Familial Risk Analyses of Attention Deficit Hyperactivity Disorder and Substance Use Disorders
Abstract
Objective: A robust and bidirectional comorbidity between attention deficit hyperactivity disorder (ADHD) and psychoactive substance use disorder (alcohol or drug abuse or dependence) has been consistently reported in the extant literature. Method: First-degree relatives from a large group of pediatrically and psychiatrically referred boys with (112 probands, 385 relatives) and without (105 probands, 358 relatives) ADHD were comprehensively assessed by blind raters with structured diagnostic interviews. Familial risk analysis examined the risks in first-degree relatives for ADHD, psychoactive substance use disorder, alcohol dependence, and drug dependence after stratifying probands by the presence and absence of these disorders. Results: ADHD in the proband was consistently associated with a significant risk for ADHD in relatives. Drug dependence in probands increased the risk for drug dependence in relatives irrespective of ADHD status, whereas alcohol dependence in relatives was predicted only by ADHD probands with comorbid alcohol dependence. In addition, ADHD in the proband predicted drug dependence in relatives, and drug dependence in comparison probands increased the risk for ADHD in relatives. Both alcohol dependence and drug dependence bred true in families without evidence for a common risk between these disorders. Conclusions: Patterns of familial risk analysis suggest that the association between ADHD and drug dependence is most consistent with the hypothesis of variable expressivity of a common risk between these disorders, whereas the association between ADHD and alcohol dependence is most consistent with the hypothesis of independent transmission of these disorders. Findings also suggest specificity for the transmission of alcohol and drug dependence.
The co-occurrence of attention deficit hyperactivity disorder (ADHD) and psychoactive substance use disorder (alcohol or drug abuse or dependence) has been reported in a variety of clinical and research settings (1 – 3) . Follow-up studies have documented a higher than expected risk for psychoactive substance use disorder in adults who had ADHD as children (4 , 5) . Studies of referred and nonreferred adults with ADHD have also documented a high risk for psychoactive substance use disorder (6 , 7) . Most recently, Kessler and colleagues (8) reported results from the National Comorbidity Survey indicating that adults with ADHD were at significantly higher risk for any substance use disorder and particularly drug dependence compared to respondents without ADHD.
Excess rates of ADHD have also been seen in adolescents and adults with psychoactive substance use disorder. DeMilio (9) reported that 25% of inpatient adolescents with psychoactive substance use disorder had ADHD. Results from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) study (10) revealed that increased substance use was associated with disruptive behavior among children and adolescents.
The available literature shows that adolescent and adult offspring of parents with psychoactive substance use disorder are at increased risk for the disorder as well as abnormal cognitive and behavioral traits suggestive of ADHD as well as elevated rates of ADHD compared with children of comparison parents (11 – 13) . Wilens et al. (14) reported that 23% of children of opioid-dependent parents had scores on the attention problems subscale of the Child Behavior Checklist that were highly suggestive of ADHD.
The link between ADHD and psychoactive substance use disorder has also been seen in family members of children with ADHD. Morrison and Stewart (15) and Cantwell (16) reported elevated rates of alcoholism in parents and second-degree relatives of children with ADHD. Similar findings have been seen in two large double-blind family genetic studies of ADHD that showed higher rates of psychoactive substance use disorder in the relatives of boys (17) and girls (18) with ADHD.
Despite the contribution of this literature suggesting a familial association between ADHD and psychoactive substance use disorder, several uncertainties remain. Many studies did not specifically examine the diagnosis of ADHD, did not use contemporaneous diagnostic criteria, did not adequately attend to the heterogeneity of psychoactive substance use disorder, and did not attempt to disentangle the type of familial association that may be operant between psychoactive substance use disorder and its subtypes with ADHD.
In one of the few studies of its kind, Milberger et al. (19) reported results from a familial risk analysis of a longitudinal sample of boys with and without ADHD followed into their adolescent years. Although the results were suggestive of independent transmission of ADHD and psychoactive substance use disorder, variable expressivity could not be ruled out. However, because the probands in this analysis were adolescents and they were still transiting through the period of risk for psychoactive substance use disorder, these findings require replication in older samples.
To this end, we reexamined patterns of familial association between ADHD and psychoactive substance use disorder in the same group that we previously studied in adolescence (19) at the 10-year follow-up into young adult years with added attention to subtypes of psychoactive substance use disorder. We conducted familial risk analyses based on models proposed by Pauls et al. (20) , testing hypotheses about the familial relationship between ADHD and psychoactive substance use disorder. Specifically, we tested three competing hypotheses: 1) ADHD and addiction to drugs and alcohol are etiologically independent, 2) ADHD and addiction to drugs and alcohol represent a distinct familial subtype, and 3) ADHD and addiction to drugs and alcohol share common familial etiologic factors (variable expressivity hypothesis).
Method
Subjects
Subjects were derived from a longitudinal case-control family study of ADHD (18 , 21 , 22) . At baseline, we ascertained Caucasian boys ages 6–17 years with (N=140) and without (N=120) DSM-III-R ADHD from pediatric and psychiatric clinics. Previously, this group was followed up at 1 year and 4 years after baseline. The present study reports on the 10-year follow-up of this group, in which 112 ADHD and 105 comparison probands were successfully reascertained. First-degree relatives of these probands included mothers (N=217), fathers (N=216), and siblings (N=310). The parents were assessed at baseline only (because they had passed the age of risk for most psychopathology), whereas the siblings were assessed at baseline (N=243), a 1-year follow-up (N=251), a 4-year follow-up (N=272), and a 10-year follow-up (N=296). At baseline, the 1-year follow-up, and the 4-year follow-up, diagnostic assessments of ADHD were based on the Schedule for Affective Disorders and Schizophrenia for School-Age Children: Epidemiologic Version (K-SADS-E) (23) for DSM-III-R. The sources providing index children and the ascertainment procedures are detailed in previous publications (18 , 21 , 22) . The parents and adult offspring provided written informed consent to participate, and the parents also provided consent for offspring under the age of 18. The children and adolescents provided written assent to participate. The human research committee at Massachusetts General Hospital approved this study.
Follow-Up Assessment Procedures
Lifetime psychiatric assessments at the 10-year follow-up relied on the K-SADS-E (Epidemiologic Version) (24) for subjects younger than 18 years of age and the Structured Clinical Interview for DSM-IV (SCID) (25) (supplemented with modules from the K-SADS-E to assess childhood diagnoses) for subjects 18 years of age and older. Ten percent of the probands (21 of 217) and 12% of the relatives (86 of 743) were younger than 18 years of age at their last assessment. We conducted direct interviews with the subjects and indirect interviews with their mothers (i.e., the mothers completed the interview about their offspring). We combined data from direct and indirect interviews by considering a diagnostic criterion positive if it was endorsed in either interview.
The interviewers were blind to the subject’s ascertainment group, the ascertainment site, and all prior assessments. Details of interviewer training and the reliability of diagnoses are provided in a previous publication (22) .
We considered a disorder positive if DSM-IV diagnostic criteria were unequivocally met. A committee of board-certified child and adult psychiatrists who were blind to the subject’s ADHD status, referral source, and all other data resolved diagnostic uncertainties. Diagnoses presented for review were considered positive only when the committee determined that diagnostic criteria were met to a clinically meaningful degree. Socioeconomic status was measured with the 5-point Hollingshead scale (26) .
Statistical Analysis
First, we compared characteristics (onset, duration, severity, etc.) of psychoactive substance use disorder (any alcohol abuse, drug abuse, alcohol dependence, or drug dependence), alcohol dependence, and drug dependence between the ADHD and comparison probands with logistic regression, linear regression, or negative binomial regression depending on the distribution of the outcome. Second, we conducted three sets of familial risk analyses. In the first set of analyses, we compared four groups of relatives defined by the probands’ ADHD and psychoactive substance use disorder status (i.e., neither, ADHD alone, psychoactive substance use disorder alone, both ADHD and psychoactive substance use disorder). Using Cox proportional hazards models, we compared the relatives of the four proband groups on estimated rates of ADHD and psychoactive substance use disorder. Using Cox models, cosegregation was established if the presence of ADHD in the relative significantly increased the risk for psychoactive substance use disorder in the same relative within the subset of families having a proband with both ADHD and psychoactive substance use disorder. We tested for nonrandom mating of ADHD and substance use disorders with Fisher’s exact test. In the second set of analyses, we repeated the analytical approach described above except that we used alcohol dependence to define the proband groups and as an outcome in the relatives. In the third set of analyses, we used drug dependence to define the proband groups and as an outcome in the relatives. Finally, we assessed whether the risk for alcohol and drug dependence in the relatives was specific to alcohol or drug dependence in the proband using Cox proportional hazards models. To account for the nonindependence of family members, we used the Huber (27) correction to produce robust variances for all statistical tests using family members. All tests were two-tailed with alpha set at 0.05.
Results
Demographic Characteristics
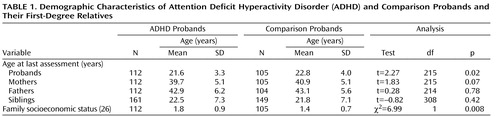
Because probands with ADHD were significantly younger than comparison probands, proband age was controlled for in all analyses ( Table 1 ). Details on attrition are provided in a previous publication (22) .
Characteristics of Substance Use in ADHD and Comparison Probands
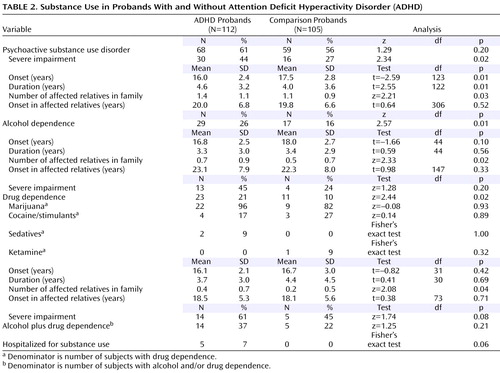
Rates of psychoactive substance use disorder (alcohol abuse, drug abuse, alcohol dependence, or drug dependence) did not differ between ADHD and comparison probands, but ADHD probands had a significantly earlier onset, a longer duration, higher rates of severe impairment associated with psychoactive substance use disorder, and more affected first-degree relatives in relation to comparison probands ( Table 2 ). Rates of both alcohol and drug dependence were significantly higher in the ADHD probands than in the comparison probands, as well as the number of first-degree relatives affected with the same disorder.
Familial Risk for ADHD and Psychoactive Substance Use Disorder
Four groups were used for the familial risk analysis of psychoactive substance use disorder and ADHD: the relatives of 46 comparison probands without psychoactive substance use disorder (comparison probands, N=153), the relatives of 59 comparison probands with psychoactive substance use disorder (comparison probands plus psychoactive substance use disorder, N=205), the relatives of 44 ADHD probands without psychoactive substance use disorder (ADHD, N=149), and the relatives of 68 ADHD probands with psychoactive substance use disorder (ADHD plus psychoactive substance use disorder, N=236).
Risk for ADHD in Relatives
Figure 1 (left) shows that age-adjusted rates of ADHD in the ADHD plus psychoactive substance use disorder and ADHD groups were significantly higher in relation to comparison probands (16% and 23% versus 5%; hazard ratio=1.7, 95% confidence interval [CI]=1.3–2.1, p<0.001, and hazard ratio=1.8, CI=1.2–2.6, p=0.002, respectively). The ADHD plus psychoactive substance use disorder group also had significantly higher rates of ADHD in relation to the comparison probands plus the psychoactive substance use disorder group (23% versus 8%, hazard ratio=1.7, CI=1.2–2.5, p=0.004).
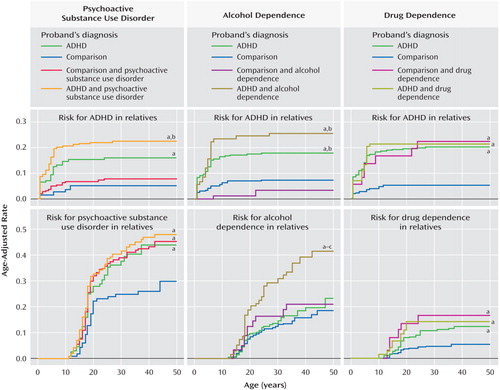
a p<0.05 versus comparison subjects.
b p<0.05 versus comparison subjects plus substance use disorder subjects.
c p<0.05 versus subjects with ADHD.
Risk for Psychoactive Substance Use Disorder in Relatives
The ADHD plus psychoactive substance use disorder, ADHD, and comparison probands plus psychoactive substance use disorder groups all had significantly higher age-adjusted rates of psychoactive substance use disorder in relation to the comparison probands (48%, 44%, and 45% versus 30%; hazard ratio=1.2, CI=1.1–1.4, p=0.001; hazard ratio=1.3, CI=1.0–1.7, p=0.02; and hazard ratio=1.7, CI=1.1–2.7, p=0.02, respectively; Figure 1 , left). There was no evidence for cosegregation in the ADHD plus psychoactive substance use disorder group (68% psychoactive substance use disorder in subjects with ADHD versus 48% psychoactive substance use disorder in subjects without ADHD, p=0.08).
Familial Risk for ADHD and Alcohol Dependence
The familial risk analysis of alcohol dependence and ADHD used four groups: the relatives of 88 comparison probands without alcohol dependence (comparison probands, N=294), the relatives of 17 comparison probands with alcohol dependence (comparison probands plus alcohol dependence, N=64), the relatives of 83 ADHD probands without alcohol dependence (ADHD, N=283), and the relatives of 29 ADHD probands with alcohol dependence (ADHD plus alcohol dependence, N=102).
Risk for ADHD in Relatives
Figure 1 , middle, shows that the rates of ADHD in the ADHD plus alcohol dependence and ADHD groups were significantly higher in relation to comparison probands (18% and 26% versus 8%; hazard ratio=1.6, CI=1.2–2.0, p<0.001, and hazard ratio=1.6, CI=1.2–2.1, p=0.001, respectively) and comparison probands plus alcohol dependence (18% and 26% versus 4%; hazard ratio=3.1, CI=1.5–6.3, p=0.002, and hazard ratio=6.6, CI=1.7–25.0, p=0.006, respectively).
Risk for Alcohol Dependence in Relatives
The ADHD plus alcohol dependence group had a significantly higher age-adjusted rate of alcohol dependence in relatives in relation to comparison probands (42% versus 18%; hazard ratio=1.4, CI=1.2–1.6, p<0.001), comparison probands plus alcohol dependence (42% versus 21%; hazard ratio=1.5, CI=1.0–2.2, p=0.05), and ADHD groups (42% versus 23%; hazard ratio=2.4, CI=1.4–4.0, p=0.002; Figure 1 , middle). There was no evidence for cosegregation in the ADHD plus alcohol dependence group (46% alcohol dependence in probands with ADHD versus 40% alcohol dependence in probands without ADHD, p=0.08).
Familial Risk for ADHD and Drug Dependence
The familial risk analysis of drug dependence and ADHD used four groups: the relatives of 94 comparison probands without drug dependence (comparison probands, N=321), the relatives of 11 comparison probands with drug dependence (comparison probands plus drug dependence, N=37), the relatives of 89 ADHD probands without drug dependence (ADHD probands, N=309), and the relatives of 23 ADHD probands with drug dependence (ADHD probands plus drug dependence, N=76).
Risk for ADHD in Relatives
Figure 1 (right) shows that the rates of ADHD in the ADHD plus drug dependence, ADHD, and comparison probands plus drug dependence groups were significantly higher in relation to the comparison probands (21%, 20%, and 22% versus 5%; hazard ratio=1.7, CI=1.3–2.2, p<0.001; hazard ratio=2.1, CI=1.5–2.7, p<0.001; and hazard ratio=8.0, CI=2.6–24.9, p<0.001, respectively).
Risk for Drug Dependence in Relatives
Likewise, Figure 1 (right) shows that the rates of drug dependence were significantly higher in the ADHD plus drug dependence, ADHD, and comparison probands plus drug dependence groups in relation to comparison probands (14%, 12%, and 17% versus 5%; hazard ratio=1.4, CI=1.1–2.0, p=0.02; hazard ratio=1.5, CI=1.0–2.3, p=0.04; and hazard ratio=5.0, CI=1.2–21.0, p=0.03, respectively). There was no evidence for cosegregation in the ADHD plus drug dependence group (21% drug dependence in subjects with ADHD versus 12% drug dependence in subjects without ADHD, p=0.34).
Nonrandom Rating Among Parents
There was no evidence for nonrandom mating of parental ADHD and parental psychoactive substance use disorder, alcohol dependence, or drug dependence (all p>0.15).
Specific Versus Common Risk for Dependence
The following four groups were used: the relatives of 156 probands without alcohol dependence and without drug dependence (no dependence, N=528), the relatives of 27 probands with alcohol dependence but without drug dependence (alcohol dependence, N=102), the relatives of 15 probands with drug dependence but without alcohol dependence (drug dependence, N=49), and the relatives of 19 probands with alcohol and drug dependence (alcohol plus drug dependence, N=64).
Risk for Alcohol Dependence in Relatives
Figure 2 (top) shows that the alcohol dependence and alcohol plus drug dependence groups had significantly higher rates of alcohol dependence compared to the no dependence group (34% and 32% versus 21%; hazard ratio=2.1, CI=1.2–3.7, p=0.009, and hazard ratio=1.2, CI=1.0–1.5, p=0.02, respectively).
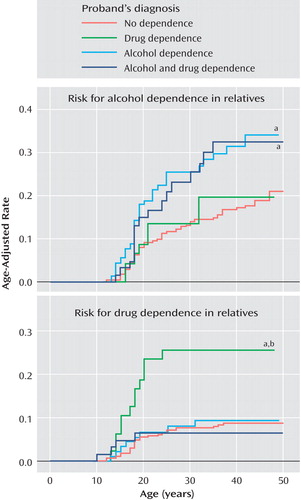
a p<0.05 versus no dependence.
b p<0.05 versus alcohol dependence.
Risk for Drug Dependence in Relatives
Figure 2 (top) shows that the drug dependence group had a significantly higher rate of drug dependence compared to the no dependence (26% versus 9%; hazard ratio=1.9, CI=1.4–2.7, p<0.001) and alcohol dependence (26% versus 10%; hazard ratio=3.0, CI=1.3–6.9, p=0.01) groups. These associations did not vary significantly by the ADHD status of the proband (both interaction effects, p>0.05).
Effect of Mood Disorders on Risk for Substance Use
A secondary analysis was conducted to determine if the risk for alcohol and drug dependence was mediated by mood disorders. Independent of proband ADHD and alcohol dependence status, neither proband major depression with severe impairment (p=0.25) nor proband bipolar disorder (p=0.09) significantly added to the risk for alcohol dependence in relatives. Independent of proband ADHD and drug dependence status, proband major depression with severe impairment did not significantly predict drug dependence in the relatives (p=0.26), but proband bipolar disorder significantly added to the risk for drug dependence in the relatives (hazard ratio=3.4, CI=1.7–7.0, p=0.001).
Discussion
In a systematic evaluation of the familial relationship between ADHD and psychoactive substance use disorder with a well-characterized longitudinal group of ADHD boys as adults and their first-degree relatives, we found the following:
ADHD in the proband was consistently associated with a significantly increased risk for ADHD in relatives irrespective of comorbidity with psychoactive substance use disorder
ADHD in the proband also predicted psychoactive substance use disorder and drug dependence in the relatives
Drug dependence in comparison probands increased the risk for ADHD in relatives
Alcohol dependence in relatives was predicted only by ADHD probands with comorbid alcohol dependence
Both alcohol dependence and drug dependence bred true in families without evidence of a common risk between these disorders.
Although probands with and without ADHD did not differ on the absolute rates of the combined category of any psychoactive substance use disorder, probands with ADHD had an earlier onset, a longer duration, and higher rates of severely impairing psychoactive substance use disorder as well as higher rates of alcohol and drug dependence. Moreover, probands with ADHD had more first-degree relatives with psychoactive substance use disorder, alcohol, and drug dependence in relation to comparison probands. Taken together, these findings indicate that the type of psychoactive substance use disorder that develops in the context of ADHD is a very morbid form of the disorder and support the hypothesis that ADHD is a familial risk factor for psychoactive substance use disorder.
The rates of substance use in the relatives of comparison probands were consistent with the National Comorbidity Survey (28) for psychoactive substance use disorder (32.1% versus 26.6%, respectively) and alcohol dependence (13.7% versus 14.1%, respectively). The relatives of comparison probands had a 5.6% prevalence of drug dependence, in between the 7.5% prevalence reported by the National Comorbidity Survey (28) and the 2.6% prevalence recently reported by Compton and colleagues (29) . For comparison probands, the rates of alcohol and drug dependence were consistent with the National Comorbidity Survey (28) , but the rate of the combined category of psychoactive substance use disorder was twice as high. Although the reasons for this high rate of psychoactive substance use disorder in comparison probands are not entirely clear, it was driven mainly by elevated rates of alcohol abuse that may reflect the high risk for binge drinking in young adults in this age range. For example, the Harvard School of Public Health 1999 College Alcohol Study (30) found that 44% of college students were binge drinkers, which was the same percentage of our comparison probands with alcohol abuse (44%, 46 of 105).
ADHD in the proband consistently increased the risk for ADHD in relatives irrespective of psychoactive substance use disorder status. The finding that the risk for psychoactive substance use disorder in relatives was increased in ADHD probands with and without psychoactive substance use disorder is consistent with the variable expressivity hypothesis that ADHD and psychoactive substance use disorder share common risk factors.
Evaluation of the subtypes of psychoactive substance use disorder revealed divergent patterns of familial transmission for alcohol and drug dependence. Findings revealed that the risk for alcohol dependence was only elevated in the relatives of probands with comorbid ADHD plus alcohol dependence. These results, together with the absence of cosegregation and nonrandom mating between ADHD and alcohol dependence, fit best with the hypothesis of independent transmission between these disorders, with the caveat that the risk for alcohol dependence exists only in the context of families with alcohol dependence and ADHD. In the event that there had been cosegregation of ADHD and alcohol dependence, the findings would have better fit with the hypothesis of family subtype.
On the other hand, the risk for drug dependence was consistent with the variable expressivity hypothesis that the two disorders share common familial determinants. Specifically, the rates of ADHD were equally elevated in the relatives of probands with ADHD, drug dependence, or both and significantly higher than the rate of ADHD in comparison relatives. Likewise, the relatives of probands with ADHD, drug dependence, or both had significantly higher rates of drug dependence compared to the relatives of comparison probands but similar rates of drug dependence compared to each other. Common risks may involve dopamine genes (31) that affect attention and arousal as well as the reward pathways associated with drug dependence. Due to our differential findings for alcohol and drug dependence, genetic studies of ADHD may benefit from understanding the biological and genetic differences between alcohol and drug dependence. Alternatively, ADHD and drug dependence may share a common environmental factor and have distinct genetic causes.
These results are also consistent with findings by our group (32) showing that in relation to comparison probands, adult subjects with ADHD exhibited a greater risk for drug abuse or dependence (8% versus 27%) than for alcohol abuse or dependence (16% versus 31%). Mannuzza et al. (5) also showed that children with ADHD as adults have a high risk for drug use disorders and not alcohol use disorders.
The rates of alcohol dependence and drug dependence were selectively higher in the relatives of probands with the same diagnosis. This finding suggests that the risk for alcohol and drug dependence in relatives is specific to the type of addiction afflicting the probands and is consistent with findings from Merikangas et al. (33) , Bierut et al. (34) , and Milberger et al. (35) , which argue for specific and independent risks for alcohol and drug dependence. However, Nurnberger and colleagues (36) suggested that alcohol and drug dependence share common mechanisms within some families. Additional work using community samples may resolve these differences because discrepant findings could be due to unique aspects of clinically ascertained samples.
Our findings should be interpreted in the context of several limitations. Despite significant differences between ADHD and comparison families in socioeconomic status, our analyses did not control for socioeconomic status. Although future studies should help elucidate the relationship between socioeconomic status, ADHD, and psychoactive substance use disorder, socioeconomic status may not be a true covariate because the outcome (substance use disorders) could affect the covariate (family socioeconomic status) on a majority of the units of analysis (parents).
Our secondary analysis further confirmed that alcohol dependence is independently transmitted because proband mood disorders did not affect the risk for alcohol dependence in the relatives. However, the same analysis suggested that the risk for drug dependence in the relatives could be accounted for by comorbid bipolar disorder in the probands. Future studies should assist in determining the precise nature of the variably expressed risk for drug dependence. In addition, because marijuana was the preponderant drug of dependence in the probands, our findings of variable expressivity may not generalize to other drugs.
Another potential source of bias stems from the indirect psychiatric assessments with the mothers about the probands and their siblings. This method may have led to an underrepresentation of psychopathology in the children. In addition, although the probands and their siblings were assessed at baseline and follow-up assessments, the parents were assessed only at baseline. Thus, it is possible that additional cases of substance use disorders emerged in the parents during the 10-year follow-up period, although the use of Cox models to calculate age-adjusted rates somewhat mitigates this concern. Our group was ascertained with DSM-III-R criteria, so the findings may have differed had DSM-IV been used. However, Biederman and colleagues (37) showed that 93% of children with a DSM-III-R diagnosis also received a DSM-IV diagnosis. Finally, community-based studies should determine if these findings extend to the general population. Studies should also determine if samples of females with ADHD would yield consistent results.
In summary, in a group of pediatrically and psychiatrically referred children and adolescents with ADHD, familial risk analyses suggest that the association between ADHD and drug and alcohol dependence are substance specific, and they are most consistent with the hypothesis of variable expressivity for drug dependence and independent transmission for alcohol dependence.
1. Carroll K, Rounsaville B: History and significance of childhood attention deficit disorder in treatment-seeking cocaine abusers. Compr Psychiatry 1993; 34:75–82Google Scholar
2. Kaminer Y: Clinical implications of the relationship between attention-deficit hyperactivity disorder and psychoactive substance use disorders. Am J Addict 1992; 1:257–264Google Scholar
3. Wilens T, Biederman J, Spencer T, Frances R: Comorbidity of attention deficit hyperactivity and psychoactive substance use disorders. Hosp Community Psychiatry 1994; 45:421–435Google Scholar
4. Hechtman L, Weiss G: Controlled prospective fifteen year follow-up of hyperactives as adults: non-medical drug and alcohol use and anti-social behaviour. Can J Psychiatry 1986; 31:557–567Google Scholar
5. Mannuzza S, Gittelman R, Klein R, Bonagura N, Malloy P, Giampino TL, Addalli KA: Hyperactive boys almost grown up, V: replication of psychiatric status. Arch Gen Psychiatry 1991; 48:77–83Google Scholar
6. Shekim WO, Asarnow RF, Hess E, Zaucha K, Wheeler N: A clinical and demographic profile of a sample of adults with attention deficit hyperactivity disorder, residual state. Compr Psychiatry 1990; 31:416–425Google Scholar
7. Biederman J, Faraone SV, Spencer T, Wilens T, Norman D, Lapey KA, Mick E, Lehman BK, Doyle A: Patterns of psychiatric comorbidity, cognition, and psychosocial functioning in adults with attention deficit hyperactivity disorder. Am J Psychiatry 1993; 150:1792–1798Google Scholar
8. Kessler RC, Adler L, Barkley R, Biederman J, Conners CK, Demler O, Faraone SV, Greenhill LL, Howes MJ, Secnik K, Spencer T, Ustun TB, Walters EE, Zaslavsky AM: The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163:716–723Google Scholar
9. DeMilio L: Psychiatric syndromes in adolescent substance abusers. Am J Psychiatry 1989; 146:1212–1214Google Scholar
10. Kandel D, Johnson J, Bird H, Canino G, Goodman S, Lahey B, Reiger D, Schwab-Stone M: Psychiatric disorders associated with substance use among children and adolescents: findings from the Methods for the Epidemiology of Child and Adolescent Mental Disorders (MECA) study. J Abnorm Child Psychol 1997; 25:121–132Google Scholar
11. Earls F, Reich W, Jung KG, Cloninger R: Psychopathology in children of alcoholic and antisocial parents. Alcohol Clin Exp Res 1988; 12:481–487Google Scholar
12. Jason Aronson H, Gilbert A: Preadolescent sons of male alcoholics. Arch Gen Psychiatry 1963; 8:235–241Google Scholar
13. Steinhausen H, Gobel D, Nestler V: Psychopathology in the offspring of alcoholic parents. J Am Acad Child Adolesc Psychiatry 1984; 23:465–471Google Scholar
14. Wilens T, Biederman J, Kiely K, Bredin E, Spencer T: Pilot study of behavioral and emotional disturbances in the high-risk children of parents with opioid dependence. J Am Acad Child Adolesc Psychiatry 1995; 34:779–785Google Scholar
15. Morrison JR, Stewart MA: A family study of the hyperactive child syndrome. Biol Psychiatry 1971; 3:189–195Google Scholar
16. Cantwell DP: Psychiatric illness in the families of hyperactive children. Arch Gen Psychiatry 1972; 27:414–417Google Scholar
17. Biederman J, Faraone SV, Keenan K, Knee D, Tsuang MT: Family-genetic and psychosocial risk factors in DSM-III attention deficit disorder. J Am Acad Child Adolesc Psychiatry 1990; 29:526–533Google Scholar
18. Biederman J, Faraone SV, Keenan K, Benjamin J, Krifcher B, Moore C, Sprich-Buckminster S, Ugaglia K, Jellinek MS, Steingard R, Spencer T, Norman D, Kolodny R, Kraus I, Perrin J, Keller MB, Tsuang MT: Further evidence for family-genetic risk factors in attention deficit hyperactivity disorder: patterns of comorbidity in probands and relatives in psychiatrically and pediatrically referred samples. Arch Gen Psychiatry 1992; 49:728–738Google Scholar
19. Milberger S, Faraone SV, Biederman J, Chu MP, Wilens T: Familial risk analysis of the association between attention-deficit/hyperactivity disorder and psychoactive substance use disorders. Arch Pediatr Adolesc Med 1998; 152:945–951Google Scholar
20. Pauls DL, Towbin KE, Leckman JF, Zahner GE, Cohen DJ: Gilles de la Tourette’s syndrome and obsessive-compulsive disorder: evidence supporting a genetic relationship. Arch Gen Psychiatry 1986; 43:1180–1182Google Scholar
21. Biederman J, Faraone S, Milberger S, Guite J, Mick E, Chen L, Mennin D, Marrs A, Ouellette C, Moore P, Spencer T, Norman D, Wilens T, Kraus I, Perrin J: A prospective 4-year follow-up study of attention-deficit hyperactivity and related disorders. Arch Gen Psychiatry 1996; 53:437–446Google Scholar
22. Biederman J, Monuteaux M, Mick E, Spencer T, Wilens T, Silva J, Snyder L, Faraone SV: Young adult outcome of attention deficit hyperactivity disorder: a controlled 10 year prospective follow-up study. Psychol Med 2006; 36:167–179Google Scholar
23. Orvaschel H, Puig-Antich J: Schedule for Affective Disorders and Schizophrenia for School-Age Children—Epidemiologic Version (K-SAD-E), 4th revision. Ft Lauderdale, Fla, Nova University, 1987Google Scholar
24. Orvaschel H: Schedule for Affective Disorder and Schizophrenia for School-Age Children—Epidemiologic Version (K-SAD-E), 5th ed. Ft Lauderdale, Fla, Nova Southeastern University, Center for Psychological Studies, 1994Google Scholar
25. First M, Spitzer R, Gibbon M, Williams J: Structured Clinical Interview for DSM-IV Axis I Disorders. Washington, DC, American Psychiatric Press, 1997Google Scholar
26. Hollingshead AB: Four Factor Index of Social Status. New Haven, Conn, Yale Press, 1975Google Scholar
27. Huber PJ: The behavior of maximum likelihood estimates under non-standard conditions. Proceedings of the Fifth Berkeley Symposium on Mathematical Statistics and Probability 1967; 1:221–233Google Scholar
28. Kessler R, McGonagle K, Zhao S, Nelson C, Hughes M, Eshleman S, Wittchen H, Kendler K: Lifetime and 12 month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch Gen Psychiatry 1994; 51:8–19Google Scholar
29. Compton WM, Thomas YF, Stinson FS, Grant BF: Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry 2007; 64:566–576Google Scholar
30. Wechsler H, Lee JE, Kuo M, Lee H: College binge drinking in the 1990s: a continuing problem: results of the Harvard School of Public Health 1999 College Alcohol Study. J Am Coll Health 2000; 48:199–210Google Scholar
31. Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick J, Holmgren MA, Sklar P: Molecular genetics of attention deficit hyperactivity disorder. Biol Psychiatry 2005; 57:1313–1323Google Scholar
32. Biederman J, Wilens T, Mick E, Milberger S, Spencer TJ, Faraone SV: Psychoactive substance use disorder in adults with attention deficit hyperactivity disorder (ADHD): effects of ADHD and psychiatric comorbidity. Am J Psychiatry 1995; 152:1652–1658Google Scholar
33. Merikangas K, Stolar M, Stevens D, Goulet J, Preisig M, Fenton B, Zhang H, O’Malley S, Rounsaville B: Familial transmission of substance use disorders. Arch Gen Psychiatry 1998; 55:973–979Google Scholar
34. Bierut L, Dinwiddie S, Begleiter H, Crowe R, Hesselbrock V, Nurnberger J, Porjesz B, Schuckit M, Reich T: Familial transmission of substance dependence: alcohol, marijuana, cocaine, and habitual smoking. Arch Gen Psychiatry 1998; 55:982–988Google Scholar
35. Milberger S, Faraone SV, Biederman J, Chu MP, Feighner JA: Substance use disorders in high-risk adolescent offspring. Am J Addict 1999; 8:211–219Google Scholar
36. Nurnberger JI Jr, Wiegand R, Bucholz K, O’Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, Petti T, Bierut L, Hinrichs AL, Kuperman S, Hesselbrock V, Porjesz B: A family study of alcohol dependence: coaggregation of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry 2004; 61:1246–1256Google Scholar
37. Biederman J, Faraone SV, Weber W, Russell RL, Rater M, Park K: Correspondence between DSM-III-R and DSM-IV attention deficit hyperactivity disorder (ADHD). J Am Acad Child Adolesc Psychiatry 1997; 36:1682–1687Google Scholar