Secretin Effects on Cerebellar-Dependent Motor Learning in Schizophrenia
Abstract
Objective: Pervasive cognitive deficits in schizophrenia are a major cause of disability among individuals with the disorder. One such deficit is the loss of effective associative learning, which is readily assessed via eye-blink conditioning procedures. The authors examined the efficacy of secretin, a hormonal agonist for the prototype group B G-protein-coupled receptors, in ameliorating eye-blink conditioning deficits in schizophrenia patients. Method: Immediately following a pretreatment delay eye-blink conditioning recording session, 25 individuals with schizophrenia received either secretin (RG1068; 20 μg/kg [N=15]) or a saline placebo (20 μg/kg [N=10]) subcutaneously in a double-blind fashion. Comparison groups were formed by yoking pairs of subjects on the basis of performance during the pretreatment baseline eye-blink conditioning recording session, and thus 20 subjects underwent further analysis. Secretin was selected because eye-blink conditioning depends on the release of Purkinje cell inhibition on deep nuclei of the cerebellum and recent findings indicate that secretin is endogenously released in the cerebellum, where it acts as a retrograde messenger and neuromodulator on basket and Purkinje cells. Results: Eye-blink conditioning was significantly improved at 2 and 24 hours after secretin administration but not after treatment with placebo. These results are consistent with evidence of intracellular signaling abnormalities in the pathophysiology of schizophrenia and indicate a possible role for secretin in modulating cerebellar-mediated classically conditioned learning. Conclusion: If cerebellar abnormalities in individuals with schizophrenia are associated with fundamental mechanisms and symptoms of the disorder, as suggested by the cognitive dysmetria model, then cerebellar-targeted treatments may provide a novel approach to treatment for schizophrenia.
Theoretical and empirical evidence suggest that the cerebellum plays a role in the mechanisms and symptoms underlying schizophrenia. Evidence of cerebellar involvement in perceptual timing, in addition to the role of the cerebellum in motor coordination, has prompted speculation that it is a dedicated system for representing time in psychological as well as motor domains (1) . The cognitive dysmetria model of schizophrenia (2 , 3) posits that some symptoms of the disorder may arise from abnormal temporal coordination of neural processes underlying cognition. Empirical support for this model derives from studies suggesting that cerebellar structure and function are abnormal in individuals with schizophrenia (4 , 5) and that cerebellar abnormalities are associated with poor long-term outcome (6) and greater cognitive dysfunction (7) among patients with the disorder.
It is unlikely that a single mechanism can explain the diverse symptoms of schizophrenia. Instead, different symptom domains probably possess unique targets for treatment. Hence, recent research has focused on targeted treatments for various cognitive symptoms of the disorder. For example, early pharmacological data suggest that nicotinic alpha 7 subunit selective cholinergic agents may improve attentional deficits (8) and muscarinic M 1 receptor agents may improve verbal learning and short-term memory (9) . However, little is known about potential therapeutic targets in the cerebellum.
An increasing volume of literature suggests that the neuropeptide secretin plays a neuromodulatory role in the cerebellum (10 , 11) . Sheitman et al. (12) reported clinical improvement in some schizophrenia patients following the administration of secretin. It is possible that enhanced cerebellar function following secretin administration contributed to this improvement.
One of the best ways to assess cerebellar function is via eye-blink conditioning, wherein a conditioned stimulus, such as a tone, is paired with an unconditioned stimulus, such as an air puff to the eye. The unconditioned stimulus air puff evokes a reflexive eye-blink, which is the unconditioned response. When the conditioned stimulus is repeatedly paired with the unconditioned stimulus, a conditioned response (an eye-blink) develops prior to the onset of the unconditioned stimulus air puff. This conditioned response requires temporal precision and is therefore an ideal test of the integrity of cerebellar timing mechanisms. Importantly, prominent abnormalities during this task have been found in individuals with schizophrenia (13 – 17) . Moreover, eye-blink conditioning responses may be mediated by neurotransmitter systems on which secretin acts.
Although secretin, a hormonal agonist for the prototype group B G-protein-coupled receptors, was the first recognized human hormone and its gastrointestinal functions have been widely studied, relatively little is known about its effects and mechanisms of action in the CNS. Animal models indicate that secretin is endogenously released in the cerebellum (10 , 11 , 18) . Yung et al. (10) showed that secretin is present in the soma and dendrites of Purkinje cells and secretin receptors are present on basket and Purkinje cells in the cerebellar cortex. Moreover, electrophysiological evidence has demonstrated that secretin increases the amplitude of spontaneous and evoked inhibitory postsynaptic currents that originate from interneurons and are recorded from Purkinje cells and decreases the paired-pulse ratio of the evoked inhibitory postsynaptic currents. Accordingly, secretin appears to increase the firing of presynaptic neurons, which then inhibit the Purkinje cells by selectively facilitating inhibitory gamma-aminobutyric acid (GABA)-ergic inputs via a presynaptic and cyclic adenosine 3′,5′-monophosphate (cAMP)-dependent mechanism. These findings suggest that secretin is synthesized in Purkinje cells and then released following depolarization to promote recurrent inhibition, which stabilizes these cells via facilitated GABA-ergic afferent activity originating from presynaptic basket cells. Secretin’s mechanism of action appears to be similar to a recently discovered endocannabinoid-mediated retrograde signaling mechanism termed depolarization-induced suppression of inhibition (19) . However, in the case of secretin, depolarization of Purkinje cells increases, rather than decreases, inhibitory input to the Purkinje cells. Taken together, this evidence constitutes a strong argument for a neuromodulatory role of secretin in the cerebellum. Additionally, given the evidence of secretin’s effects in cerebellar cellular function and its proposed mechanism of action on Purkinje cells, eye-blink conditioning methodology is well suited for examining the CNS effects of secretin in humans. This objective is an important one, since secretin has not been demonstrated to influence human CNS function, although other members of the same peptide family act as neuropeptides (10) . The centrality of the cerebellum in acquiring eye-blink responses has been demonstrated in both nonhuman (20 – 24) and human studies (25 – 27) . Although the hippocampus, amygdala, and frontal regions may modulate conditioned response acquisition and performance, cerebellar lesions alone prevent learning of conditioned responses and abolish previously learned responses (28) . Moreover, eye-blink conditioning magnitude correlates with cerebellar volume (26) . The cerebellar cortex acts on the deep cerebellar nuclei—the only source of cerebellar output—exclusively through the inhibitory effects of Purkinje cells on these nuclei. Consequently, populations of Purkinje cells are thought to directly affect conditioned response rates by facilitating or depressing output of the deep nuclei. Given evidence that secretin inhibits Purkinje cells by facilitating GABA-ergic effects of basket cells on Purkinje cells (10) , we predicted that secretin administration would enhance eye-blink conditioning. Specifically, secretin should act to inhibit Purkinje cells, thus releasing their inhibition (i.e., increasing the gain) on the conditioned response generating deep nuclei. Abnormalities in the acquisition and timing of eye-blink conditioning have been widely reported in studies of schizophrenia (13 – 17) . Although studies of delay eye-blink conditioning with small sample sizes of schizophrenia patients have reported no differences in the percentage of conditioned responses (15) or facilitation (16 , 17) , a recent study in our laboratory (unpublished data available upon request from Bolbecker AR et al.) found impaired conditioning in a large sample of schizophrenia patients (N=62), replicating previous studies of eye-blink conditioning in schizophrenia (13 , 14) . Importantly, repeated eye-blink conditioning training across multiple independent sessions does not appear to significantly improve eye-blink conditioning in patients with schizophrenia, pointing to the refractory nature of the cerebellar-mediated learning deficit in the disorder. Previous research indicates specific roles for secretin and its receptors in the inhibition of cerebellar Purkinje cells (10) . This intriguing finding, combined with extensive knowledge of cerebellar mechanisms underlying eye-blink conditioning, led to the important prediction that secretin administration would increase conditioned eye-blink responses, which would be clearly demonstrated in populations such as schizophrenia patients, in whom profound eye-blink conditioning deficits have been reported. In the present study, a double-blind, placebo-controlled, between-subjects design indicated that subcutaneous administration of secretin significantly increased eye-blink conditioning in individuals diagnosed with schizophrenia.
Method
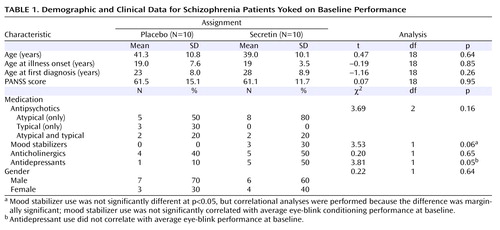
Participants
Twenty-five individuals who met criteria for schizophrenia using the Structured Clinical Interview for DSM-IV (29) were recruited. Participants provided written informed consent and completed study procedures. Since this was a purely mechanistic study, the effects of secretin on neurocognitive and symptom status were not evaluated. The Positive and Negative Syndrome Scale (PANSS [30] ) was used to assess symptom severity at baseline. An additional 12 subjects were recruited but not examined, since these subjects failed at least one screening test (e.g., urine drug screen) or completed only the pretreatment baseline assessments. In a single case, one subject who was randomly assigned to secretin administration showed significant elevations in blood pressure following subcutaneous injection. This subject was monitored until the blood pressure returned to preinjection levels, which occurred the same day, and completed participation in the study. Subjects were randomly assigned to receive either secretin (N=15) or a saline placebo (N=10) subcutaneously in a double-blind design. After all subjects were tested and eye-blink conditioning was quantified, the blind was lifted and two comparison groups (secretin versus saline) were formed by yoking pairs of subjects on the basis of performance during only the pretreatment baseline eye-blink conditioning recording session. This procedure, commonly used in nonhuman animal pharmacological trials, ensures that comparison groups are equivalent on the primary dependent variable before treatment is initiated. Subjects were yoked within 10 percentage points on the primary dependent variable, which was the percentage of conditioned eye-blink responses. Thus, data from 20 subjects were submitted to statistical analyses. These subjects were medically stable outpatients whose prescribed medications were unchanged for at least 4 weeks before and during the study. Among these subjects, all passed urine toxicology screens for illicit substance use. Table 1 provides clinical, demographic, and medication data for both treatment groups. Chi square and parametric (t test) analyses revealed that the two groups did not statistically differ on any of these variables, with the exception of antidepressant medication, which was more prevalent in the secretin group. However, antidepressant use did not correlate with eye-blink conditioning performance at baseline (r= –0.06, df=20, p=0.80). On average, subjects had been pre-exposed to eye-blink conditioning 1.7 times (SD=2.1; range=0–7). In the secretin group, six out of 10 subjects had been pre-exposed compared with five out of 10 subjects in the placebo group. The number of previous eye-blink conditioning sessions did not differ between groups (t=0.63, df=18, p=0.54) or correlate with the mean percentage of conditioned eye-blink responses at pretreatment baseline (r=0.25, df=20, p=0.46). Moreover, subjects who had been pre-exposed to eye-blink conditioning (N=11) and those who had not (N=9) did not differ on the pretreatment baseline mean percentage of conditioned eye-blink responses (t=1.06, df=18, p=0.31). Taken together with our published report (13) and our recent large unpublished study (data available upon request from Bolbecker AR et al.), both of which showed profound eye-blink conditioning deficits in similarly stable schizophrenia patients relative to age-matched nonpsychiatric comparison subjects, these analyses demonstrate the refractory nature of this cerebellar-mediated learning deficit among individuals with schizophrenia. Exclusion criteria were 1) concurrent DSM-IV comorbidity with any substance dependence, excluding nicotine; 2) positive urine drug screen for controlled substances; 3) active suicidal ideation; 4) abnormal hepatic function (aspartate transaminase or alanine transaminase >2.5 times the upper limit of normal or bilirubin >1.5 times the upper limit of normal); 5) abnormal renal function (blood urea nitrogen or creatinine >1.5 times the upper limit of normal); 6) abnormal bone marrow function (white blood cell count <4×103/mm 3 , platelets <100×103/mm 3 , and hemoglobin <10 g/dl); 7) a history of sensitivity to any study drug ingredient; 8) clinically significant organic disease, including cardiovascular, hepatic, pulmonary, neurological, or renal disease or other medical condition; and 9) pregnancy, breast-feeding, or refusal to use adequate birth control.
Design and Treatment Dosing
Subjects were randomly assigned in a double-blind fashion to either secretin or saline placebo prior to the baseline eye-blink conditioning recording session. Treatment was administered immediately after the baseline session. The eye-blink conditioning recording session was repeated 2 and 24 hours following this single treatment. In a previous study of the effects of secretin on schizophrenia, an intravenous dose of 0.4 μg/kg produced equivocal results, with some patients showing improvement clinically (12) . It was suggested that future studies use different doses. In the present study, a dose of 20 μg/kg of secretin was selected for subcutaneous administration, which approximates the maximum capacity of intravenous administration of 1.0 μg/kg. Depending on treatment assignment, each subject received 20 mg/kg of either RG1068 (Repligen Corp., Waltham, Mass.) or saline subcutaneously. Comprised of 27 amino acids, RG1068 is a 3,039.4- dalton synthetic peptide, and it is identical in amino acid sequence to naturally occurring human secretin and differs from porcine secretin in two amino acids.
Delay Eye-Blink Conditioning Task and Stimuli
Subjects completed a 28-minute, 108-trial, single-cue tone-delay eye-blink conditioning procedure. (Although not reported in the present study, ancillary electromyograph [EMG] measures of acoustic startle modulation were also collected but were not reliably affected by secretin administration.) At the onset, eight unconditioned stimuli (unconditioned stimulus: corneal air puff, 50 msec, 10 psi at source) were presented with an intertrial interval of 15 seconds. The acquisition phase followed, consisting of five blocks of trials (mean intertrial interval=15 seconds; range=10–20 seconds). Each block contained 18 trials in which the conditioned stimulus (1 KHz tone; 400 msec; sound pressure level: 80 dB) was paired with the unconditioned stimulus and two trials in which the conditioned stimulus was presented alone. In trials in which the conditioned and unconditioned stimuli were paired, the unconditioned stimulus air puff coterminated with the conditioned stimulus tone. In order to maintain the subjects’ attention throughout the experiment, neutral photographic images (e.g., landscapes, objects) selected from the International Affective Picture System (31) were presented (2-second duration) between each trial during the acquisition and extinction phases. Subjects rated the pleasantness of the pictures on a scale of 1 to 4 using a button response pad. Additionally, they were observed via a closed-circuit monitor to ensure that their eyes remained open. The experiment was briefly suspended if signs of fatigue were found in order for the examiner to interact with the subjects. These procedures were identical for each of the following three experimental sessions: pretreatment baseline, 2 hours posttreatment, and 24 hours posttreatment.
Eye-Blink Recording Procedures
Eye-blinks were recorded using pairs of bipolar EMG recording electrodes (Ag/Ag-Cl=4 mm; model TD-23; MedAssociated, St. Albans, Vt.) placed on the orbicularis palpebrarum muscle below each eye of the subject, with a ground electrode on the forehead. The air puff unconditioned stimulus was presented to the left eye via copper tubing (diameter=1/16 inch) affixed to eyeglass rims and placed 1 cm away from the inner canthus of the eye, just beyond the reach of the eyelashes. A plastic tube (120 inch) connected the copper tubing on the glasses to a regulator receiving medical grade air. Foam ear inserts (E-A-RLINK, Aearo Company Auditory Systems, Indianapolis) were used for presentation of the tone conditioned stimulus. EMG signals were recorded continuously throughout each recording session at 2.5 kHz, with a SynAmps bioamplifier (high-pass filter=1 Hz; low-pass filter=300 Hz; gain=2,500), and stored for off-line analysis.
Data Reduction
The continuous EMG recordings were segmented to create 1,086-msec epochs during which each trial occurred. These epochs comprised EMG activity beginning 500 msec prior to presentation of the conditioned stimulus, continuing for 400 msec during the conditioned stimulus, and terminating 186 msec after the conditioned/unconditioned stimuli offset in order to capture the unconditioned response. A high-pass filter (10 Hz, 6 dB/octave) was applied to the data before being rectified and smoothed using a 41-point Gaussian-weighted moving average. Data were entered into DataMunch, a Matlab computer program purpose written for eye-blink conditioning data analysis (unpublished data by King DAT and Tracy J, available upon request from Hetrick WP), for further analysis. On a subject-by-subject and trial-by-trial basis, responses were recorded as blinks if the amplitude exceeded five standard deviations above the baseline (window=125 msec prior to conditioned stimulus presentation). Conditioned responses were recorded if the blink occurred between 100 msec and 350 msec after conditioned stimulus onset, which corresponded to a period beginning 250 msec before the onset of the unconditioned stimulus. Alpha responses, which are reflexive nonassociative orienting EMG responses to the conditioned stimulus tone, were assessed between 25 and 100 msec after the conditioned stimulus. Trials in which spontaneous blinks occurred within a window from 75 msec prior to conditioned tone stimulus presentation to 25 msec following the conditioned stimulus were labeled “bad” trials and excluded from further analysis.
Statistical Analyses
The effects of treatment on conditioning within each of the three sessions (pretreatment baseline, 2 hours posttreatment, and 24 hours posttreatment) were examined in a series of two- (drug: secretin, saline) -by-five (block: 1–5) repeated-measures analyses of variance (ANOVAs). The effects of treatment across sessions were examined with a two- (drug: secretin, saline) -by-three (session: pretreatment, 2-hours posttreatment, 24-hours posttreatment) repeated-measures ANOVAs. Finally, separate one-way ANOVAs were conducted for each treatment group to examine the main effect of session (three levels), and pairwise comparisons were used to explore any main effects. In all analyses, sex was entered as a covariate, since female subjects exhibited significantly greater conditioning than male subjects. Additionally, the number of cigarettes smoked by subjects in 1) the last 24 hours, 2) an average week, and 3) the past month were entered as covariates in the analyses and did not alter reported results. Effect size estimates are reported in the form of partial eta 2 (η P2 ) values, in which small effect sizes are <0.06, moderate effect sizes range from 0.06 to 0.14, and large effect sizes are >0.14 (32) .
Results
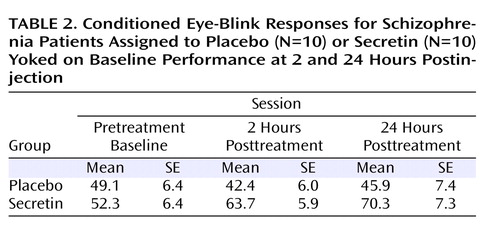
Table 2 shows the average percentage of conditioned eye-blink responses for each session. As expected, given the baseline yoking procedure, statistical analyses indicated that the overall mean percentage of conditioned eye-blink responses at pretreatment baseline did not differ between treatment groups (F=0.12, df=1, p=0.73; η P2 =0.01). Additionally, there was no main effect of block, nor was there a block-by-treatment group interaction. However, at 2 hours posttreatment, overall conditioning in the secretin group was significantly higher compared with the saline group (F=6.4, df=1, p=0.02; η P2 =0.27). Neither the main effect of block nor the block-by-treatment group interaction was significant. At 24 hours posttreatment, overall conditioning in the secretin group remained significantly higher (F=5.5, df=1, p=0.03; η P2 =0.25). To examine the effect of treatment across sessions (pretreatment baseline, 2 hours posttreatment, and 24 hours posttreatment), the mean percentage of conditioned eye-blink responses was computed for each subject within each of the three sessions. Secretin administration resulted in higher levels of conditioning at 2 and 24 hours posttreatment compared with levels during the pretreatment baseline recording session, whereas conditioning in the saline-treated group remained relatively unchanged ( Figure 1 ). Statistical analyses revealed a main effect of session (F=5.7, df=1.9, p=0.01; η P2 =0.25), and, importantly, a session-by-treatment group interaction (F=3.5, df=1.9, p=0.04; η P2 =0.17). Although the main effect of treatment only approached statistical significance (F=3.9, df=1, p=0.06), it was associated with a large effect size estimate (η P2 =0.17) (32) . In the secretin group, the mean increase in conditioning from baseline to 2 hours posttreatment (mean=10.3% [SE=5.5%]) approached significance (p=0.10) and became statistically significant (p=0.03) at 24 hours posttreatment (mean=14.7% [SE=5.4%]). In the saline group, mean conditioning differences between baseline and 2 (mean=5.7% [SE=5.4%]) and 24 (mean=3.4% [SE=5.4%]) hours posttreatment were not statistically different. Importantly, analysis of the entire nonyoked sample (placebo group: N=10; secretin group: N=15) produced the same pattern of results. Within each posttreatment session, including the mean percentage of conditioned eye-blink responses at pretreatment baseline as a covariate produced significant group differences at 2 (p=0.007) and 24 hours (p=0.03) posttreatment. When preinjection baseline percentage of conditioned eye-blink response scores were subtracted from postinjection percentage of conditioned eye-blink responses, significant group differences were observed at 2 hours posttreatment (p=0.007) but not 24 hours posttreatment (p=0.28). The similarity of mean percentage of conditioned eye-blink responses at 2 and 24 hours posttreatment suggests that this is likely attributable to increased variance at 24 hours posttreatment ( Table 3 ). In two separate two- (drug: secretin, saline) -by-two (session: 2 hours posttreatment, 24 hours posttreatment) repeated-measures ANOVAs, a main effect of group was observed both when the individual baseline percentage of conditioned eye-blink response average was used as a covariate (p=0.006) and when the pretreatment baseline percentage of conditioned eye-blink response scores were subtracted from the posttreatment percentage of conditioned eye-blink responses at 2 and 24 hours posttreatment (p=0.04). The consistency of group differences, both within and across sessions, using these different methods of analysis makes a compelling argument that there were true differences between the groups.

a The top graph illustrates the mean percentage of conditioned eye-blink responses and standard errors for each of the five blocks across the three sessions (pretreatment baseline, 2 hours posttreatment, and 24 hours posttreatment) of eye-blink conditioning. The bottom graph illustrates the mean percentage of conditioned eye-blink responses for each session.
Discussion
Our finding that subcutaneous secretin administration increased the rate of conditioned responses in a well-established associative learning task among individuals diagnosed with schizophrenia is important for two reasons. First, to the best of our knowledge, these results provide the first behavioral evidence that secretin affects the function of the human CNS. Moreover, the observed protherapeutic effects of secretin on this cerebellar-dependent measure of conditioning are consistent with the putative role of secretin in the cerebellum, as established in a rodent model (10 , 11 , 18) . Second, the fact that these improvements in cerebellar-dependent conditioning were observed in individuals with schizophrenia, a psychiatric disorder previously shown to have eye-blink conditioning deficits (13 , 14) , is important because—taken together with the animal model (10 , 11 , 18) —the results suggest a mechanism for these deficits. The apparent beneficial effects of secretin administration on conditioning rate in our study are especially noteworthy given that 1) a majority of the patients in the secretin group had been pre-exposed to the eye-blink conditioning task but exhibited no practice effects and 2) patients in the saline-treated group exhibited no evidence of increased rates of conditioning, even after exposure to 270 conditioning trials in a span of 24 hours. In other words, secretin’s effects were noteworthy given the apparent refractory nature of this cerebellar-mediated conditioning deficit in schizophrenia patients. The fact that secretin was shown to affect cerebellar-dependent learning in schizophrenia is significant given compelling evidence suggesting that the cerebellum plays a role in a wide variety of psychological functions, including cognitive and affective processes (33 , 34) . As detailed in a review by Schmahmann (34) , reciprocal topographic projections connect the cerebellum to brain areas implicated in schizophrenia, such as the thalamus, limbic system, motor region, prefrontal region, and posterior parietal cortex. Hence, it has been suggested that the cerebellum integrates and coordinates information from different functional domains (2 , 33 , 34) . Cerebellar abnormalities have been reported in schizophrenia (5 , 35) , including decreased cerebellar size (7 , 36 , 37) . Interestingly, cerebellar volume deficits have been shown to be reliable indicators of poor long-term outcome (6) . Moreover, observed cerebellar volume deficits are correlated with greater cognitive dysfunction (7) , lending support to the theory that cerebellar dysfunction contributes to cognitive dysmetria (2 , 3) . The role of the cerebellum in timing suggests that its overarching role may be to integrate and coordinate neural signals in time. Thus, cerebellar abnormalities in schizophrenia may lead to discoordination of temporal information that gives rise to symptoms of schizophrenia. Taken in the context of the present finding that secretin improved eye-blink conditioning performance in individuals with schizophrenia during a cerebellar-dependent task, this neuropeptide system may prove useful as a therapeutic target for improving the cerebellar components of cognition by remediating timing deficits in schizophrenia.
Although the present results are novel and theoretically interesting, there are several experimental limitations to the study that warrant follow-up in subsequent research. First, although we attempted to control for baseline differences in conditioning by yoking subjects on the basis of baseline performance, the source of these differences and their effects on the results are unclear. Additionally, the yoking procedure resulted in the loss of subjects in these analyses. Nevertheless, the same pattern of results was seen for the full sample when alternative statistical methods were employed in order to take baseline differences into account. Furthermore, there were performance differences between male and female subjects, limiting the interpretability of the analyses. However, these differences existed at baseline and persisted throughout the study, which suggests that they were not related to drug condition. Moreover, no sex effects on eye-blink conditioning were found in our earlier study (13) or in our more recent, larger schizophrenia study (unpublished data available upon request from Bolbecker AR et al.). Hence, these differences may be an artifact of small sample size.
The present study was notable because it showed an increase in eye-blink conditioning rates in a group of medically stable schizophrenia patients following secretin administration. This finding is particularly striking, since this group had not shown an increase in conditioned responses even after repeated exposure to the cerebellar-mediated task. The present results add to the increasing evidence of a cerebellar contribution to the pathophysiology of schizophrenia and point to a possible role for secretin in modulating cerebellar-mediated classically conditioned learning.
1. Ivry RB, Spencer RM: The neural representation of time. Curr Opin Neurobiol 2004; 14:225–232Google Scholar
2. Andreasen NC: A unitary model of schizophrenia: Bleuler’s “fragmented phrene” as schizencephaly. Arch Gen Psychiatry 1999; 56:781–787Google Scholar
3. Andreasen NC, Pierson R: The role of the cerebellum in schizophrenia. Biol Psychiatry 2008; 64:81–88Google Scholar
4. Ho BC, Mola C, Andreasen NC: Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry 2004; 55:1146–1153Google Scholar
5. Wiser AK, Andreasen NC, O’Leary DS, Watkins GL, Boles Ponto LL, Hichwa RD: Dysfunctional cortico-cerebellar circuits cause “cognitive dysmetria” in schizophrenia. Neuroreport 1998; 9:1895–1899Google Scholar
6. Wassink TH, Andreasen NC, Nopoulos P, Flaum M: Cerebellar morphology as a predictor of symptom and psychosocial outcome in schizophrenia. Biol Psychiatry 1999; 45:41–48Google Scholar
7. Nopoulos PC, Ceilley JW, Gailis EA, Andreasen NC: An MRI study of cerebellar vermis morphology in patients with schizophrenia: evidence in support of the cognitive dysmetria concept. Biol Psychiatry 1999; 46:703–711Google Scholar
8. Freedman R, Olincy A, Buchanan RW, Harris JG, Gold JM, Johnson L, Allensworth D, Guzman-Bonilla A, Clement B, Ball MP, Kutnick J, Pender V, Martin LF, Stevens KE, Wagner BD, Zerbe GO, Soti F, Kem WR: Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry 2008; 165:1040–1047Google Scholar
9. Shekhar AS, Potter WZ, Lightfoot J, Lienemann J, Dube S, Mellincrodt C, Bymaster FP, McKinzie DL, Felder CC: Selective muscarinic receptor agonist xanolemine as a novel treatment approach for schizophrenia. Am J Psychiatry 2008; 165:1033–1039Google Scholar
10. Yung WH, Leung PS, Ng SS, Zhang J, Chan SC, Chow BK: Secretin facilitates GABA transmission in the cerebellum. J Neurosci 2001; 21:7063–7068Google Scholar
11. Lee M, Chen L, Chow BKC, Yung WH: Endogenous release and multiple actions of secretin in the rat cerebellum. J Neurosci 2005; 144:377–386Google Scholar
12. Sheitman BB, Knable MB, Jarskog LF, Chakos M, Boyce LH, Early J, Lieberman JA: Secretin for refractory schizophrenia. Schizophr Res 2004; 66:177–181Google Scholar
13. Brown SM, Kieffaber PD, Carroll CA, Vohs JL, Tracy JA, Shekhar A, O’Donnell BF, Steinmetz JE, Hetrick WP: Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn 2005; 58:94–108Google Scholar
14. Hofer E, Doby D, Anderer P, Dantendorfer K: Impaired conditional discrimination learning in schizophrenia. Schizophr Res 2001; 51:127–136Google Scholar
15. Marenco S, Weinberger DR, Schreurs BG: Single-cue delay and trace classical conditioning in schizophrenia. Biol Psychiatry 2003; 53:390–402Google Scholar
16. Spain B: Eyelid conditioning and arousal in schizophrenic and normal subjects. J Abnorm Psychol 1966; 71:260–266Google Scholar
17. Sears LL, Andreasen NC, O’Leary DS: Cerebellar functional abnormalities in schizophrenia are suggested by classical eyeblink conditioning. Biol Psychiatry 2000; 48:204–209Google Scholar
18. Yung WH, Chan YS, Chow BKC, Wang JJ: The role of secretin in the cerebellum. Cerebellum 2006; 5:43–48Google Scholar
19. Kreitzer AC, Regehr WG: Cerebellar depolarization-induced suppression of inhibition is mediated by endogenous cannabinoids. J Neurosci 2001; 21:RC174Google Scholar
20. McCormick DA, Thompson RF: Cerebellum: essential involvement in the classically conditioned eyelid response. Science 1984; 223:296–299Google Scholar
21. McCormick DA, Lavond DG, Clark GA, Kettner RE, Rising CE, Thompson RF: The engram found? Role of the cerebellum in classical conditioning of the nictitating membrane and eyelid response. Bull Psychon Soc 1981; 18:103–105Google Scholar
22. Thompson RF, Donegan NH: The search for the engram, in Learning and Memory. Edited by Martinez JL, Kesner RP. San Diego, Academic Press, 1986, pp 3–44Google Scholar
23. Yeo CH, Hardiman MJ, Glickstein M: Classical conditioning of the nictitating membrane response of the rabbit, II: lesions of the cerebellar cortex. Exp Brain Res 1985; 60:99–113Google Scholar
24. Yeo CH, Hardiman MJ, Glickstein M: Classical conditioning of the nictitating membrane response of the rabbit, I: lesions of the cerebellar nuclei. Exp Brain Res 1985; 60:87–98Google Scholar
25. Woodruff-Pak DS, Goldenberg G, Downey-Lamb MM, Boyko OB, Lemieux SK: Cerebellar volume in humans related to magnitude of classical conditioning. Neuroreport 2000; 11:609–615Google Scholar
26. Woodruff-Pak DS, Vogel RW 3rd, Ewers M, Coffey J, Boyko OB, Lemieux SK: MRI-assessed volume of cerebellum correlates with associative learning. Neurobiol Learn Mem 2001; 76:342–357Google Scholar
27. Dimitrova A, Gerwig M, Brol B, Gizewski ER, Forsting M, Beck A, Aurich V, Kolb FP, Timmann D: Correlation of cerebellar volume with eyeblink conditioning in healthy subjects and in patients with cerebellar cortical degeneration. Brain Res 2008; 1198:73–84Google Scholar
28. Christian KM, Thompson RF: Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem 2003; 10:427–455Google Scholar
29. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version—Patient Edition (SCID-I/P). New York, New York State Psychiatric Institute, Biometrics Research, 2001Google Scholar
30. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Google Scholar
31. Lang PJ, Greenwald MK: The International Affective Picture System Standardization Procedure and Initial Group Results for Affective Judgments (Tech Rep No 1A) Gainesville, Fla, University of Florida, Center for Research in Psychophysiology, 1988Google Scholar
32. Cohen J: Eta-squared and partial eta-squared in communication science. Hum Commun Res 1973; 28:473–490Google Scholar
33. Katz DB, Steinmetz JE: Psychological functions of the cerebellum. Behav Cogn Neurosci Rev 2002; 1:229–241Google Scholar
34. Schmahmann JD: Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci 2004; 16:367–378Google Scholar
35. Andreasen NC, Nopoulos P, O’Leary DS, Miller DD, Wassink T, Flaum: Defining the phenotype of schizophrenia: cognitive dysmetria and its neural mechanisms. Biol Psychiatry 1999; 46:908–920Google Scholar
36. Ichimiya T, Okubo Y, Suhara T, Sudo Y: Reduced volume of the cerebellar vermis in neuroleptic-naive schizophrenia. Biol Psychiatry 2000; 49:20–27Google Scholar
37. Loeber RT, Cintron CM, Yurgelun-Todd DA: Morphometry of individual cerebellar lobules in schizophrenia. Am J Psychiatry 2001; 158:952–954Google Scholar