Reduced Caudate and Nucleus Accumbens Response to Rewards in Unmedicated Individuals With Major Depressive Disorder
Abstract
Objective: Major depressive disorder is characterized by impaired reward processing, possibly due to dysfunction in the basal ganglia. However, few neuroimaging studies of depression have distinguished between anticipatory and consummatory phases of reward processing. Using functional MRI (fMRI) and a task that dissociates anticipatory and consummatory phases of reward processing, the authors tested the hypothesis that individuals with major depression would show reduced reward-related responses in basal ganglia structures. Method: A monetary incentive delay task was presented to 30 unmedicated individuals with major depressive disorder and 31 healthy comparison subjects during fMRI scanning. Whole-brain analyses focused on neural responses to reward-predicting cues and rewarding outcomes (i.e., monetary gains). Secondary analyses focused on the relationship between anhedonic symptoms and basal ganglia volumes. Results: Relative to comparison subjects, participants with major depression showed significantly weaker responses to gains in the left nucleus accumbens and the caudate bilaterally. Group differences in these regions were specific to rewarding outcomes and did not generalize to neutral or negative outcomes, although relatively reduced responses to monetary penalties in the major depression group emerged in other caudate regions. By contrast, evidence for group differences during reward anticipation was weaker, although participants with major depression showed reduced activation to reward cues in a small sector of the left posterior putamen. In the major depression group, anhedonic symptoms and depression severity were associated with reduced caudate volume bilaterally. Conclusions: These results suggest that basal ganglia dysfunction in major depression may affect the consummatory phase of reward processing. Additionally, morphometric results suggest that anhedonia in major depression is related to caudate volume.
Anhedonia—lack of reactivity to pleasurable stimuli—is a core symptom of major depressive disorder (1 , 2) . Relative to healthy comparison subjects, depressed individuals display reduced positive attentional biases (3) , weaker positive affect in response to pleasant stimuli (4) , and reduced reward responsiveness (5) . Neuroimaging indicates that these deficits may reflect dysfunction in the basal ganglia, including the striatum (nucleus accumbens, caudate, putamen) and the globus pallidus (6 – 11) . However, the functional significance of basal ganglia dysfunction in major depression remains poorly understood. Specifically, whether dysfunction is more closely associated with deficits in the anticipatory or the consummatory phase of reward processing is unclear.
Dissociating these phases is important for two reasons (12) . First, they reflect different psychological states: anticipation is characterized by goal-directed behavior, whereas consummation involves pleasure experience (13) . Second, they make separable contributions to goal-directed behavior (14) . In nonhuman primates, unexpected rewards elicit phasic bursts in dopamine neurons projecting from the midbrain to the basal ganglia (14) . However, the bursts eventually shift from the rewards to reward-predicting cues. Because the basal ganglia are critical for motor control (15) , this constitutes a mechanism by which reward-predicting cues can elicit motivated behavior. Given dopamine abnormalities in major depression (16) , depression may involve impairments in the anticipatory and/or consummatory components of this mechanism.
To explore this issue, a recent study (17) used a monetary incentive delay task to investigate anticipatory versus consummatory phases of reward processing in 14 participants with major depression and 12 comparison subjects. Surprisingly, there were no group differences in basal ganglia responses to reward cues. Furthermore, although participants with major depression showed bilateral reductions in putamen responses to gains, no outcome-related differences emerged in the accumbens or the caudate, regions implicated in processing reward feedback (18 , 19) , particularly when reward delivery is unpredictable (20) . However, there were also no group differences in behavior. Thus, these null results may have reflected intact reward processing in that particular sample of patients with major depression and/or limited statistical power.
In this study, we used a similar task to probe anticipatory and consummatory phases of reward processing in a larger group of unmedicated depressed individuals (N=30) and healthy comparison subjects (N=31). To permit a balanced design, the task was modified such that 50% of reward and loss trials ended in monetary gains and penalties, respectively (21) . Given the role of dopamine and the basal ganglia in reward anticipation (22) , we predicted that depressed individuals would show blunted responses to reward cues, particularly in the ventral striatum. However, based on prior findings (17) , and because gains would be delivered in only 50% of reward trials (20) , we hypothesized that participants with major depression might primarily show impaired striatal responses to rewarding outcomes. Finally, in light of recent work (23) , we predicted that greater anhedonic symptoms would be associated with smaller caudate volumes.
Method
Participants
Depressed individuals were recruited from a treatment study comparing the effectiveness of the dietary supplement S -adenosyl- l -methionine and escitalopram. Comparison subjects were recruited from the community. Participants with major depression had a DSM-IV diagnosis of major depressive disorder according to the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID; 24 ) and had a score ≥16 on the 21-item Hamilton Depression Rating Scale (HAM-D; 25 ). Exclusion criteria were any psychotropic medication in the past 2 weeks, fluoxetine in the past 6 weeks, or dopaminergic drugs or neuroleptics in the past 6 months; a current or past history of major depressive disorder with psychotic features; and presence of other axis I diagnoses (including lifetime substance dependence and any substance use disorder in the past year), with the exception of anxiety disorders. Comparison subjects reported no medical or neurological illness, no current or past psychopathology (according to the SCID), and no use of psychotropic medications. All participants were right-handed.
The final sample included 30 participants with major depression and 31 demographically matched comparison subjects ( Table 1 ). Participants with major depression were moderately depressed, as assessed by the Beck Depression Inventory–II (BDI; 26 ) (mean score=27.48 [SD=10.60]) and the 17-item HAM-D (mean score=17.97 [SD=4.19]). Eleven depressed participants had a current anxiety disorder, and three had subthreshold anxiety symptoms. In the major depression group, 11 participants (37%) had never received antidepressants and 16 (53%) reported prior antidepressant use; information about prior antidepressant treatment was unavailable for three individuals. Only three individuals reported resistance to a prior antidepressant. All participants provided written informed consent to a protocol approved by the Committee on the Use of Human Subjects in Research at Harvard University and the Partners Human Research Committee.
Monetary Incentive Delay Task
The task has been described previously (21) . Trials began with a visual cue (1.5 seconds) indicating the potential outcome (reward: +$; loss: –$; no incentive: 0$). After a variable interstimulus interval (3–7.5 seconds), a red target square was briefly presented, to which subjects responded by pressing a button. After a second delay (4.4–8.9 seconds), visual feedback (1.5 seconds) indicated trial outcome (gain, penalty, no change). A variable interval (3–12 seconds) separated the trials. The task involved five blocks with 24 trials (eight per cue), yielding 40 and 20 trials for cue- and outcome-related analyses, respectively.
Participants were told that responding rapidly would maximize their chances of obtaining gains and avoiding penalties. However, gains and penalties were actually delivered in a predetermined pattern to allow a balanced design. For each block, half the reward trials yielded a monetary gain (range=$1.96–$2.34; mean=$2.15) and half ended with no-change feedback. Similarly, half the loss trials yielded a monetary penalty (range=$1.81–$2.19; mean=$2.00), and half resulted in no change. No-incentive trials always ended with no-change feedback. To maximize feedback believability, target duration was longer for trials scheduled to be successful (i.e., gains on reward trials) than for those scheduled to be unsuccessful (i.e., no-change feedback on reward trials). Furthermore, target durations were individually titrated on the basis of data collected on reaction time during a practice session (see the data supplement that accompanies the online edition of this article).
Procedure
Data collection occurred prior to start of treatment. After blocks 2 and 4, participants rated their affective response to cues and outcomes for valence (on a scale ranging from 1=most negative to 5=most positive) and arousal (from 1=low intensity to 5=high intensity). Participants were compensated $80 for their time and “earned” $20–$22 from the task.
Data Acquisition
Data were collected on a 1.5-T Symphony/Sonata scanner (Siemens Medical Systems, Iselin, N.J.) and consisted of a T 1 -weighted MPRAGE acquisition (repetition time=2730 msec; echo time=3.39 msec; field of view=256 mm; voxel dimensions=1×1×1.33 mm; 128 slices) and gradient echo T 2 *-weighted echoplanar images, which were acquired using an optimized pulse sequence (21) (repetition time=2500 msec; echo time=35 msec; field of view=200 mm; voxel dimensions=3.125×3.125×3 mm; 35 interleaved slices).
Data Reduction and Statistical Analyses
Reaction time and affective ratings
After removal of outliers (responses exceeding three standard deviations from the mean), reaction time data were entered into a group-by-cue-by-block analysis of variance (ANOVA). For brevity, only effects involving group or cue are reported. Affective ratings were averaged across the two assessments and entered into group-by-cue or group-by-outcome ANOVAs.
Functional and structural MRI
Analyses were conducted using FreeSurfer and FreeSurfer Functional Analysis Stream (FS-FAST) ( 27 ; http://surfer.nmr.mgh.harvard.edu). Preprocessing included slice-time and motion correction, removal of slow trends with a second-order polynomial, intensity normalization, and spatial smoothing (6 mm full width at half maximum); a temporal whitening filter was used to correct for autocorrelation in the noise. Data for four participants in the major depression group were lost because of excessive motion (>5 mm), leaving 26 individuals with major depression and 31 comparison subjects for fMRI analysis. Before group analyses were conducted, the data were resampled into the Montreal Neurological Institute MNI305 space (voxel size=2 mm 3 ).
Functional data were analyzed using the general linear model. The hemodynamic response was modeled as a gamma function and convolved with stimulus onsets; motion parameters were included as nuisance regressors. Between-group whole-brain random-effects comparisons were computed for reward anticipation (reward cue versus no-incentive cue) and reward outcome (gain versus no-change feedback on no-incentive trials) contrasts. Note that because of the double subtraction, clusters exceeding the statistical threshold show a significant group-by-condition interaction. Secondary analyses of loss-related contrasts are reported in the online data supplement. Because of a priori hypotheses about the basal ganglia, activation maps were thresholded using a peak voxel criterion of p<0.005 with a minimum cluster extent of 12 voxels; Monte Carlo simulations were performed to confirm that the primary findings held after correction for multiple comparisons (see the online data supplement). Findings emerging outside the basal ganglia should be considered preliminary. To assess whether findings in a priori regions were specific to rewards, follow-up group-by-condition ANOVAs were conducted on averaged beta weights (including for penalties) extracted from clusters showing group differences.
Structural MRI
Morphometric analyses used FreeSurfer’s automated parcellation approach (27 , 28 ; see Table S1 in the online data supplement) and focused on the basal ganglia. To account for differences in cranial size, volumes were divided by the intracranial volume and entered into a group-by-hemisphere-by-region (nucleus accumbens, caudate, putamen, and globus pallidus) ANOVA. Significant effects were followed up with post hoc t tests. For participants with major depression, Pearson correlations and hierarchical regressions (controlling for age and gender) were conducted to examine relationships between volumes and anhedonic symptoms or depression severity. As in prior work (29) , anhedonia was assessed by computing a BDI anhedonia subscore (loss of pleasure, interest, energy, and libido; reliability coefficient: a=0.85).
Results
Reaction Time
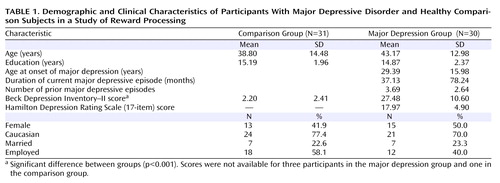
A main effect of cue emerged (F=30.15, df=2, 118, p<0.0001), reflecting motivated responding (shorter reaction time) on reward and loss trials versus no-incentive trials. The main effect of group was not significant, indicating that the comparison and major depression groups showed similar overall reaction time (mean=350.38 msec [SD=68.91] and mean=357.01 msec [SD=75.60], respectively; see the online data supplement). These effects were qualified by a significant group-by-cue interaction (F=3.98, df=2, 118, p<0.045). As evident from Figure 1 A, the interaction reflected smaller reaction time differences on incentive versus no-incentive trials in the major depression group. Relative to comparison subjects, participants with major depression showed weaker reward-related reaction time modulation (reaction time in no-incentive trials minus reaction time in reward trials; t=–2.09, df=59, p<0.047), with a similar tendency for loss-related reaction time modulation (t=–1.97, df=59, p=0.053) ( Figure 1 B). However, no group differences in reaction time emerged for reward, loss, or no-incentive trials (p values >0.21). Moreover, both groups showed the shortest reaction time to reward cues, followed by loss and no-incentive cues (p values <0.002).
a Panel A, reaction time in response to the target as a function of reward, loss, or no-incentive cue. Panel B, reaction time difference scores (no-incentive minus reward cue; no-incentive minus loss cue) reveal significantly reduced relative reaction time speed in the major depression group for reward trials (p<0.047) and a similar tendency for loss trials (p=0.053).
Mirroring the lack of group effect in reaction times collected during scanning, groups did not differ in target durations linked to successful or unsuccessful outcomes, which were selected on the basis of reaction time during practice (see the online data supplement). There were also no group differences in the percentage of reward trials ending in gains or of loss trials ending in penalties, or in total money earned (see Table S2 in the online data supplement). Thus, fMRI findings were not confounded by group differences in task difficulty.
Affective Ratings
Participants’ ratings data indicated that the cues and outcomes elicited the intended responses (see Figure S1 in the online data supplement). Critically, relative to the comparison group, the major depression group reported overall reduced positive affect in response to both cue (group: F=5.62, df=1, 58, p<0.021) and feedback (group: F=12.26, df=1, 59, p<0.001) stimuli, as well as reduced arousal in response to gains (p<0.045) but not to penalties or no-change feedback (p values >0.42; group-by-outcome interaction, F=3.20, df=2, 118, p<0.045).
Functional MRI Data
Reward anticipation (reward cue minus no-incentive cue)
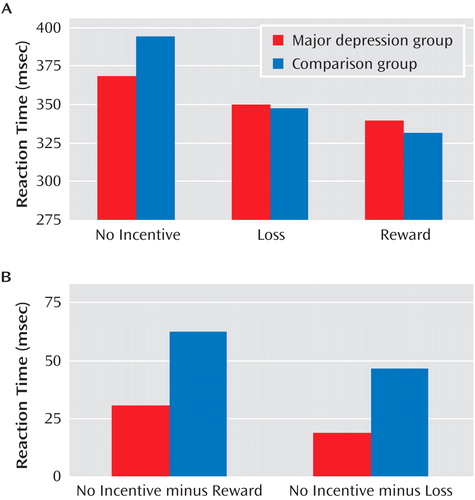
A complete list of regions showing group differences is provided in Table S3 of the online data supplement. Surprisingly, both groups showed robust basal ganglia responses to reward cues ( Figure 2 A). However, the major depression group showed relatively weaker activation in the left posterior putamen ( Figure 2 B and C).
a Coronal (panels A, C) and axial (panel B) slices showing anticipatory reward activity (reward cue minus no-incentive cue) in basal ganglia regions are displayed for both groups as well as for the random-effects analyses comparing the two groups. Panel A shows robust activation of ventral striatal regions, including the nucleus accumbens, in both groups, leading to a lack of group differences. In panels B and C, relative to the comparison group, the major depression group shows significantly reduced activation during reward anticipation in the left putamen (x=–28, y=–13, z=–2). All contrasts are thresholded at p<0.005. Left hemisphere is displayed on the right.
Reward outcome (gain minus no-change feedback)
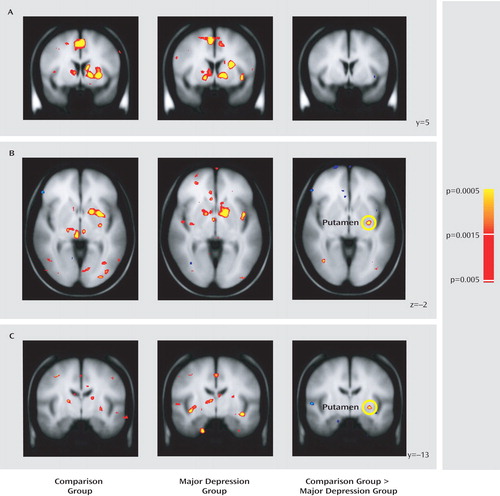
Relative to comparison subjects, the major depression group showed significantly weaker responses to gain versus no-change feedback in the left nucleus accumbens and the dorsal caudate bilaterally, including two subregions in the right caudate and two in the left caudate ( Figure 3 A and B). Both clusters in the right caudate and one in the left caudate remained significant after correction for multiple comparisons (see Table S4 in the online data supplement); accordingly, differences in the nucleus accumbens should be considered preliminary. To test whether group differences were specific to reward outcomes, mean beta weights were extracted from each cluster and entered into group-by-condition (gains, penalties, and no-change feedback) ANOVAs; for the caudate regions of interest, the factor subregion was added. For brevity, only effects involving group are reported.
a Coronal slices showing consummatory reward activity (gain feedback minus no-change feedback) in basal ganglia regions are displayed for both comparison subjects and participants with major depression as well as for the random-effects analyses comparing the two groups. Relative to the comparison group, the major depression group showed significantly reduced activation in response to gain feedback in the left nucleus accumbens (panel A) and the caudate bilaterally (panel B). Follow-up analyses on beta weights extracted from the nucleus accumbens (panel C) and caudate regions bilaterally (panel D) (averaged across three clusters that survived correction for multiple comparisons) indicated that group differences were specific to reward outcome. All contrasts are thresholded at p<0.005. Left hemisphere is displayed on the right.
In the accumbens ( Figure 3 C), a main effect of condition (F=3.46, df=2, 110, p<0.040) was qualified by the group-by-condition interaction (F=2.94, df=2, 110, p=0.063); the main effect of group was not significant. Because of a priori hypotheses regarding the accumbens, and given the significant group-by-condition interaction in the whole-brain analysis, follow-up tests were performed to clarify the source of the interaction. Relative to the comparison group, the major depression group showed significantly weaker responses to gains (p<0.005) but not to penalties or no-change feedback. Furthermore, within-group tests showed that while comparison subjects responded more strongly to gains compared with both penalties (p<0.004) and no-change (p<0.001) feedback, in participants with major depression left accumbens activation was not modulated by condition.
In the caudate ( Figure 3 D), the ANOVA revealed significant main effects of subregion, condition, and group (p values <0.013), a significant condition-by-subregion interaction and, most important, a significant group-by-condition interaction (F=7.89, df=2, 110, p<0.002). This interaction was due to significantly greater activation in the comparison versus the major depression group in response to gains (p<0.0002) but not to penalties or no-incentive feedback. Moreover, whereas comparison subjects showed increased caudate activation bilaterally in response to both gains and losses (p values <0.0002) relative to no-change feedback, participants with major depression failed to show any feedback-dependent caudate modulation. No correlations emerged between activation in the left putamen, left accumbens, or caudate and anhedonic symptoms in either group.
Morphometric Data
The group-by-hemisphere-by-region ANOVA revealed no group differences (see Table S5 in the online data supplement). In the major depression group, correlations were run between 1) proportional left accumbens and bilateral caudate volumes and 2) anhedonic symptoms and depression severity. For the left accumbens, no significant effects emerged. For the left and right caudate, volume was inversely related to total BDI score (left: r=–0.489, p<0.015; right: r=–0.579, p<0.002) and BDI anhedonia subscore (left: r=–0.553, p<0.004; right: r=–0.635, p<0.0001) ( Figure 4 ). Critically, both left and right caudate volumes predicted total BDI score and BDI anhedonia subscore after adjusting for age and gender (total BDI score: left caudate, ΔR 2 =0.203; right caudate, ΔR 2 =0.309; BDI anhedonia subscore: left caudate, ΔR 2 =0.281; right caudate, ΔR 2 =0.387; all ΔF >6.09, p values <0.025).
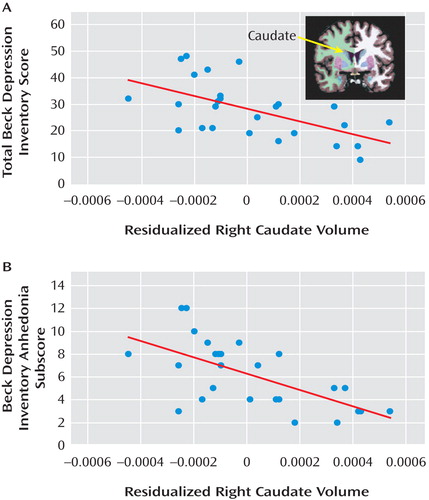
a Scatterplot and Pearson correlation between residualized right caudate volume (adjusted for age and gender) and (panel A) total score of the Beck Depression Inventory–II (BDI) (r=–0.579, p<0.002) and (panel B) BDI anhedonia subscore (r=–0.635, p<0.0001) for participants with major depression. Similar correlations emerged for the left caudate (total BDI: r=–0.489, p<0.015; BDI anhedonia subscore: r=–0.553, p<0.004). The BDI anhedonia subscore was computed by summing items 4 (loss of pleasure), 12 (loss of interest), 15 (loss of energy), and 21 (loss of interest in sex).
Control Analyses
In light of group differences in valence ratings for reward cues and valence and arousal ratings for gains, control analyses evaluated whether group differences in left putamen reward cue responses and left accumbens and bilateral caudate gain responses remained after controlling for affective ratings (see the online data supplement). Regression analyses confirmed that this was the case. Moreover, group differences in accumbens and caudate gain responses remained after controlling for the volumes of these structures and group differences in reward-related reaction time modulation. In addition, no significant correlations between reward-related accumbens and caudate activation and the volume of these regions emerged. Finally, there were no differences in basal ganglia activation for participants with major depression with comorbid anxiety (N=14) compared to those without (N=16).
Discussion
This study investigated anticipatory and consummatory phases of reward processing in depression. Behaviorally, the major depression group showed evidence of anhedonia, reporting generally reduced positive affect to reward stimuli and less arousal following gains. These findings were mirrored by group differences in basal ganglia responses to rewarding outcomes, as participants with major depression showed weaker responses to gains in the caudate bilaterally and in the left nucleus accumbens. By contrast, there was less evidence of differences during reward anticipation. Both groups showed robust basal ganglia responses to reward cues, and although comparison subjects activated the left posterior putamen more strongly than did participants with major depression, the size of the cluster was relatively small. Also, groups did not differ in reaction time as a function of cue, although relatively weaker modulation by reward was seen in participants with major depression (see difference scores). Finally, negative correlations between anhedonic symptoms (and depression severity) and caudate volume emerged in participants with major depression. These findings, which extend previous reports of basal ganglia dysfunction in major depression (6 – 11 , 30) , suggest that this dysfunction is more closely associated with consummatory than anticipatory deficits and emphasize a role for reduced caudate volume in anhedonia.
Reduced Basal Ganglia Response to Rewarding Outcomes in Major Depression
The strong caudate response to gains in comparison subjects fits human (18 , 20 , 31) and animal (32) studies demonstrating this structure’s sensitivity to reward-related information. The caudate responds maximally when rewards are unpredictable (e.g., when delivered on 50% of reward trials, as was done here) and subjects believe that outcomes are contingent on their actions (31) . Accordingly, the between-group caudate difference suggests a weaker perceived action-outcome relationship and/or weaker responses to unpredictable rewards in depression.
Evidence for the first interpretation is mixed. Although groups differed in reward-related reaction time modulation (reaction time difference scores), there was no group difference in reactions on reward trials, and both groups responded faster on reward trials than on loss or no-incentive trials. Thus, both groups behaved as though their responses influenced the chances of receiving gains. Alternatively, the impact of the gains may have been weaker in participants with major depression. This is consistent with the fact that participants with major depression reported overall blunted affective responses and decreased arousal to gains. In addition, group differences were also observed in the left nucleus accumbens, a region that responds strongly to rewarding stimuli (33) . Activity in the accumbens appears to track the hedonic value of outcomes (31 , 34) . Thus, while the group difference in caudate responses suggests a depression-related deficit in expressing goal-directed behaviors, the finding in the accumbens indicates a more primary deficit in hedonic coding. These results are consistent with evidence indicating that deep brain stimulation to the accumbens (35) and ventral capsule/ventral striatum (36) significantly reduced symptom severity and anhedonia in treatment-resistant patients with major depression. Collectively, these findings indicate that dysfunction in regions mediating hedonic impact (accumbens) and reinforcement of actions (caudate) plays an important role in the pathophysiology of major depression.
The group differences in gain responses are intriguing in light of reports of reduced ability to modulate behavior as a function of intermittent rewards in major depression (5) . Using a probabilistic reward task, we found that depressed subjects, particularly those reporting anhedonic symptoms, showed a reduced response bias toward a more frequently rewarded stimulus relative to comparison subjects. Furthermore, healthy comparison subjects with blunted response bias in the probabilistic task also generated weak basal ganglia responses to gains in the fMRI task used here (37) . These considerations suggest that weak basal ganglia responses to unpredictable rewards may contribute to poor learning of action-reward contingencies in major depression.
Intact Basal Ganglia Responses to Reward Cues in Major Depression
Surprisingly, both groups showed robust basal ganglia responses to reward cues. However, in contrast to results in a prior study (17) , the major depression group in the present study showed weaker reward-related reaction time modulation and affective responses to reward-related stimuli relative to comparison subjects. Thus, behavioral evidence of reward processing deficits can coexist with significant basal ganglia responses to reward-predicting cues.
The nature of the intact basal ganglia response to reward cues in individuals with major depression is unclear. In incentive delay tasks, anticipatory ventral striatal activity is typically regarded as related to the dopamine signal seen in response to reward cues in electrophysiological studies (38) . In nonhuman primates, this signal is first elicited by unpredicted rewards and travels back to cues only when a cue-outcome contingency is learned (14) . In our study, the comparison group showed a significantly stronger basal ganglia response to gains than the major depression group, yet the two groups showed few differences in response to reward cues. This suggests two possibilities: 1) the unlikely possibility that the dopamine signal traveled from the gains (consummatory phase) to the cues (anticipatory phase) more rapidly in individuals with major depression or 2) the more likely possibility that the reward cues elicited a ventral striatal response on their own that was similar across groups and possibly independent of transmission of the dopamine signal elicited by gains. This possibility is rarely considered in studies using incentive delay tasks, but because participants know that reward cues can lead to gains, it is possible that the cues elicit ventral striatal activation from the outset. However, even if this is the case, a group difference in ventral striatal response to reward cues might still be expected (8) . Studies in which participants learn cue-reward associations over time are needed to investigate this issue.
Reduced Caudate Volume and Anhedonia
Replicating findings with nonclinical subjects (23) , participants with major depression who had elevated anhedonic symptoms showed reduced caudate volumes bilaterally. This relationship provides impetus for continued investigation of depressive endophenotypes (1 , 2) , because it is unclear whether reduced caudate volume predisposes individuals to anhedonic or more severe depression or instead represents a state-related correlate of these symptoms.
Limitations
Several limitations of this study should be emphasized. First, although the a priori hypothesis about the nucleus accumbens (8 , 10 , 11) was confirmed by a group-by-condition interaction at p<0.005, this difference was not significant after correction for multiple comparisons because of the small cluster size (see the online data supplement). Moreover, no correlations between striatal activation and anhedonic symptoms emerged. Consequently, additional studies are needed to confirm the role of the nucleus accumbens in reward dysfunction in major depression. Given mounting interest in the role of the accumbens in the pathophysiology of major depression, as exemplified by recent deep brain stimulation studies targeting this region (35 , 36) , our finding of reduced reward-related accumbal responses is nevertheless intriguing. Second, correlations between caudate volume and depression severity emerged for BDI score but not HAM-D score. Although the reason for this discrepancy is unclear, it is possible that several BDI items tapping anhedonia contributed to this finding. In spite of these limitations, the current findings indicate that anhedonia, a core component of major depression, may reflect weak reward consummatory responses in the basal ganglia, particularly the nucleus accumbens and the caudate, and is related to reduced caudate size.
1. Hasler G, Drevets WC, Manji HK, Charney DS: Discovering endophenotypes for major depression. Neuropsychopharmacology 2004; 29:1765–1781Google Scholar
2. Pizzagalli DA, Jahn AL, O’Shea JP: Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol Psychiatry 2005; 57:319–327Google Scholar
3. Joormann J, Gotlib IH: Selective attention to emotional faces following recovery from depression. J Abnorm Psychol 2007; 116:80–85Google Scholar
4. Berenbaum H, Oltmanns TF: Emotional experience and expression in schizophrenia and depression. J Abnorm Psychol 1992; 101:37–44Google Scholar
5. Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M: Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J Psychiatr Res 2009; 43:76–87Google Scholar
6. Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME: A functional anatomical study of unipolar depression. J Neurosci 1992; 12:3628–3641Google Scholar
7. Elliott R, Sahakian BJ, Michael A, Paykel ES, Dolan RJ: Abnormal neural response to feedback on planning and guessing tasks in patients with unipolar depression. Psychol Med 1998; 28:559–571Google Scholar
8. Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA: Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 2006; 163:1784–1790Google Scholar
9. Keedwell PA, Andrew C, Williams SCR, Brammer MJ, Phillips ML: The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 2005; 58:843–853Google Scholar
10. Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD: Abnormal temporal difference reward-learning signals in major depression. Brain 2008; 131:2084–2093Google Scholar
11. Steele JD, Kumar P, Ebmeier KP: Blunted response to feedback information in depressive illness. Brain 2007; 130:2367–2374Google Scholar
12. Berridge KC, Robinson TE: What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 1998; 28:309–369Google Scholar
13. Gard DE, Germans Gard M, Kring AM, John OP: Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Person 2006; 40:1086–1102Google Scholar
14. Schultz W: Multiple reward signals in the brain. Nat Rev Neurosci 2000; 1:199–207Google Scholar
15. Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci 1986; 9:357–381Google Scholar
16. Dunlop BW, Nemeroff CB: The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 2007; 64:327–337Google Scholar
17. Knutson B, Bhanji JP, Cooney RE, Atlas LY, Gotlib IH: Neural responses to monetary incentives in major depression. Biol Psychiatry 2008; 63:686–692Google Scholar
18. Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA: Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol 2000; 84:3072–3077Google Scholar
19. Delgado MR, Locke HM, Stenger VA, Fiez JA: Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognit Affect Behav Neurosci 2003; 3:27–38Google Scholar
20. Delgado MR, Miller MM, Inati S, Phelps EA: An fMRI study of reward-related probability learning. Neuroimage 2005; 24:862–873Google Scholar
21. Dillon DG, Holmes AJ, Jahn AL, Bogdan R, Wald LL, Pizzagalli DA: Dissociation of neural regions associated with anticipatory versus consummatory phases of incentive processing. Psychophysiology 2008; 45:36–49Google Scholar
22. Knutson B, Cooper JC: Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol 2005; 18:411–417Google Scholar
23. Harvey PO, Pruessner J, Czechowska Y, Lepage M: Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol Psychiatry 2007; 12:767–775Google Scholar
24. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research, 2002Google Scholar
25. Hamilton M: A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Google Scholar
26. Beck AT, Steer RA, Brown GK: Beck Depression Inventory Manual, 2nd ed. San Antonio, Tex, Psychological Corp, 1996Google Scholar
27. Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM: Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002; 33:341–355Google Scholar
28. Tae WS, Kim SS, Lee KU, Nam EC, Kim KW: Validation of hippocampal volumes measured using a manual method and two automated methods (FreeSurfer and IBASPM) in chronic major depressive disorder. Neuroradiology 2008; 50:569–581Google Scholar
29. Pizzagalli DA, Goetz E, Ostacher M, Iosifescu D, Perlis RH: Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol Psychiatry 2008; 64:162–168Google Scholar
30. Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE: Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry 2005; 62:1228–1236Google Scholar
31. Tricomi EM, Delgado MR, Fiez JA: Modulation of caudate activity by action contingency. Neuron 2004; 41:281–292Google Scholar
32. Kawagoe R, Takikawa Y, Hikosaka O: Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci 1998; 1:411–416Google Scholar
33. Berns GS, McClure SM, Pagnoni G, Montague PR: Predictability modulates human brain response to reward. J Neurosci 2001; 21:2793–2798Google Scholar
34. O’Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ: Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science 2004; 304:452–454Google Scholar
35. Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Joe AY, Kreft M, Lenartz D, Sturm V: Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 2008; 33:368–377Google Scholar
36. Malone DA Jr, Dougherty DD, Rezai AR, Carpenter LL, Friehs GM, Eskandar EN, Rauch SL, Rasmussen SA, Machado AG, Kubu CS, Tyrka AR, Price LH, Stypulkowski PH, Giftakis JE, Rise MT, Malloy PF, Salloway SP, Greenberg BD: Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry 2009; 65:267–275Google Scholar
37. Santesso DL, Dillon DG, Birk JL, Holmes AJ, Goetz E, Bogdan R, Pizzagalli DA: Individual differences in reinforcement learning: behavioral, electrophysiological, and neuroimaging correlates. Neuroimage 2008; 42:807–816Google Scholar
38. Knutson B, Gibbs SE: Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology 2007; 191:813–822Google Scholar