2003; 54:1465–1468Google Scholar
14. Schweitzer JB, Lee DO, Hanford RB, Zink CF, Ely TD, Tagamets MA, Hoffman JM, Grafton ST, Kilts CD: Effect of methylphenidate on executive functioning in adults with attention-deficit/hyperactivity disorder: normalization of behavior but not related brain activity. Biol Psychiatry 2004; 56:597–606Google Scholar
15. Bush G, Spencer TJ, Holmes J, Shin LM, Valera EM, Seidman LJ, Makris N, Surman C, Aleardi M, Mick E, Biederman J: Functional magnetic resonance imaging of methylphenidate and placebo in attention-deficit/hyperactivity disorder during the multi-source interference task. Arch Gen Psychiatry 2008; 65:102–114Google Scholar
16. MacLeod CM: Half a century of research on the Stroop effect: an integrative review. Psychol Bull 1991; 109:163–203Google Scholar
17. Plessen K, Peterson BS: The neurobiology of impulsivity and self-regulatory control in children with attention-deficit/hyperactivity disorder, in Neurobiology of Mental Illness, 3rd ed. Edited by Charney D, Nestler EJ. Oxford, England, Oxford University Press, 2008, pp 1129–1152Google Scholar
18. Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC: An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry 1999; 45:1237–1258Google Scholar
19. Nigg JT: Neuropsychologic theory and findings in attention-deficit/hyperactivity disorder: the state of the field and salient challenges for the coming decade. Biol Psychiatry 2005; 57:1424–1435Google Scholar
20. van Mourik R, Oosterlaan J, Sergeant JA: The Stroop revisited: a meta-analysis of interference control in AD/HD. J Child Psychol Psychiatry 2005; 46:150–165Google Scholar
21. Wilens TE: Effects of methylphenidate on the catecholaminergic system in attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2008; 28:S46–S53Google Scholar
22. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Google Scholar
23. DuPaul GJ: Parent and teacher ratings of ADHD symptoms: psychometric properties in a community-based sample. J Clin Child Psychology 1991; 20:245–253Google Scholar
24. Connors C: Psychological assessment of children with minimal brain dysfuntion. Ann NY Acad Sci 1973; 205:283–302Google Scholar
25. Poline JB, Worsley KJ, Evans AC, Friston KJ: Combining spatial extent and peak intensity to test for activations in functional imaging. Neuroimage 1997; 5:83–96Google Scholar
26. Chen Y, Rangarajan G, Feng J, Ding M: Analyzing multiple nonlinear time series with extended Granger causality. Physics Letters A 2004; 324:26–35Google Scholar
27. Gusnard DA, Akbudak E, Shulman GL, Raichle ME: Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA 2001; 98:4259–4264Google Scholar
28. Marsh R, Zhu H, Schultz RT, Quackenbush G, Royal J, Skudlarski P, Peterson BS: A developmental fMRI study of self-regulatory control. Hum Brain Mapp 2006; 27:848–863Google Scholar
29. McKiernan KA, D’Angelo BR, Kaufman JN, Binder JR: Interrupting the “stream of consciousness”: an fMRI investigation. Neuroimage 2006; 29:1185–1191Google Scholar
30. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN: Wandering minds: the default network and stimulus-independent thought. Science 2007; 315:393–395Google Scholar
31. Simpson JR Jr, Snyder AZ, Gusnard DA, Raichle ME: Emotion-induced changes in human medial prefrontal cortex, I: during cognitive task performance. Proc Natl Acad Sci USA 2001; 98:683–687Google Scholar
32. Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biederman J: Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biol Psychiatry 2006; 60:1071–1080Google Scholar
33. Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, Giedd J, Castellanos FX, Rapoport J: Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2006; 63:540–549Google Scholar
34. Overmeyer S, Bullmore ET, Suckling J, Simmons A, Williams SC, Santosh PJ, Taylor E: Distributed grey and white matter deficits in hyperkinetic disorder: MRI evidence for anatomical abnormality in an attentional network. Psychol Med 2001; 31:1425–1435Google Scholar
35. Sowell ER, Thompson PM, Welcome SE, Henkenius AL, Toga AW, Peterson BS: Cortical abnormalities in children and adolescents with attention-deficit hyperactivity disorder. Lancet 2003; 362:1699–1707Google Scholar
36. Morris R, Pandya DN, Petrides M: Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J Comp Neurol 1999; 407:183–192Google Scholar
37. Castellanos FX, Margulies DS, Kelly C, Uddin LQ, Ghaffari M, Kirsch A, Shaw D, Shehzad Z, Di Martino A, Biswal B, Sonuga-Barke EJ, Rotrosen J, Adler LA, Milham MP: Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol Psychiatry 2008; 63:332–337Google Scholar
38. Volkow ND, Fowler JS, Wang GJ, Telang F, Logan J, Wong C, Ma J, Pradhan K, Benveniste H, Swanson JM: Methylphenidate decreased the amount of glucose needed by the brain to perform a cognitive task. PLoS One 2008; 3:e2017Google Scholar
39. Buschman TJ, Miller EK: Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science 2007; 315:1860–1862Google Scholar
40. Clare Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP: Competition between functional brain networks mediates behavioral variability. Neuroimage 2008; 39:527–537Google Scholar
41. Sridharan D, Levitin DJ, Menon V: A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 2008; 105:12569–12574Google Scholar
42. Laird AR, McMillan KM, Lancaster JL, Kochunov P, Turkeltaub PE, Pardo JV, Fox PT: A comparison of label-based review and ALE meta-analysis in the Stroop task. Hum Brain Mapp 2005;25:6–21Google Scholar
43. MacDonald AW 3rd, Cohen JD, Stenger VA, Carter CS: Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288:1835–1838Google Scholar
44. Sonuga-Barke EJ, Castellanos FX: Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci Biobehav Rev 2007; 31:977–986Google Scholar